Use of the Serum Wisteria floribunda Agglutinin-Positive Mac2 Binding Protein as a Marker of Gastroesophageal Varices and Liver-Related Events in Chronic Hepatitis C Patients
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Endoscopic Evaluation of GEV
2.3. WFA+–M2BP and other Serum Marker Measurements
2.4. Occurrence of Liver-Related Events
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
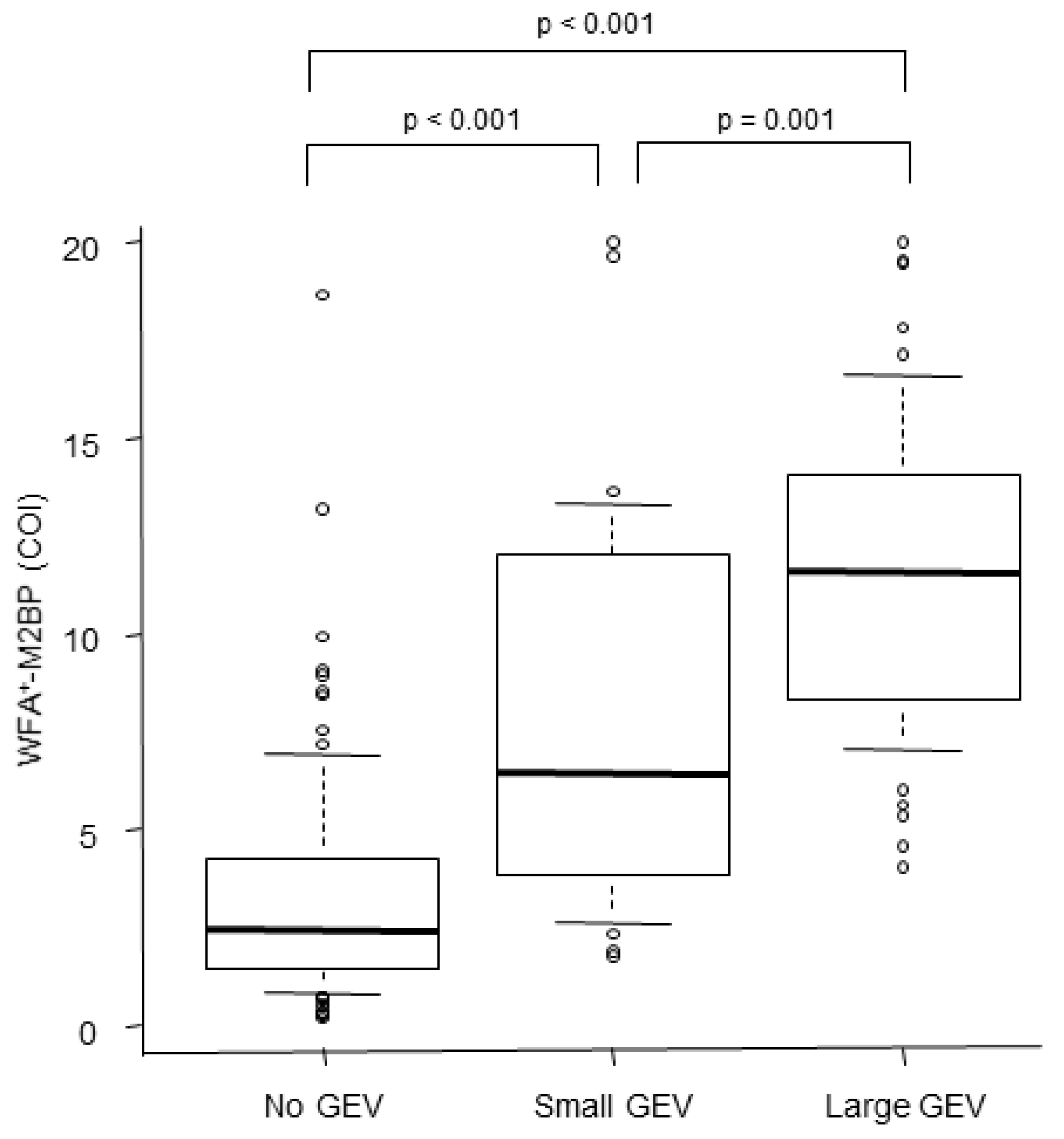
3.2. Serum WFA+–M2BP Levels and the Presence or Grade of GEV
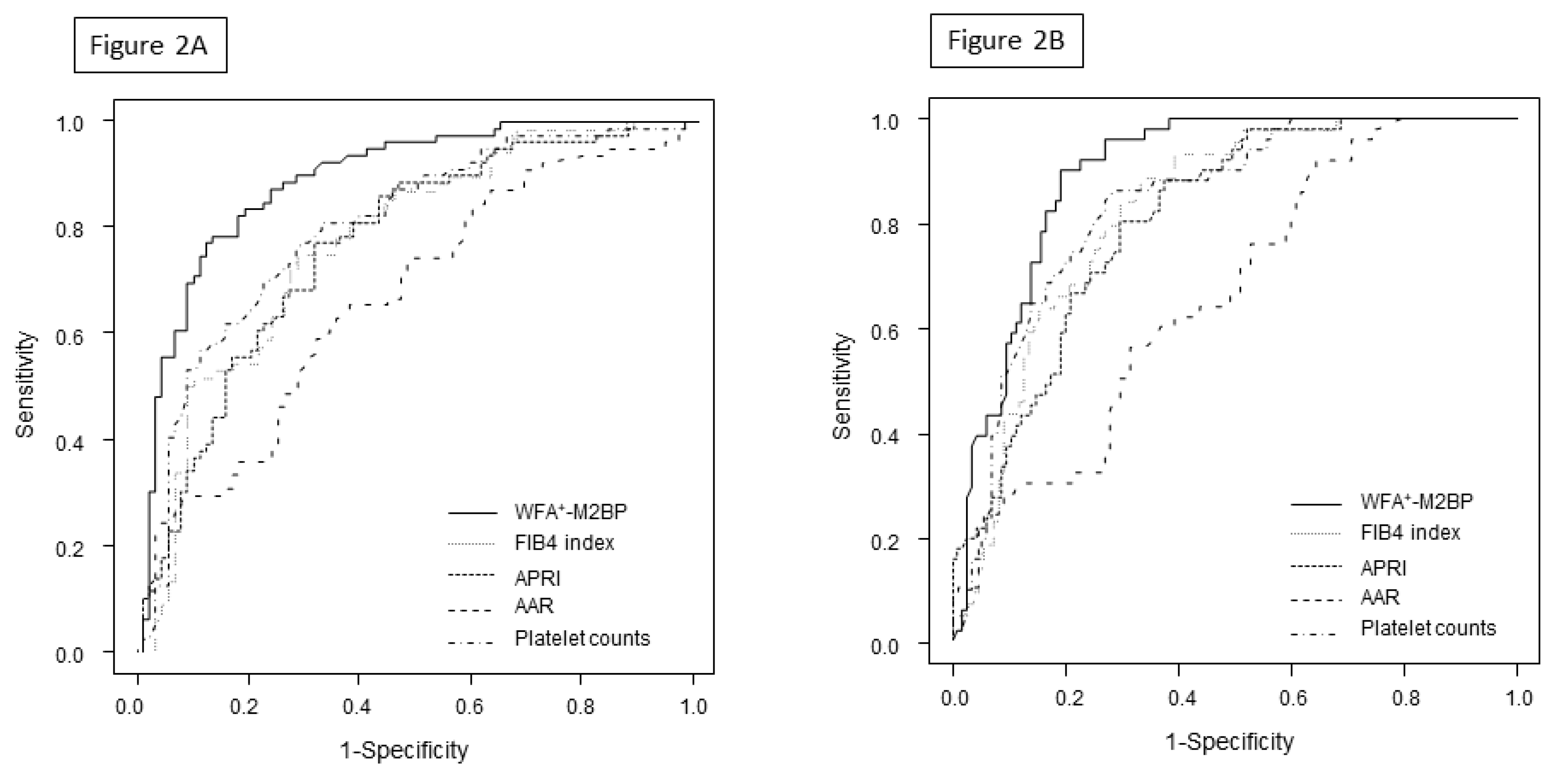
3.3. Factors Associated with the Presence of GEV and Large GEV
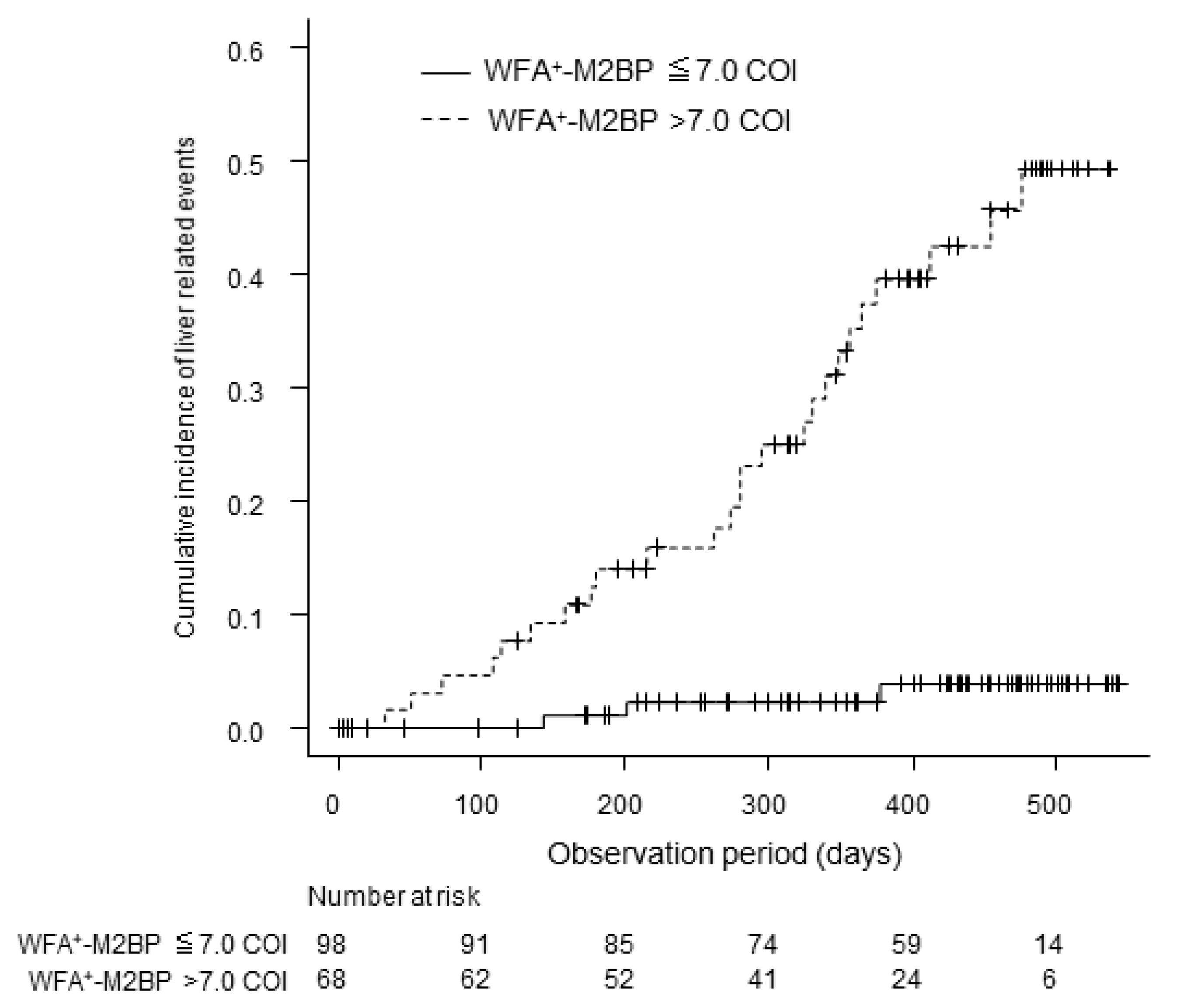
3.4. WFA+–M2BP Levels and Liver-Related Events
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Kahi, C.; Francois, F.; Pinto, A.; Marathe, A.; Bini, E.J.; Pandya, P.; Sitaraman, S.; Shen, J. Improved patient survival after acute variceal bleeding: A multicenter, cohort study. Am. J. Gastroenterol. 2003, 98, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, N.; Pauwels, A.; Serfaty, L.; Fourdan, O.; Lévy, V.G.; Poupon, R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology 2004, 40, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Morishita, N.; Hiramatsu, N.; Oze, T.; Harada, N.; Yamada, R.; Miyazaki, M.; Yakushijin, T.; Miyagi, T.; Yoshida, Y.; Tatsumi, T.; et al. Liver stiffness measurement by acoustic radiation force impulse is useful in predicting the presence of esophageal varices or high-risk esophageal varices among patients with HCV-related cirrhosis. J. Gastroenterol. 2013, 49, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.M.; Omran, D.; El Beshlawey, M.L.; Abdo, M.; El Askary, A. Can transient elastography, Fib-4, Forns Index, and Lok Score predict esophageal varices in HCV-related cirrhotic patients? Gastroenterología y Hepatología 2014, 37, 58–65. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Chen, T.W.; Zhang, X.-M.; Zeng, N.-L.; Zhou, L.; Tang, H.-J.; Wang, D.; Jian, S.; Liao, J.; Xiang, J.-Y.; et al. Diameters of left gastric vein and its originating vein on magnetic resonance imaging in liver cirrhosis patients with hepatitis B: Association with endoscopic grades of esophageal varices. Hepatol. Res. 2013, 44, E110–E117. [Google Scholar] [CrossRef]
- Kazemi, F.; Kettaneh, A.; N’Kontchou, G.; Pinto, E.; Ganne-Carrié, N.; Trinchet, J.; Beaugrand, M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J. Hepatol. 2006, 45, 230–235. [Google Scholar] [CrossRef]
- Takuma, Y.; Nouso, K.; Morimoto, Y.; Tomokuni, J.; Sahara, A.; Toshikuni, N.; Takabatake, H.; Shimomura, H.; Doi, A.; Sakakibara, I.; et al. Measurement of Spleen Stiffness by Acoustic Radiation Force Impulse Imaging Identifies Cirrhotic Patients With Esophageal Varices. Gastroenterology 2013, 144, 92–101.e2. [Google Scholar] [CrossRef]
- Deng, H.; Qi, X.; Guo, X. Diagnostic Accuracy of APRI, AAR, FIB-4, FI, King, Lok, Forns, and FibroIndex Scores in Predicting the Presence of Esophageal Varices in Liver Cirrhosis. Medicine 2015, 94, 1795. [Google Scholar] [CrossRef]
- Castera, L.; Foucher, J.; Bernard, P.-H.; Carvalho, F.; Allaix, D.; Merrouche, W.; Couzigou, P.; De Lédinghen, V. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369 examinations. Hepatology 2010, 51, 828–835. [Google Scholar] [CrossRef]
- Kuno, A.; Ikehara, Y.; Tanaka, Y.; Ito, K.; Matsuda, A.; Sekiya, S.; Hige, S.; Sakamoto, M.; Kage, M.; Mizokami, M.; et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci. Rep. 2013, 3, 1065. [Google Scholar] [CrossRef] [PubMed]
- Toshima, T.; Shirabe, K.; Ikegami, T.; Yoshizumi, T.; Kuno, A.; Togayachi, A.; Gotoh, M.; Narimatsu, H.; Korenaga, M.; Mizokami, M.; et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA+-M2BP), for assessing liver fibrosis. J. Gastroenterol. 2014, 50, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Tateyama, M.; Abiru, S.; Komori, A.; Nagaoka, S.; Saeki, A.; Hashimoto, S.; Sasaki, R.; Bekki, S.; Kugiyama, Y.; et al. Elevated serum levels ofWisteria floribundaagglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014, 60, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Kurosaki, M.; Kuno, A.; Korenaga, M.; Togayachi, A.; Gotoh, M.; Nakakuki, N.; Takada, H.; Matsuda, S.; Hattori, N.; et al. Wisteria floribundaagglutinin positive human Mac-2-binding protein as a predictor of hepatocellular carcinoma development in chronic hepatitis C patients. Hepatol. Res. 2015, 45, E82–E88. [Google Scholar] [CrossRef]
- Nishikawa, H.; Takata, R.; Enomoto, H.; Kazunori, Y.; Kishino, K.; Shimono, Y.; Iwata, Y.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Proposal of a predictive model for advanced fibrosis containing Wisteria floribunda agglutinin-positive Mac-2-binding protein in chronic hepatitis C. Hepatol. Res. 2016, 47, 12724. [Google Scholar] [CrossRef]
- Vizzutti, F.; Arena, U.; Romanelli, R.; Rega, L.; Foschi, M.; Colagrande, S.; Petrarca, A.; Moscarella, S.; Belli, G.; Zignego, A.L.; et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 2007, 45, 1290–1297. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, U.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Wai, C.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Giannini, E.G.; Risso, D.; Botta, F.; Chiarbonello, B.; Fasoli, A.; Malfatti, F.; Romagnoli, P.; Testa, E.; Ceppa, P.; Testa, R. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch. Intern. Med. 2003, 163, 218–224. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2012, 48, 452–458. [Google Scholar] [CrossRef]
- Ishii, N.; Harimoto, N.; Araki, K.; Muranushi, R.; Hoshino, K.; Hagiwara, K.; Gantumur, D.; Yamanaka, T.; Tsukagoshi, M.; Igarashi, T.; et al. Preoperative Mac-2 binding protein glycosylation isomer level predicts postoperative ascites in patients with hepatic resection for hepatocellular carcinoma. Hepatol. Res. 2019, 49, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Ohnishi, S.; Miyazaki, T.; Ideta, T.; Kochi, T.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; et al. Impact of serum glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein levels on liver functional reserves and mortality in patients with liver cirrhosis. Hepatol. Res. 2015, 45, 1083–1090. [Google Scholar] [CrossRef]
- Toyoda, H.; Kumada, T.; Tada, T.; Kaneoka, Y.; Maeda, A.; Korenaga, M.; Mizokami, M.; Narimatsu, H. Serum WFA+-M2BP levels as a prognostic factor in patients with early hepatocellular carcinoma undergoing curative resection. Liver Int. 2015, 36, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Tempesta, D.; Fattovich, G.; Castera, L.; Halfon, P.; Bourlière, M.; Noventa, F.; Angeli, P.; Saggioro, A.; Alberti, A. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: Results of a multicenter, large-scale study. J. Hepatol. 2010, 53, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, S.U.; Park, S.Y.; Kim, B.K.; Park, J.Y.; Kim, Y.; Ahn, S.H.; Tak, W.Y.; Kweon, Y.O.; Han, K.-H. A Novel Model to Predict Esophageal Varices in Patients with Compensated Cirrhosis Using Acoustic Radiation Force Impulse Elastography. PLoS ONE 2015, 10, e0121009. [Google Scholar] [CrossRef]
- Sporea, I.; Raţiu, I.; Sirli, R.; Popescu, A.; Bota, S. Value of transient elastography for the prediction of variceal bleeding. World J. Gastroenterol. 2011, 17, 2206–2210. [Google Scholar] [CrossRef]
- Castera, L.; Le Bail, B.; Roudot-Thoraval, F.; Bernard, P.-H.; Foucher, J.; Merrouche, W.; Couzigou, P.; De Lédinghen, V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: Comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J. Hepatol. 2009, 50, 59–68. [Google Scholar] [CrossRef]
- Thabut, D.; Trabut, J.-B.; Massard, J.; Rudler, M.; Muntenau, M.; Messous, D.; Poynard, T. Non-invasive diagnosis of large oesophageal varices with FibroTest in patients with cirrhosis: A preliminary retrospective study. Liver Int. 2006, 26, 271–278. [Google Scholar] [CrossRef]
- Qamar, A.A.; Grace, N.D.; Groszmann, R.J.; Garcia-Tsao, G.; Bosch, J.; Burroughs, A.K.; Maurer, R.; Planas, R.; Escorsell, A.; García-Pagán, J.C.; et al. Platelet count is not a predictor of the presence or development of gastroesophageal varices in cirrhosis. Hepatology 2007, 47, 153–159. [Google Scholar] [CrossRef]
- De Franchis, R. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J. Hepatol. 2010, 53, 762–768. [Google Scholar] [CrossRef]
- Mak, L.-Y.; Wong, D.K.-H.; Cheung, K.S.; Seto, W.-K.; Lai, C.; Yuen, M.-F. Role of serum M2BPGi levels on diagnosing significant liver fibrosis and cirrhosis in treated patients with chronic hepatitis B virus infection. Clin. Transl. Gastroenterol. 2018, 9, e163. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Higuchi, M.; Kurosaki, M.; Kirino, S.; Osawa, L.; Watakabe, K.; Wang, W.; Okada, M.; Shimizu, T.; Takaura, K.; et al. Wisteria floribunda agglutinin-positive mac-2 binding protein as an age-independent fibrosis marker in nonalcoholic fatty liver disease. Sci. Rep. 2019, 9, 10109. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Nishimura, T.; Yoh, K.; Aizawa, N.; Sakai, Y.; et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level and high-sensitivity C-reactive protein concentration in autoimmune hepatitis. Hepatol. Res. 2015, 46, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Nishimura, T.; Yoh, K.; Aizawa, N.; Sakai, Y.; et al. Impact of serum Wisteria floribunda agglutinin positive Mac-2-binding protein and serum interferon-gamma-inducible protein-10 in primary biliary cirrhosis. Hepatol Res. 2016, 46, 575–583. [Google Scholar] [CrossRef]
- Abe, M.; Miyake, T.; Kuno, A.; Imai, Y.; Sawai, Y.; Hino, K.; Hara, Y.; Hige, S.; Sakamoto, M.; Yamada, G.; et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J. Gastroenterol. 2014, 50, 776–784. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, M.; Yu, D.; Li, W.; Zhang, D.; Lu, F.; Gong, Q.-M.; Liu, F.; Jiang, J.-H.; Zheng, M.; et al. Serum WFA + -M2BP levels for evaluation of early stages of liver fibrosis in patients with chronic hepatitis B virus infection. Liver Int. 2016, 37, 35–44. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Kishino, K.; Shimono, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Nishimura, T.; Yoh, K.; et al. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein for patients with chronic hepatitis B and C: A comparative study. J. Viral Hepat. 2016, 23, 977–984. [Google Scholar] [CrossRef]
| Factors | Category | No GEV | Present GEV | p-Value |
|---|---|---|---|---|
| Number | 87 | 79 | ||
| Gender | male/female | 37/50 | 44/35 | 0.09 |
| Age (years) | 72.5 ± 9.1 | 71.3 ± 10 | 0.42 | |
| AST (IU/L) | 52.1 ± 34 | 71.6 ± 44 | 0.002 | |
| ALT (IU/L) | 47.2 ± 45 | 48.9 ± 34 | 0.78 | |
| Albumin (g/dl) | 3.86 ± 0.6 | 3.22 ± 0.5 | <0.001 | |
| Platelet counts (×104/μL) | 16.2 ± 21 | 8.7 ± 4.0 | 0.003 | |
| Prothrombin Time (INR) | 1.06 ± 0.14 | 1.16 ± 0.14 | <0.001 | |
| Child-Pughclass | A/B/C | 79/8/0 | 46/33/0 | <0.001 |
| History of HCC | No/Yes | 60/27 | 35/44 | 0.001 |
| Factors | AUROC | Cut-Off Value | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| Presence of GEV | ||||||
| WFA+–M2BP | 0.90 | 6.0 | 78.5 | 87.4 | 84.9 | 81.7 |
| APRI | 0.78 | 1.4 | 77.2 | 69.0 | 68.6 | 75.0 |
| FIB-4 Index | 0.81 | 6.0 | 78.5 | 72.4 | 72.0 | 76.2 |
| Platelet counts | 0.81 | 11.0 | 75.9 | 72.4 | 71.4 | 76.8 |
| AAR | 0.69 | 1.4 | 65.8 | 63.2 | 61.5 | 64.8 |
| Presence of large GEV | ||||||
| WFA+–M2BP | 0.90 | 7.0 | 90.2 | 80.9 | 66.7 | 93.0 |
| APRI | 0.81 | 1.8 | 80.4 | 70.4 | 53.4 | 87.1 |
| FIB-4 Index | 0.84 | 6.4 | 82.4 | 72.2 | 56.2 | 89.2 |
| Platelet counts | 0.84 | 10.0 | 84.3 | 73.0 | 58.1 | 91.3 |
| AAR | 0.67 | 1.5 | 56.9 | 67.8 | 42.4 | 75.7 |
| Presence of GEV | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95%CI | p Value | Odds Ratio | 95%CI | p Value | |
| WFA+–M2BP (>6.0 COI) | 25.2 | 11.0–57.7 | <0.001 | 30.7 | 9.1–104.0 | <0.001 |
| Child-Pughclass (B) | 7.1 | 3.0–16.6 | <0.001 | |||
| age (>70 years) | 0.7 | 0.4–1.4 | 0.3 | |||
| gender (male) | 1.7 | 0.9–3.1 | 0.09 | |||
| AST (>40 IU/L) | 3.3 | 1.7–6.5 | <0.001 | |||
| ALT (>40 IU/L) | 2.1 | 1.1–3.8 | 0.02 | |||
| PT-INR (>1.1) | 4.5 | 2.4–8.7 | <0.001 | |||
| albumin (<3.5 g/dl) | 5.2 | 2.7–10.2 | <0.001 | |||
| Platelet counts (<10.0 × 104/μL) | 7.7 | 3.9–15.5 | <0.001 | 4.4 | 1.7–11.2 | 0.002 |
| History of HCC | 2.8 | 1.5–5.3 | 0.001 | |||
| WFA+-M2BP (>7.0 COI) | 38.9 | 13.8–109.0 | <0.001 | 28.4 | 7.8–103.0 | <0.001 |
| Child-Pughclass (B) | 4.4 | 2.1–9.3 | <0.001 | |||
| age (>70 years) | 0.4 | 0.2–0.9 | 0.02 | |||
| gender (male) | 1.1 | 0.6–2.2 | 0.7 | |||
| AST (>40 IU/L) | 4.6 | 2.0–10.6 | <0.001 | |||
| ALT (>40 IU/L) | 2 | 1.0–3.8 | 0.04 | |||
| PT-INR (>1.1) | 6.4 | 3.1–13.5 | <0.001 | |||
| albumin (<3.5 g/dl) | 4.2 | 2.1–8.4 | <0.001 | |||
| Platelet counts (<10.0 × 104/μL) | 12.6 | 5.5–29.0 | <0.001 | 6 | 2.1–17.6 | 0.001 |
| History of HCC | 1.8 | 0.9–3.5 | 0.08 | |||
| Factors | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95%CI | p Value | Odds Ratio | 95%CI | p Value | |
| WFA+–M2BP (>7.0 COI) | 14.8 | 4.5–49 | <0.001 | 6.7 | 1.8–24 | 0.004 |
| Child–Pughclass (B) | 11.5 | 5.1–26 | <0.001 | 5 | 2.0–12 | <0.001 |
| age (>70 years) | 0.96 | 0.5–2.0 | 0.9 | |||
| gender (male) | 2.3 | 1.04–4.9 | 0.04 | |||
| AST (>40 IU/L) | 17.4 | 2.3–127 | 0.006 | |||
| ALT (>40 IU/L) | 2.7 | 1.3–6.0 | 0.01 | |||
| Platelet counts (<10.0 × 104/μL) | 3.4 | 1.5–7.4 | 0.002 | |||
| History of HCC | 3.6 | 1.6–8.0 | 0.001 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, T.; Tamaki, N.; Kurosaki, M.; Wang, W.; Okada, M.; Higuchi, M.; Takaura, K.; Takada, H.; Yasui, Y.; Tsuchiya, K.; et al. Use of the Serum Wisteria floribunda Agglutinin-Positive Mac2 Binding Protein as a Marker of Gastroesophageal Varices and Liver-Related Events in Chronic Hepatitis C Patients. Diagnostics 2020, 10, 173. https://doi.org/10.3390/diagnostics10030173
Hayashi T, Tamaki N, Kurosaki M, Wang W, Okada M, Higuchi M, Takaura K, Takada H, Yasui Y, Tsuchiya K, et al. Use of the Serum Wisteria floribunda Agglutinin-Positive Mac2 Binding Protein as a Marker of Gastroesophageal Varices and Liver-Related Events in Chronic Hepatitis C Patients. Diagnostics. 2020; 10(3):173. https://doi.org/10.3390/diagnostics10030173
Chicago/Turabian StyleHayashi, Tsuguru, Nobuharu Tamaki, Masayuki Kurosaki, Wan Wang, Mao Okada, Mayu Higuchi, Kenta Takaura, Hitomi Takada, Yutaka Yasui, Kaoru Tsuchiya, and et al. 2020. "Use of the Serum Wisteria floribunda Agglutinin-Positive Mac2 Binding Protein as a Marker of Gastroesophageal Varices and Liver-Related Events in Chronic Hepatitis C Patients" Diagnostics 10, no. 3: 173. https://doi.org/10.3390/diagnostics10030173
APA StyleHayashi, T., Tamaki, N., Kurosaki, M., Wang, W., Okada, M., Higuchi, M., Takaura, K., Takada, H., Yasui, Y., Tsuchiya, K., Nakanishi, H., Itakura, J., Harada, M., & Izumi, N. (2020). Use of the Serum Wisteria floribunda Agglutinin-Positive Mac2 Binding Protein as a Marker of Gastroesophageal Varices and Liver-Related Events in Chronic Hepatitis C Patients. Diagnostics, 10(3), 173. https://doi.org/10.3390/diagnostics10030173





