Increased Circulating Levels of Tissue-Type Plasminogen Activator Are Associated with the Risk of Spontaneous Abortion During the First Trimester of Pregnancy
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Settings
2.2. Plasma Collection
2.3. Extracellular Vesicles Isolation from Maternal Circulation
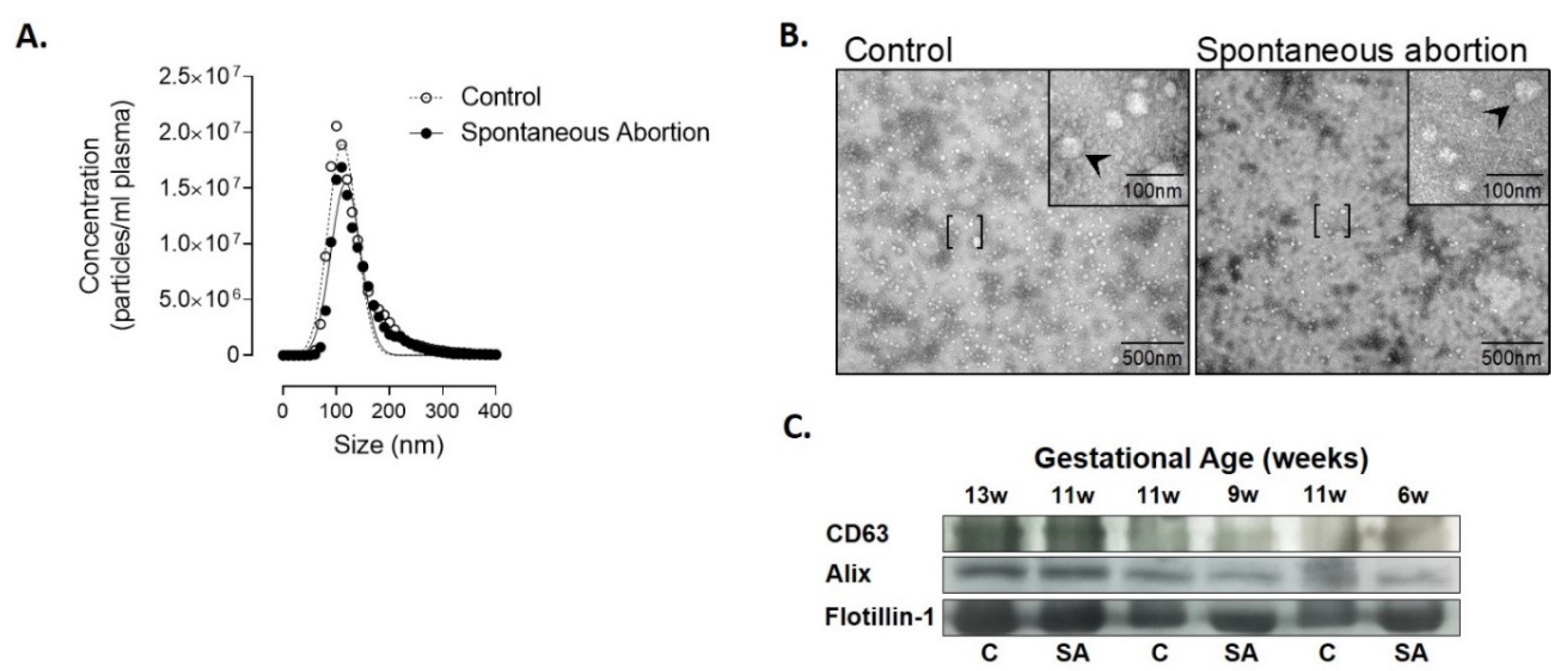
2.4. Nanotracking Particle Analysis
2.5. Transmission Electron Microscopy
2.6. Western Blot
2.7. ELISA Assay
2.8. Statistical Analyses
3. Results
3.1. Clinical and Biochemical Characteristics of the Study Population
3.2. Characterization and Quantification of Extracellular Vesicles in Plasma
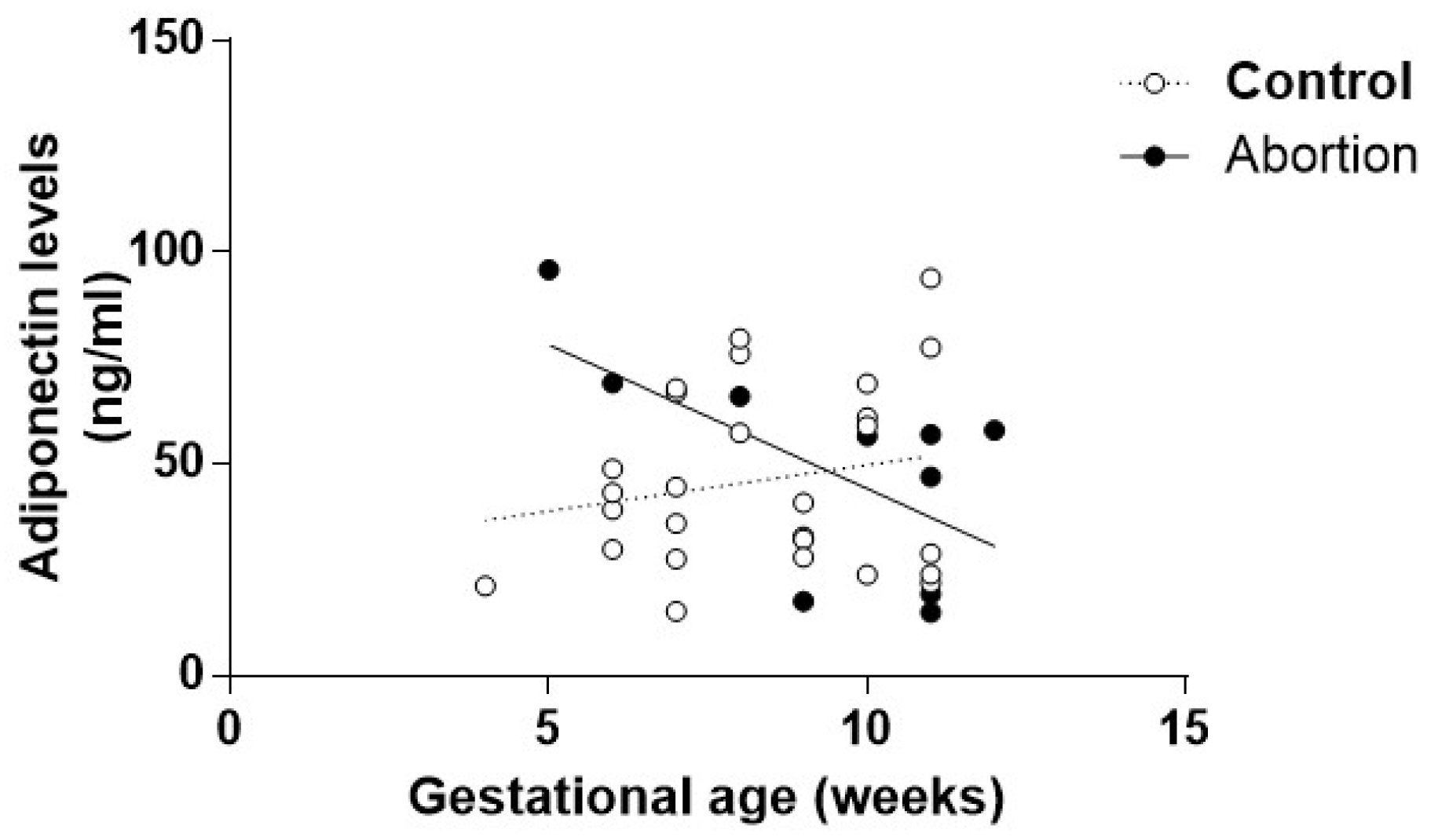
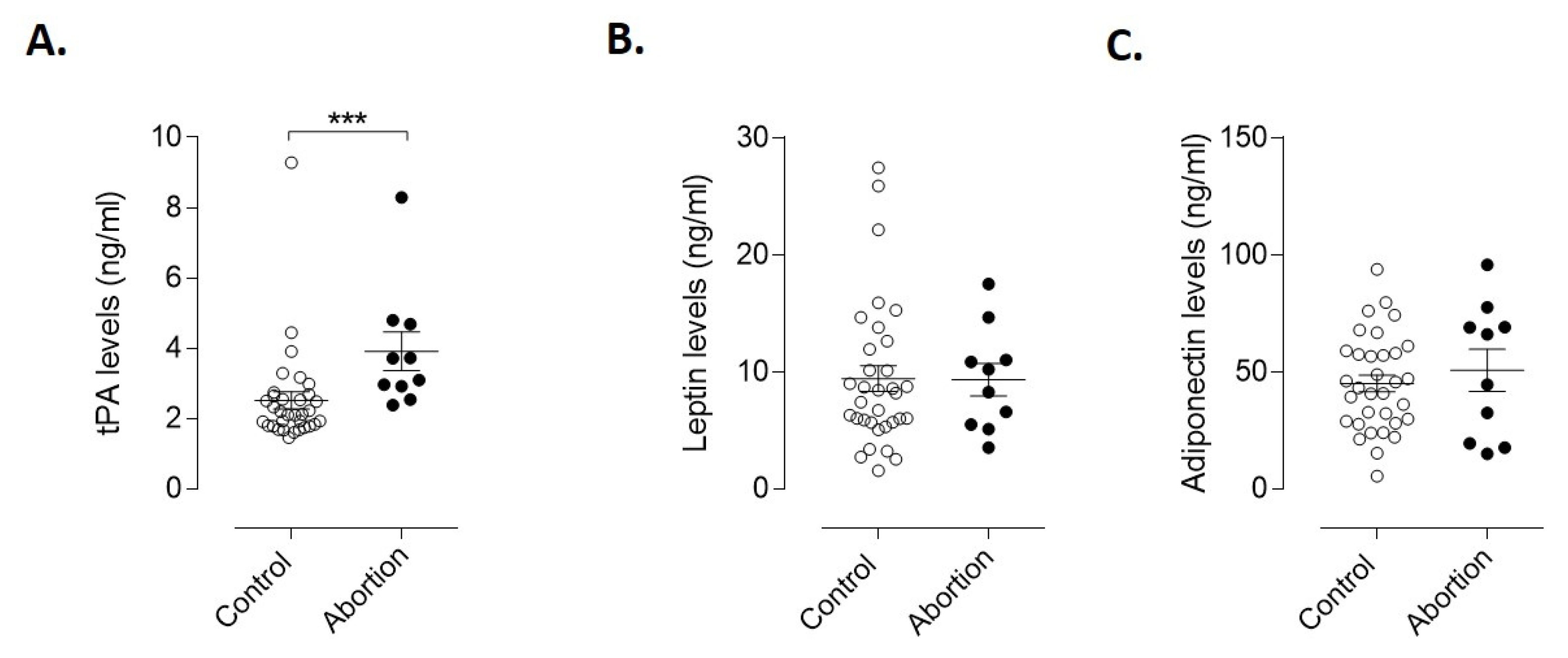
3.3. Endothelial and Adipogenic Markers as Early Predictors of Spontaneous Abortion
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Robinson, G.E. Pregnancy loss. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, C.; Wang, L.; Chen, D.; Guang, W.; French, J. Conception, early pregnancy loss, and time to clinical pregnancy: A population-based prospective study. Fertil. Steril. 2003, 79, 577–584. [Google Scholar] [CrossRef]
- Giakoumelou, S.; Wheelhouse, N.; Cuschieri, K.; Entrican, G.; Howie, S.E.; Horne, A.W. The role of infection in miscarriage. Hum. Reprod. Update 2016, 22, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Bourne, T.; Bottomley, C. When is a pregnancy nonviable and what criteria should be used to define miscarriage? Fertil. Steril. 2012, 98, 1091–1096. [Google Scholar] [CrossRef]
- Seeber, B.E. What serial hcg can tell you, and cannot tell you, about an early pregnancy. Fertil. Steril. 2012, 98, 1074–1077. [Google Scholar] [CrossRef]
- Ong, C.Y.; Liao, A.W.; Spencer, K.; Munim, S.; Nicolaides, K.H. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein a as predictors of pregnancy complications. BJOG 2000, 107, 1265–1270. [Google Scholar] [CrossRef]
- Mol, B.W.; Lijmer, J.G.; Ankum, W.M.; van der Veen, F.; Bossuyt, P.M. The accuracy of single serum progesterone measurement in the diagnosis of ectopic pregnancy: A meta-analysis. Hum. Reprod. 1998, 13, 3220–3227. [Google Scholar] [CrossRef]
- Verhaegen, J.; Gallos, I.D.; van Mello, N.M.; Abdel-Aziz, M.; Takwoingi, Y.; Harb, H.; Deeks, J.J.; Mol, B.W.; Coomarasamy, A. Accuracy of single progesterone test to predict early pregnancy outcome in women with pain or bleeding: Meta-analysis of cohort studies. BMJ 2012, 345, e6077. [Google Scholar] [CrossRef]
- Revenfeld, A.L.; Baek, R.; Nielsen, M.H.; Stensballe, A.; Varming, K.; Jorgensen, M. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin. Ther. 2014, 36, 830–846. [Google Scholar] [CrossRef]
- Ghiran, I.; Kuo, W.P. Examining the role of microvesicles to develop prognostic and diagnostic assays. J. Appl. Oral. Sci. 2010, 18. [Google Scholar] [CrossRef]
- Katsuda, T.; Kosaka, N.; Ochiya, T. The roles of extracellular vesicles in cancer biology: Toward the development of novel cancer biomarkers. Proteomics 2014, 14, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Van der Pol, E.; Boing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Scholz-Romero, K.; Perez, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E.; Salomon, C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Scholz-Romero, K.; Sarker, S.; Sweeney, E.; Kobayashi, M.; Correa, P.; Longo, S.; Duncombe, G.; Mitchell, M.D.; Rice, G.E.; et al. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes 2016, 65, 598–609. [Google Scholar] [CrossRef]
- Salomon, C.; Guanzon, D.; Scholz-Romero, K.; Longo, S.; Correa, P.; Illanes, S.E.; Rice, G.E. Placental exosomes as early biomarker of preeclampsia: Potential role of exosomal micrornas across gestation. J. Clin. Endocrinol. Metab. 2017, 102, 3182–3194. [Google Scholar] [CrossRef]
- Luo, Z.C.; Nuyt, A.M.; Delvin, E.; Fraser, W.D.; Julien, P.; Audibert, F.; Girard, I.; Shatenstein, B.; Deal, C.; Grenier, E.; et al. Maternal and fetal leptin, adiponectin levels and associations with fetal insulin sensitivity. Obesity 2013, 21, 210–216. [Google Scholar] [CrossRef]
- Mazaki-Tovi, S.; Kanety, H.; Pariente, C.; Hemi, R.; Wiser, A.; Schiff, E.; Sivan, E. Maternal serum adiponectin levels during human pregnancy. J. Perinatol. 2007, 27, 77–81. [Google Scholar] [CrossRef]
- Lage, M.; Garcia-Mayor, R.V.; Tome, M.A.; Cordido, F.; Valle-Inclan, F.; Considine, R.V.; Caro, J.F.; Dieguez, C.; Casanueva, F.F. Serum leptin levels in women throughout pregnancy and the postpartum period and in women suffering spontaneous abortion. Clin. Endocrinol. 1999, 50, 211–216. [Google Scholar] [CrossRef]
- Laird, S.M.; Quinton, N.D.; Anstie, B.; Li, T.C.; Blakemore, A.I. Leptin and leptin-binding activity in women with recurrent miscarriage: Correlation with pregnancy outcome. Hum. Reprod. 2001, 16, 2008–2013. [Google Scholar] [CrossRef]
- Hong, Y.; Xie, Q.X.; Chen, C.Y.; Yang, C.; Li, Y.Z.; Chen, D.M.; Xie, M.Q. Insulin resistance in first-trimester pregnant women with pre-pregnant glucose tolerance and history of recurrent spontaneous abortion. J. Biol. Regul. Homeost. Agents 2013, 27, 225–231. [Google Scholar]
- Cseh, K.; Baranyi, E.; Melczer, Z.; Kaszas, E.; Palik, E.; Winkler, G. Plasma adiponectin and pregnancy-induced insulin resistance. Diabetes Care 2004, 27, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Gris, J.C.; Schved, J.F.; Neveu, S.; Mares, P.; Aguilar-Martinez, P.; Dupaigne, D. Impaired fibrinolytic capacity and early recurrent spontaneous abortion. BMJ 1990, 300. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.E.; Haubelt, H.; Pauer, H.U.; Hinney, B.; Krome-Cesar, C.; Legler, T.J.; Hellstern, P.; Emons, G.; Zoll, B.; Kohler, M. Recurrent pregnancy loss and its relation to fv leiden, fii g20210a and polymorphisms of plasminogen activator and plasminogen activator inhibitor. Pathophysiol. Haemost. Thromb. 2003, 33, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Stirling, Y.; Woolf, L.; North, W.R.; Seghatchian, M.J.; Meade, T.W. Haemostasis in normal pregnancy. Thromb. Haemost. 1984, 52, 176–182. [Google Scholar] [CrossRef]
- Higgins, J.R.; Walshe, J.J.; Darling, M.R.; Norris, L.; Bonnar, J. Hemostasis in the uteroplacental and peripheral circulations in normotensive and pre-eclamptic pregnancies. Am. J. Obstet. Gynecol. 1998, 179, 520–526. [Google Scholar] [CrossRef]
- Belo, L.; Santos-Silva, A.; Rumley, A.; Lowe, G.; Pereira-Leite, L.; Quintanilha, A.; Rebelo, I. Elevated tissue plasminogen activator as a potential marker of endothelial dysfunction in pre-eclampsia: Correlation with proteinuria. BJOG 2002, 109, 1250–1255. [Google Scholar] [CrossRef]
- Bu, C.; Zhang, C.; Li, Z.; Gao, L.; Xie, Z.; Cai, G. Autoantibodies to plasminogen and tissue plasminogen activator in women with recurrent pregnancy loss. Clin. Exp. Immunol. 2007, 149, 31–39. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Peiris, H.N.; Kobayashi, M.; Koh, Y.Q.; Duncombe, G.; Illanes, S.E.; Rice, G.E.; Salomon, C. Placental exosomes in normal and complicated pregnancy. Am. J. Obstet. Gynecol. 2015, 213, S173–S181. [Google Scholar] [CrossRef]
- Chong, S.Y.; Lee, C.K.; Huang, C.; Ou, Y.H.; Charles, C.J.; Richards, A.M.; Neupane, Y.R.; Pavon, M.V.; Zharkova, O.; Pastorin, G.; et al. Extracellular vesicles in cardiovascular diseases: Alternative biomarker sources, therapeutic agents, and drug delivery carriers. Int. J. Mol. Sci. 2019, 20, 3272. [Google Scholar] [CrossRef] [PubMed]
- Mustapic, M.; Eitan, E.; Werner, J.K., Jr.; Berkowitz, S.T.; Lazaropoulos, M.P.; Tran, J.; Goetzl, E.J.; Kapogiannis, D. Plasma extracellular vesicles enriched for neuronal origin: A potential window into brain pathologic processes. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Miranda, J.; Paules, C.; Nair, S.; Lai, A.; Palma, C.; Scholz-Romero, K.; Rice, G.E.; Gratacos, E.; Crispi, F.; Salomon, C. Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction —Liquid biopsies to monitoring fetal growth. Placenta 2018, 64, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, C.J.; Hoggard, N.; Thomas, L.; Mercer, J.G.; Wallace, J.M.; Lea, R.G. Placental leptin. Rev. Reprod. 2000, 5, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Haghiac, M.; Basu, S.; Presley, L.; Serre, D.; Catalano, P.M.; Hauguel-de Mouzon, S. Patterns of adiponectin expression in term pregnancy: Impact of obesity. J. Clin. Endocrinol. Metab. 2014, 99, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Hauguel-de Mouzon, S.; Catalano, P. Adiponectin: Are measurements clinically useful in pregnancy? Diabetes Care 2013, 36, 1434–1436. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Characteristics | Abortion (n = 10) | Controls (n = 34) | p-Value |
|---|---|---|---|
| Maternal age (years), mean (SD) | 32.80 (6.14) | 29.82 (5.20) | 0.1716 |
| Maternal weight (kg), mean (SD) | 64.45 (2.71) | 67.68 (14.25) | 0.4829 |
| Maternal height (cm), mean (SD) | 161.10 (6.62) | 162.70 (6.01) | 0.4759 |
| Maternal BMI (kg/m2), mean (SD) | 24.93 (2.05) | 25.78 (4.94) | 0.5992 |
| GA at time of blood sampling (weeks) | 9.95 (5.04) | 9.37 (2.25) | 0.7137 |
| GA at abortion (weeks) | 7.90 (1.97) | ||
| GA at delivery (weeks) | 38.42 (1.28) | ||
| Nullipara (%), n | 70.00 (7) | 38.24 (13) |
| Characteristics | Abortion (n = 10) | Controls (n = 34) | p-Value |
|---|---|---|---|
| Hematocrit (%), mean (SD) | 35.40 (6.43) | 36.83 (2.19) | 0.2859 |
| Hemoglobin (g/dL), mean (SD) | 12.93 (1.21) | 12.76 (0.77) | 0.6027 |
| Total Cholesterol (mg/dL), mean (SD) | 179.60 (24.22) | 169.30 (27.68) | 0.2953 |
| HDL (mg/dL), mean (SD) | 63.90 (11.14) | 63.71 (14.16) | 0.9685 |
| LDL (mg/dL), mean (SD) | 114.70 (30.94) | 98.56 (24.40) | 0.0905 |
| Triglycerides (mg/dL), mean (SD) | 99.10 (28.57) | 108.20 (54.74) | 0.6185 |
| Insulin (IU/L), mean (SD) | 7.811 (2.26) | 10.11 (5.89) | 0.2594 |
| Glycemia (mg/dL), mean (SD) | 74.60 (5.38) | 77.24 (5.66) | 0.1975 |
| HbA1c (mg/dL), mean (SD) | 5.07 (0.23) | 5.09 (0.17) | 0.7172 |
| Uric Acid (mg/dL), mean (SD) | 3.10 (0.74) | 3.03 (0.85) | 0.8061 |
| AST (IU/L), mean (SD) | 19.90 (3.04) | 21.65 (7.52) | 0.4800 |
| ALT (IU/L), mean (SD) | 24.70 (6.02) | 28.56 (11.16) | 0.3026 |
| ALP (IU/L), mean (SD) | 67.80 (14.54) | 63.26 (11.69) | 0.3134 |
| SHBG (nm/L), mean (SD) | 249.20 (103.80) | 259.70 (136.40) | 0.8240 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, L.J.; Varas-Godoy, M.; Acuña-Gallardo, S.; Correa, P.; Passalacqua, G.; Monckeberg, M.; Rice, G.E.; Illanes, S.E. Increased Circulating Levels of Tissue-Type Plasminogen Activator Are Associated with the Risk of Spontaneous Abortion During the First Trimester of Pregnancy. Diagnostics 2020, 10, 197. https://doi.org/10.3390/diagnostics10040197
Monteiro LJ, Varas-Godoy M, Acuña-Gallardo S, Correa P, Passalacqua G, Monckeberg M, Rice GE, Illanes SE. Increased Circulating Levels of Tissue-Type Plasminogen Activator Are Associated with the Risk of Spontaneous Abortion During the First Trimester of Pregnancy. Diagnostics. 2020; 10(4):197. https://doi.org/10.3390/diagnostics10040197
Chicago/Turabian StyleMonteiro, Lara J., Manuel Varas-Godoy, Stephanie Acuña-Gallardo, Paula Correa, Gianluca Passalacqua, Max Monckeberg, Gregory E. Rice, and Sebastián E. Illanes. 2020. "Increased Circulating Levels of Tissue-Type Plasminogen Activator Are Associated with the Risk of Spontaneous Abortion During the First Trimester of Pregnancy" Diagnostics 10, no. 4: 197. https://doi.org/10.3390/diagnostics10040197
APA StyleMonteiro, L. J., Varas-Godoy, M., Acuña-Gallardo, S., Correa, P., Passalacqua, G., Monckeberg, M., Rice, G. E., & Illanes, S. E. (2020). Increased Circulating Levels of Tissue-Type Plasminogen Activator Are Associated with the Risk of Spontaneous Abortion During the First Trimester of Pregnancy. Diagnostics, 10(4), 197. https://doi.org/10.3390/diagnostics10040197





