The Influence of Thyroid Pathology on Osteoporosis and Fracture Risk: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Bone Health
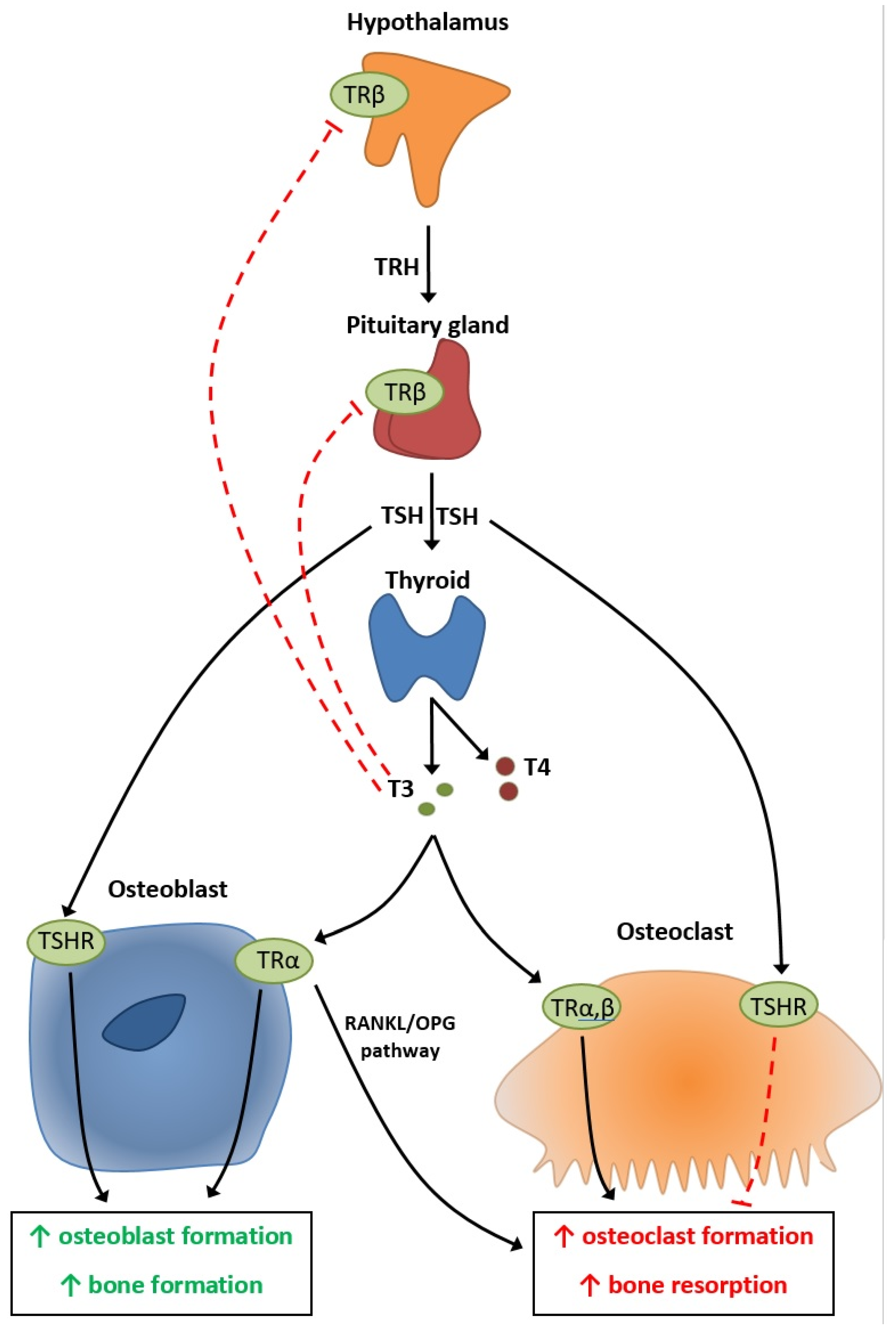
4. Physiopathology of Thyroid’s Effect on Bone Metabolism
5. Thyroid Pathology
6. Conclusions
Funding
Conflicts of Interest
References
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Rotatori, S.; Bianciardi, S.; Nuti, R.; Merlotti, D. Treatment needs and current options for postmenopausal osteoporosis. Expert Opin. Pharm. 2016, 17, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, M.P.; Unwin, B.K.; Greenawald, M.H.; Casiano, V.E. Diagnosis and management of osteoporosis. Am. Fam. Physician 2015, 92, 261–268. [Google Scholar] [PubMed]
- Piciu, D.; Irimie, A.; Kontogeorgos, G.; Piciu, A.; Buiga, R. Highly aggressive pathology of non-functional parathyroid carcinoma. Orphanet J. Rare Dis. 2013, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Mester, A.; Apostu, D.; Ciobanu, L.; Piciu, A.; Lucaciu, O.; Campian, R.S.; Taulescu, M.; Bran, S. The impact of proton pump inhibitors on bone regeneration and implant osseointegration. Drug Metab. Rev. 2019, 51, 330–339. [Google Scholar] [CrossRef]
- Unnanuntana, A.; Gladnick, B.P.; Donnelly, E.; Lane, J.M. The assessment of fracture risk. J. Bone Jt. Surg. Ser. Am. Vol. 2010, 92, 743–753. [Google Scholar] [CrossRef]
- Williams, G.R. Thyroid hormone actions in cartilage and bone. Eur. Thyroid J. 2013, 2, 3–13. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physioloy 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Bassett, J.H.D.; Williams, G.R. Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr. Rev. 2016, 37, 135–187. [Google Scholar] [CrossRef]
- Baliram, R.; Latif, R.; Zaidi, M.; Davies, T.F. Expanding the Role of Thyroid-Stimulating Hormone in Skeletal Physiology. Front. Endocrinol. 2017, 8, 252. [Google Scholar] [CrossRef]
- Reddy, P.A.; Harinarayan, C.V.; Sachan, A.; Suresh, V.; Rajagopal, G. Bone disease in thyrotoxicosis. Indian J. Med. Res. 2012, 135, 277–286. [Google Scholar] [PubMed]
- Downey, C.; Kelly, M.; Quinlan, J.F. Changing trends in the mortality rate at 1-year post hip fracture—A systematic review. World J. Orthop. 2019, 10, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Tsevis, K.; Trakakis, E.; Pergialiotis, V.; Alhazidou, E.; Peppa, M.; Chrelias, C.; Papantoniou, N.; Panagopoulos, P. The influence of thyroid disorders on bone density and biochemical markers of bone metabolism. Horm. Mol. Biol. Clin. Investig. 2018, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Zhang, Y.; Li, Q.; Hu, Y.; Tao, X.-J.; Liu, B.-L.; Ma, J.-H.; Li, D.-M. Low Thyroid Stimulating Hormone Levels Are Associated with Low Bone Mineral Density in Femoral Neck in Elderly Women. Arch. Med. Res. 2016, 47, 310–314. [Google Scholar] [CrossRef]
- Tuchendler, D.; Bolanowski, M. Assessment of bone metabolism in premenopausal females with hyperthyroidism and hypothyroidism. Endokrynol. Pol. 2013, 64, 40–44. [Google Scholar]
- Svare, A.; Nilsen, T.I.L.; Bjoro, T.; Forsmo, S.; Schei, B.; Langhammer, A. Hyperthyroid levels of TSH correlate with low bone mineral density: The HUNT 2 study. Eur. J. Endocrinol. 2009, 161, 779–786. [Google Scholar] [CrossRef]
- Kim, D.J.; Khang, Y.H.; Koh, J.-M.; Shong, Y.K.; Kim, G.S. Low normal TSH levels are associated with low bone mineral density in healthy postmenopausal women. Clin. Endocrinol. 2006, 64, 86–90. [Google Scholar] [CrossRef]
- Babu, R.P.; Christy, A.; Hegde, A.; Manjrekar, P.; D’Souza, V. Do premenopausal hypothyroid women on levothyroxine therapy need bone status monitoring? Clin. Med. Insights Women’s 2015, 8, CMWH-S22114. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Kim, J.Y.; Lee, J.; Song, H.-J.; Kim, J.-Y.; Choi, N.-K.; Park, B.-J. Levothyroxine dose and fracture risk according to the osteoporosis status in elderly women. J. Prev. Med. Public Health 2014, 47, 36–46. [Google Scholar] [CrossRef]
- Karimifar, M.; Esmaili, F.; Salari, A.; Kachuei, A.; Faragzadegan, Z.; Karimifar, M. Effects of Levothyroxine and thyroid stimulating hormone on bone loss in patients with primary hypothyroidism. J. Res. Pharm. Pr. 2014, 3, 83–87. [Google Scholar] [CrossRef]
- Konca Degertekin, C.; Turhan Iyidir, O.; Aktas Yılmaz, B.; Elbeg, S.; Pasaoglu, O.T.; Pasaoglu, H.; Cakır, N.; Arslan, M. RANKL/Osteoprotegerin System and Bone Turnover in Hashimoto Thyroiditis. Calcif. Tissue Int. 2016, 99, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lim, S.; Park, H.; Woo, H.Y.; Chang, Y.; Sung, E.; Jung, H.S.; Yun, K.E.; Kim, C.W.; Ryu, S.; et al. Subclinical thyroid dysfunction, bone mineral density, and osteoporosis in a middle-aged Korean population. Osteoporos. Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Siru, R.; Alfonso, H.; Chubb, S.A.P.; Golledge, J.; Flicker, L.; Yeap, B.B. Subclinical thyroid dysfunction and circulating thyroid hormones are not associated with bone turnover markers or incident hip fracture in older men. Clin. Endocrinol. 2018, 89, 93–99. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Garg, M.K.; Tandon, N.; Kanwar, R.; Narang, A.; Sastry, A.; Bhadra, K. Thyroid function and bone mineral density among Indian subjects. Indian J. Endocrinol. Metab. 2012, 16, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Oh, K.W.; Rhee, E.J.; Jung, C.H.; Kim, S.W.; Yun, E.J.; Tae, H.J.; Baek, K.H.; Kang, M.I.; Choi, M.G.; et al. Relationship between subclinical thyroid dysfunction and femoral neck bone mineral density in women. Arch. Med. Res. 2006, 37, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, A.; Fusco, A.; Andreoli, A.; Magnani, A.; Tulli, A.; Lauro, D.; De Lorenzo, A. Effect of subclinical hypothyroidism and obesity on whole-body and regional bone mineral content. Horm. Res. 2002, 57, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Rodriguez, E.; Stuber, M.; Del Giovane, C.; Feller, M.; Collet, T.-H.; Lowe, A.L.; Blum, M.R.; van Vliet, N.A.; van Heemst, D.; Kearney, P.M.; et al. Skeletal Effects of Levothyroxine for Subclinical Hypothyroidism in Older Adults: A TRUST Randomized Trial Nested Study. J. Clin. Endocrinol. Metab. 2020, 105, 6–26. [Google Scholar] [CrossRef]
- Pedrera-Zamorano, J.D.; Roncero-Martin, R.; Calderon-Garcia, J.F.; Santos-Vivas, M.; Vera, V.; Martinez-Alvarez, M.; Rey-Sanchez, P. Treatment of subclinical hypothyroidism does not affect bone mass as determined by dual-energy X-ray absorptiometry, peripheral quantitative computed tomography and quantitative bone ultrasound in Spanish women. Arch. Med. Sci. 2015, 11, 1008–1014. [Google Scholar]
- Tarraga Lopez, P.J.; Lopez, C.F.; de Mora, F.N.; Montes, J.A.R.; Albero, J.S.; Manez, A.N.; Casas, A.G. Osteoporosis in patients with subclinical hypothyroidism treated with thyroid hormone. Clin. Cases Min. Bone Metab. 2011, 8, 44–48. [Google Scholar]
- Meier, C.; Beat, M.; Guglielmetti, M.; Christ-Crain, M.; Staub, J.-J.; Kraenzlin, M. Restoration of euthyroidism accelerates bone turnover in patients with subclinical hypothyroidism: A randomized controlled trial. Osteoporos. Int. 2004, 15, 209–216. [Google Scholar] [CrossRef]
- Rosario, P.W. Radioiodine therapy in elderly patients with subclinical hyperthyroidism due to non-voluminous nodular goiter and its effect on bone metabolism. Arq. Bras. Endocrinol. Metab. 2013, 57, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Thayakaran, R.; Adderley, N.J.; Sainsbury, C.; Torlinska, B.; Boelaert, K.; Sumilo, D.; Price, M.; Thomas, G.N.; Toulis, K.A.; Nirantharakumar, K. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: Longitudinal study. BMJ 2019, 366, l4892. [Google Scholar] [CrossRef] [PubMed]
- Rapacki, E.; Lauritzen, J.B.; Madsen, C.M.; Jorgensen, H.L.; Norring-Agerskov, D. Thyroid-stimulating hormone (TSH) is associated with 30-day mortality in hip fracture patients. Eur. J. Trauma Emerg. Surg. 2019, 1–7. [Google Scholar] [CrossRef]
- Maccagnano, G.; Notarnicola, A.; Pesce, V.; Mudoni, S.; Tafuri, S.; Moretti, B. The Prevalence of Fragility Fractures in a Population of a Region of Southern Italy Affected by Thyroid Disorders. BioMed Res. Int. 2016, 2016, 6017165. [Google Scholar] [CrossRef]
- Vestergaard, P.; Mosekilde, L. Fractures in patients with hyperthyroidism and hypothyroidism: A nationwide follow-up study in 16,249 patients. Thyroid 2002, 12, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsen, B.; Jorgensen, H.L.; Laulund, A.S.; Nybo, M.; Bauer, D.C.; Brix, T.H.; Hegedus, L. The excess risk of major osteoporotic fractures in hypothyroidism is driven by cumulative hyperthyroid as opposed to hypothyroid time: An observational register-based time-resolved cohort analysis. J. Bone Min. Res. 2015, 30, 898–905. [Google Scholar] [CrossRef]
- Polovina, S.P.; Miljic, D.; Zivojinovic, S.; Milic, N.; Micic, D.; Brkic, V.P. The impact of thyroid autoimmunity (TPOAb) on bone density and fracture risk in postmenopausal women. Hormones 2017, 16, 54–61. [Google Scholar]
- Lambrinoudaki, I.; Armeni, E.; Pliatsika, P.; Rizos, D.; Kaparos, G.; Augoulea, A.; Alexandrou, A.; Flokatoula, M.; Creatsa, M.; Panoulis, C.; et al. Thyroid function and autoimmunity are associated with the risk of vertebral fractures in postmenopausal women. J. Bone Miner. Metab. 2017, 35, 227–233. [Google Scholar] [CrossRef]
- Kim, K.-C.; Lee, Y.-K.; Lee, Y.J.; Ha, Y.-C.; Koo, K.-H. Bone health and clinical results after hip fracture surgery in patients with subclinical hypothyroidism. J. Bone Metab. 2014, 21, 213–216. [Google Scholar] [CrossRef][Green Version]
- Garin, M.C.; Arnold, A.M.; Lee, J.S.; Robbins, J.; Cappola, A.R. Subclinical thyroid dysfunction and hip fracture and bone mineral density in older adults: The cardiovascular health study. J. Clin. Endocrinol. Metab. 2014, 99, 2657–2664. [Google Scholar] [CrossRef]
- Polovina, S.; Popovic, V.; Duntas, L.; Milic, N.; Micic, D. Frax score calculations in postmenopausal women with subclinical hypothyroidism. Hormones 2013, 12, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Buzkova, P.; Fink, H.A.; Vu, J.; Carbone, L.; Chen, Z.; Cauley, J.; Bauer, D.C.; Cappola, A.R.; Robbins, J. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch. Intern. Med. 2010, 170, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, J.; Wang, J.; Zhao, X.; Gu, M. Association of subclinical thyroid dysfunction with bone mineral density and fracture: A meta-analysis of prospective cohort studies. Endocrine 2019, 67, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Huang, H.; Li, J.; Wang, J. Relationship between subclinical thyroid dysfunction and the risk of fracture: A meta-analysis of prospective cohort studies. Osteoporos. Int. 2016, 27, 115–125. [Google Scholar] [CrossRef]
- Blum, M.R.; Bauer, D.C.; Collet, T.-H.; Fink, H.A.; Cappola, A.R.; da Costa, B.R.; Wirth, C.D.; Peeters, R.P.; Asvold, B.O.; den Elzen, W.P.J.; et al. Subclinical thyroid dysfunction and fracture risk: A meta-analysis. JAMA 2015, 313, 2055–2065. [Google Scholar] [CrossRef]
- Segna, D.; Bauer, D.C.; Feller, M.; Schneider, C.; Fink, H.A.; Aubert, C.E.; Collet, T.-H.; da Costa, B.R.; Fischer, K.; Peeters, R.P.; et al. Association between subclinical thyroid dysfunction and change in bone mineral density in prospective cohorts. J. Intern. Med. 2018, 283, 56–72. [Google Scholar] [CrossRef]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet Lond. Engl. 2017, 390, 1550–1562. [Google Scholar] [CrossRef]
- Redford, C.; Vaidya, B. Subclinical hypothyroidism: Should we treat? Post Reprod. Heal. 2017, 23, 55–62. [Google Scholar] [CrossRef]
- Donangelo, I.; Suh, S.Y. Subclinical Hyperthyroidism: When to Consider Treatment. Am. Fam. Physician 2017, 95, 710–716. [Google Scholar]
- Piciu, D.; Irimie, A. Diagnosis and treatment guidelines in thyroid carcinoma. European and American consensus adapted to Romania. Acta Endocrinol. Buchar. 2007, 3, 103–115. [Google Scholar] [CrossRef]
- Piciu, D.; Irimie, A.; Piciu, A. Investigation of thyroid carcinoma over 40 years, using the database of the Ion Chiricuta Institute of Oncology Cluj-Napoca. J. BUON 2014, 19, 524–529. [Google Scholar] [PubMed]
| Type of Study | Number of Patients | Conclusion | Year | References |
|---|---|---|---|---|
| Hypothyroidism | ||||
| Prospective | 188 | Hypothyroidism is associated with lower BMD | 2018 | [13] |
| Cross-sectional | 1097 | Lower TSH is related to decreased BMD | 2016 | [14] |
| Prospective | 119 | Hypothyroidism does not affect bone density | 2013 | [15] |
| Cross-sectional | 6722 | Lower TSH was related to the lowest BMD in the forearm | 2009 | [16] |
| Cross-sectional | 959 | Lower TSH was related to lower BMD | 2006 | [17] |
| Hypothyroidism treatment | ||||
| Prospective | 75 | Levothyroxine increases bone turnover, but it does not lead to frank osteoporosis | 2015 | [18] |
| Retrospective | 11,155 | Overtreatment of hypothyroidism is associated with an increased rate of osteoporosis | 2014 | [19] |
| Retrospective | 150 | Levothyroxine is associated with a lower BMD | 2014 | [20] |
| Autoimmune thyroid pathology | ||||
| Prospective | 80 | Hashimoto disease can further activate the OPG/RANKL system leading to additional bone loss | 2016 | [21] |
| Subclinical hypothyroidism | ||||
| Retrospective | 25,510 | Subclinical hypothyroidism does not affect BMD | 2019 | [22] |
| Prospective | 4248 | Subclinical hypothyroidism is not predictive of incident hip fracture | 2018 | [23] |
| Cross-sectional | 1290 | TSH does not affect BMD | 2012 | [24] |
| Prospective | 413 | Subclinical hypothyroidism is associated with lower BMD | 2006 | [25] |
| Prospective | 32 | Subclinical hypothyroidism was related to a reduced leg BMD | 2002 | [26] |
| Subclinical hypothyroidism treatment | ||||
| Prospective | 196 | Levothyroxine does not affect bone health | 2020 | [27] |
| Prospective | 45 | Levothyroxine treatment does not affect bone mass. | 2015 | [28] |
| Retrospective | 182 | Levothyroxine is associated with a higher bone loss | 2011 | [29] |
| Cross-sectional retrospective | 66 | Levothyroxine treatment is related to accelerated bone loss | 2004 | [30] |
| Subclinical hyperthyroidism | ||||
| Prospective | 413 | Subclinical hyperthyroidism is associated with lower BMD | 2006 | [25] |
| Subclinical hyperthyroidism treatment | ||||
| Prospective | 36 | Radioiodine has beneficial effects on BMD | 2013 | [31] |
| Type of Study | Number of Patients | Conclusion | Year | References |
|---|---|---|---|---|
| Hypothyroidism | ||||
| Retrospective | 162,369 | TSH above the upper reference value increases the risk of fractures | 2019 | [32] |
| Retrospective | 914 | TSH above the median increases the early mortality rate in the case of surgically treated hip fractures | 2019 | [33] |
| Retrospective | 108,977 | Hypothyroidism is associated with increased risk of fracture | 2016 | [34] |
| Retrospective | 16,249 | Hypothyroidism is associated with increased fracture risk | 2002 | [35] |
| Hypothyroidism treatment | ||||
| Retrospective | 230,552 | Overtreatment of hypothyroidism has a similar fracture risk, as seen in hyperthyroidism | 2015 | [36] |
| Autoimmune thyroid pathology | ||||
| Prospective | 189 | TPOAb can be considered a marker for increased risk of fracture in euthyroid post-menopausal women | 2017 | [37] |
| Retroscpective | 335 | TPOAb can be considered a marker for increased risk of fracture | 2017 | [38] |
| Subclinical hypothyroidism | ||||
| Prospective | 4248 | Subclinical hypothyroidism is not predictive of incident hip fracture | 2018 | [23] |
| Retrospective | 471 | Subclinical hypothyroidism is not associated with lower BMD | 2014 | [39] |
| Cross-sectional | 4963 | Subclinical hypothyroidism is not associated with increased hip fracture rate | 2014 | [40] |
| Prospective | 82 | Subclinical hypothyroidism has an increased fracture risk | 2013 | [41] |
| Prospective | 3567 | Subclinical hypothyroidism has an increased risk of hip fracture | 2010 | [42] |
| Subclinical hyperthyroidism | ||||
| Prospective | 4248 | Subclinical hyperthyroidism is not predictive of incident hip fracture | 2018 | [23] |
| Cross-sectional | 4963 | Subclinical hyperthyroidism is not associated with increased hip fracture rate | 2014 | [40] |
| Prospective | 3567 | Subclinical hyperthyroidism has an increased risk of hip fracture | 2010 | [42] |
| Bone-Related Outcome | Number of Patients | Conclusion | Year | References |
|---|---|---|---|---|
| Subclinical hypothyroidism | ||||
| Osteoporosis Fracture risk | 313,557 | Subclinical hypothyroidism has no impact on fracture risk. | 2019 | [43] |
| Fracture risk | 314,146 | Subclinical hypothyroidism is not associated with an increased risk of fracture. | 2016 | [44] |
| Fracture risk | 70,298 | Subclinical hypothyroidism is not associated with an increased risk of fractures. | 2015 | [45] |
| Subclinical hyperthyroidism | ||||
| Osteoporosis Fracture risk | 313,557 | Subclinical hyperthyroidism could increase the risk of fractures and decrease BMD. | 2019 | [40] |
| Osteoporosis | 5458 | Subclinical hyperthyroidism is associated with lower BMD. | 2018 | [46] |
| Fracture risk | 314,146 | Subclinical hyperthyroidism is associated with an increased risk of fracture. | 2016 | [44] |
| Fracture risk | 70,298 | Subclinical hyperthyroidism is associated with an increased risk of fractures. | 2015 | [45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostu, D.; Lucaciu, O.; Oltean-Dan, D.; Mureșan, A.-D.; Moisescu-Pop, C.; Maxim, A.; Benea, H. The Influence of Thyroid Pathology on Osteoporosis and Fracture Risk: A Review. Diagnostics 2020, 10, 149. https://doi.org/10.3390/diagnostics10030149
Apostu D, Lucaciu O, Oltean-Dan D, Mureșan A-D, Moisescu-Pop C, Maxim A, Benea H. The Influence of Thyroid Pathology on Osteoporosis and Fracture Risk: A Review. Diagnostics. 2020; 10(3):149. https://doi.org/10.3390/diagnostics10030149
Chicago/Turabian StyleApostu, Dragos, Ondine Lucaciu, Daniel Oltean-Dan, Alexandru-Dorin Mureșan, Cristina Moisescu-Pop, Andrei Maxim, and Horea Benea. 2020. "The Influence of Thyroid Pathology on Osteoporosis and Fracture Risk: A Review" Diagnostics 10, no. 3: 149. https://doi.org/10.3390/diagnostics10030149
APA StyleApostu, D., Lucaciu, O., Oltean-Dan, D., Mureșan, A.-D., Moisescu-Pop, C., Maxim, A., & Benea, H. (2020). The Influence of Thyroid Pathology on Osteoporosis and Fracture Risk: A Review. Diagnostics, 10(3), 149. https://doi.org/10.3390/diagnostics10030149








