Aberrant CD3-Positive, CD8-Low, CD7-Negative Lymphocytes May Appear During Viral Infections and Mimic Peripheral T-Cell Lymphoma
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Specimens for Flow Cytometric Analysis
2.2. Panel for T-Cell Immunophenotyping
2.3. Panel for B-Cell Immunophenotyping
2.4. Cell Sorting with MoFlo
2.5. PCR Analysis for Virus Detection
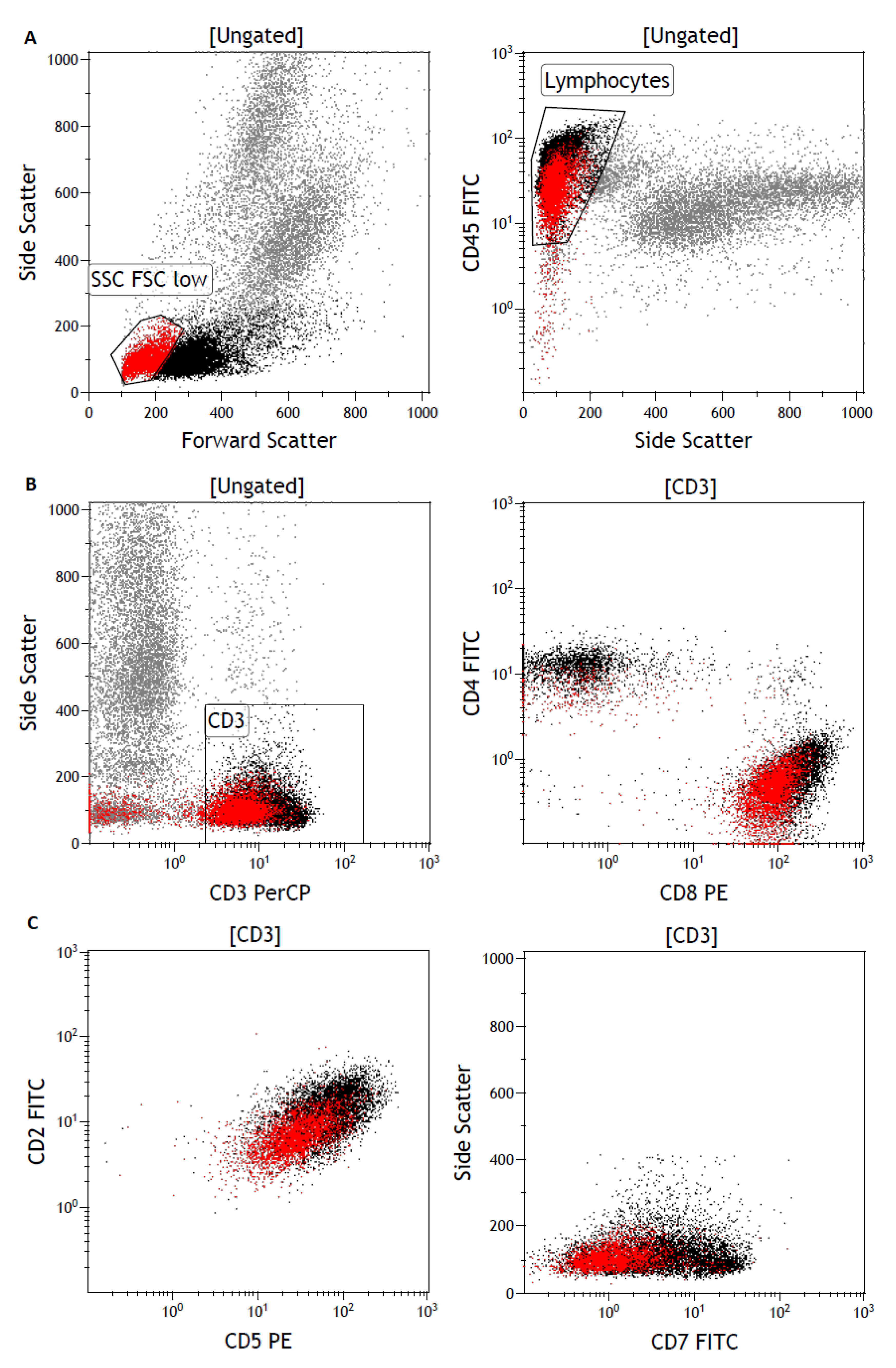
3. Results
3.1. Patient Characteristics
3.2. Flow Cytometry Detects a Lymphocyte Subpopulation with Low T-Cell Marker Expression
3.3. Analysis for T-cell Receptor Repertoire and Viral DNA
3.4. Follow-Up Monitoring of the SSC/FSC-Low T-Cell Population
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; Volume 2, pp. 345–421. [Google Scholar]
- Gorczyca, W.; Weisberger, J.; Liu, Z.; Tsang, P.; Hossein, M.; Wu, C.D.; Dong, H.; Wong, J.Y.L.; Tugulea, S.; Dee, S.; et al. An approach to diagnosis of t-cell lymphoproliferative disorders by flow cytometry. Cytometry 2002, 50, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.; Picker, L.J.; Aquino, D.B.; McKenna, R.W.; Dawson, D.B.; Kroft, S.H. Immunophenotypic analysis of peripheral t-cell neoplasms. A multiparameter flow cytometric approach. Am. J. Clin. Pathol. 2001, 116, 512–526. [Google Scholar] [CrossRef] [PubMed]
- IOTest® Beta Mark TCR Vß Repertoire Kit PN IM3497, Data Sheet, Beckman Coulter, Brea, California, USA. Available online: https://www.bc-cytometry.com/PDF/DataSheet/IM3497DS.pdf (accessed on 20 January 2020).
- Lazarovits, A.I.; White, M.J.; Karsh, J. Cd7-t cells in rheumatoid arthritis. Arthritis Rheum 1992, 35, 615–624. [Google Scholar] [CrossRef]
- Moll, M.; Reinhold, U.; Kukel, S.; Abken, H.; Müller, R.; Oltermann, I.; Kreysel, H.W. Cd7-negative helper t cells accumulate in inflammatory skin lesions. J. Investig. Dermatol. 1994, 102, 328–332. [Google Scholar] [CrossRef]
- Smith, K.J.; Skelton, H.G.; Chu, W.S.; Yeager, J.; Angritt, P.; Wagner, K.F. Decreased cd7 expression in cutaneous infiltrates of hiv-1+ patients. Military medical consortium for the advancement of retroviral research (mmcarr). Am. J. Dermatopathol. 1995, 17, 564–569. [Google Scholar] [CrossRef]
- Weisberger, J.; Cornfield, D.; Gorczyca, W.; Liu, Z. Downregulation of pan t-cell antigens, particularly cd7, in acute infectious mononucleosis. Am. J. Clin. Pathol. 2003, 120, 49–55. [Google Scholar] [CrossRef]
- Esau, D. Viral Causes of Lymphoma: The History of Epstein-Barr Virus and Human T-Lymphotropic Virus 1. Virology (Auckl.) 2017, 8, 1178122X17731772. [Google Scholar] [CrossRef]
- International T-Cell Lymphoma Project. International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study: Pathology Findings and Clinical Outcomes. J. Clin. Oncol. 2008, 25, 4124–4130. [Google Scholar]
- Tomkinson, B.E.; Wagner, D.K.; Nelson, D.L.; Sullivan, J.L. Activated lymphocytes during acute Epstein-Barr virus infection. J. Immunol. 1987, 139, 3802–3807. [Google Scholar] [PubMed]
- Ebihara, T.; Sakai, N.; Koyama, S. CD8+ T cell subsets of cytotoxic T lymphocytes induced by Epstein-Barr virus infection in infectious mononucleosis. Tohoku J. Exp. Med. 1990, 162, 213–224. [Google Scholar] [CrossRef]
- Bharadwaj, M.; Burrows, S.; Burrows, S.J.; Moss, D.J.; Catalina, M.; Khanna, R. Longitudinal dynamics of antigen-specific CD8+ cytotoxic T lymphocytes following Epstein-Barr virus infection. Blood 2001, 98, 2588–2589. [Google Scholar] [CrossRef] [PubMed]
- Zidovec, S.; Culig, Z.; Begovac, J.; Jeren, T. Comparison of lymphocyte subpopulations in the peripheral blood of patients with infectious mononucleosis and human immunodeficiency virus infections: A preliminary report. J. Clin. Lab. Immunol. 1998, 50, 63–69. [Google Scholar]
- Moss, P.; Khan, N. CD8(+) t-cell immunity to cytomegalovirus. Hum. Immunol. 2004, 65, 456–464. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Esgalhado, A.J.; Patrão, L.; Santos, M.; Neves, V.P.; Martinez, J.; Patto, M.A.V.; Silva, H.; Arosa, F.A. Distinctive CD8+ T cell and MHC class I signatures in polycythemia vera patients. Ann. Hematol. 2018, 97, 1563–1575. [Google Scholar] [CrossRef]
- Li, Z.; Xia, Y.; Feng, L.-N.; Chen, J.-R.; Li, H.-M.; Cui, J.; Cai, Q.-Q.; Sim, K.S.; Nairismägi, M.-L.; Laurensia, Y.; et al. Genetic risk of extranodal natural killer T-cell lymphoma: A genome-wide association study. The Lancet. Oncology 2016, 17, 1240–1247. [Google Scholar] [PubMed]
- Tortorella, D.; Gewurz, B.E.; Furman, M.H.; Schust, D.J.; Ploegh, H.L. Viral Subversion of the Immune System. Annu. Rev. Immunol. 2000, 18, 861–926. [Google Scholar] [CrossRef] [PubMed]
- Ressing, M.E.; van Leeuwen, D.; Verreck, F.A.W.; Gomez, R.; Heemskerk, B.; Toebes, M.; Mullen, M.M.; Jardetzky, T.S.; Longnecker, R.; Schilham, M.W.; et al. Interference with T cell receptor-HLA-DR interactions by Epstein-Barr virus gp42 results in reduced T helper cell recognition. Proc. Natl. Acad. Sci. USA 2003, 100, 11583–11588. [Google Scholar] [CrossRef] [PubMed]



| Patient (no.) | Age (years) | Sex | % of WBC | % of Lymphocytes | Diagnosis |
|---|---|---|---|---|---|
| 1 | 15 | M | 11 | 16 | EBV |
| 2 | 17 | M | 25 | 33 | EBV |
| 3 | 46 | M | 14 | 19 | EBV |
| 4* | 77 | M | 17 | 28 | Unknown viral infection |
| 5* | 31 | M | 24 | 31 | EBV |
| 6 | 65 | M | 4 | 8 | Unknown viral infection |
| 7 | 22 | F | 29 | 37 | EBV |
| 8 | 71 | F | 16 | 25 | CMV |
| 9 | 20 | M | 21 | 31 | EBV |
| 10 | 59 | F | – | – | EBV |
| 11* | 60 | F | 20 | 37 | EBV |
| 12 | 59 | M | 4 | 5 | CMV |
| 13 | 26 | F | 14 | 31 | EBV |
| 14 | 45 | F | 14 | 22 | CMV |
| 15 | 18 | M | 2 | 2 | EBV |
| 16 | 17 | M | 7 | 12 | EBV |
| 17 | 37 | F | 10 | 24 | MMR |
| 18 | 37 | M | 1 | 1.3 | EBV |
| Patient (no.) | SSC/FSC | CD3 | CD8 | CD4 | CD5 | CD2 | CD7 | HLA-DR | TCR α/β | CD4/CD8 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Low | ++ | ++ | - | ++ | ++ | - | + | ++ | 0.07 |
| 2 | Low | ++ | + | - | ++ | ++ | - | n.i. | + | 0.18 |
| 3 | Low | + | + | - | + | + | - | + | + | 0.09 |
| 4 | Low | ++ | + | - | + | ++ | - | n.i. | + | 0.4 |
| 5 | Low | ++ | ++ | - | ++ | ++ | - | n.i. | + | 0.1 |
| 6 | Low | ++ | + | - | ++ | ++ | - | n.i. | + | 0.8 |
| 7 | Low | ++ | ++ | - | n.i. | n.i. | n.i. | n.i. | n.i. | 0.49 |
| 8 | Low | ++ | ++ | - | ++ | ++ | - | n.i. | ++ | 0.31 |
| 9 | Low | ++ | ++ | - | +++ | ++ | - | n.i. | + | 0.21 |
| 10 | Low | +++ | ++ | - | ++ | ++ | - | n.i. | +++ | 1.8 |
| 11 | Low | ++ | + | - | + | ++ | - | n.i. | + | 0.32 |
| 12 | Low | +++ | ++ | - | ++ | +++ | - | n.i. | ++ | 0.17 |
| 13 | Low | +++ | ++ | - | ++ | ++ | - | + | + | 0.18 |
| 14 | Low | +++ | ++ | - | ++ | ++ | - | n.i. | ++ | 0.25 |
| 15 | Low | +++ | ++ | - | ++ | +++ | + | n.i. | +++ | 0.04 |
| 16 | Low | +++ | ++ | - | ++ | +++ | - | ++ | n.i. | 0.11 |
| 17 | Low | ++ | + | - | + | + | - | n.i. | + | 0.28 |
| 18 | Low | ++ | ++ | - | + | + | - | n.i. | n.i. | 0.29 |
| Population | CD8-High | CD4+ | CD8-Low | CD19+ | Granulocytes |
|---|---|---|---|---|---|
| EBV-PCR | negative | negative | positive | positive | negative |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klameth, A.; Neubauer, A.; Keller, C.; Aepinus, C.; Kaiser, U.; Hoffmann, J.; Brendel, C. Aberrant CD3-Positive, CD8-Low, CD7-Negative Lymphocytes May Appear During Viral Infections and Mimic Peripheral T-Cell Lymphoma. Diagnostics 2020, 10, 204. https://doi.org/10.3390/diagnostics10040204
Klameth A, Neubauer A, Keller C, Aepinus C, Kaiser U, Hoffmann J, Brendel C. Aberrant CD3-Positive, CD8-Low, CD7-Negative Lymphocytes May Appear During Viral Infections and Mimic Peripheral T-Cell Lymphoma. Diagnostics. 2020; 10(4):204. https://doi.org/10.3390/diagnostics10040204
Chicago/Turabian StyleKlameth, Andreas, Andreas Neubauer, Christian Keller, Christian Aepinus, Ulrich Kaiser, Jörg Hoffmann, and Cornelia Brendel. 2020. "Aberrant CD3-Positive, CD8-Low, CD7-Negative Lymphocytes May Appear During Viral Infections and Mimic Peripheral T-Cell Lymphoma" Diagnostics 10, no. 4: 204. https://doi.org/10.3390/diagnostics10040204
APA StyleKlameth, A., Neubauer, A., Keller, C., Aepinus, C., Kaiser, U., Hoffmann, J., & Brendel, C. (2020). Aberrant CD3-Positive, CD8-Low, CD7-Negative Lymphocytes May Appear During Viral Infections and Mimic Peripheral T-Cell Lymphoma. Diagnostics, 10(4), 204. https://doi.org/10.3390/diagnostics10040204





