Photoacoustic Spectral Sensing Technique for Diagnosis of Biological Tissue Coagulation: In-Vitro Study
Abstract
1. Introduction
2. Materials and Methods
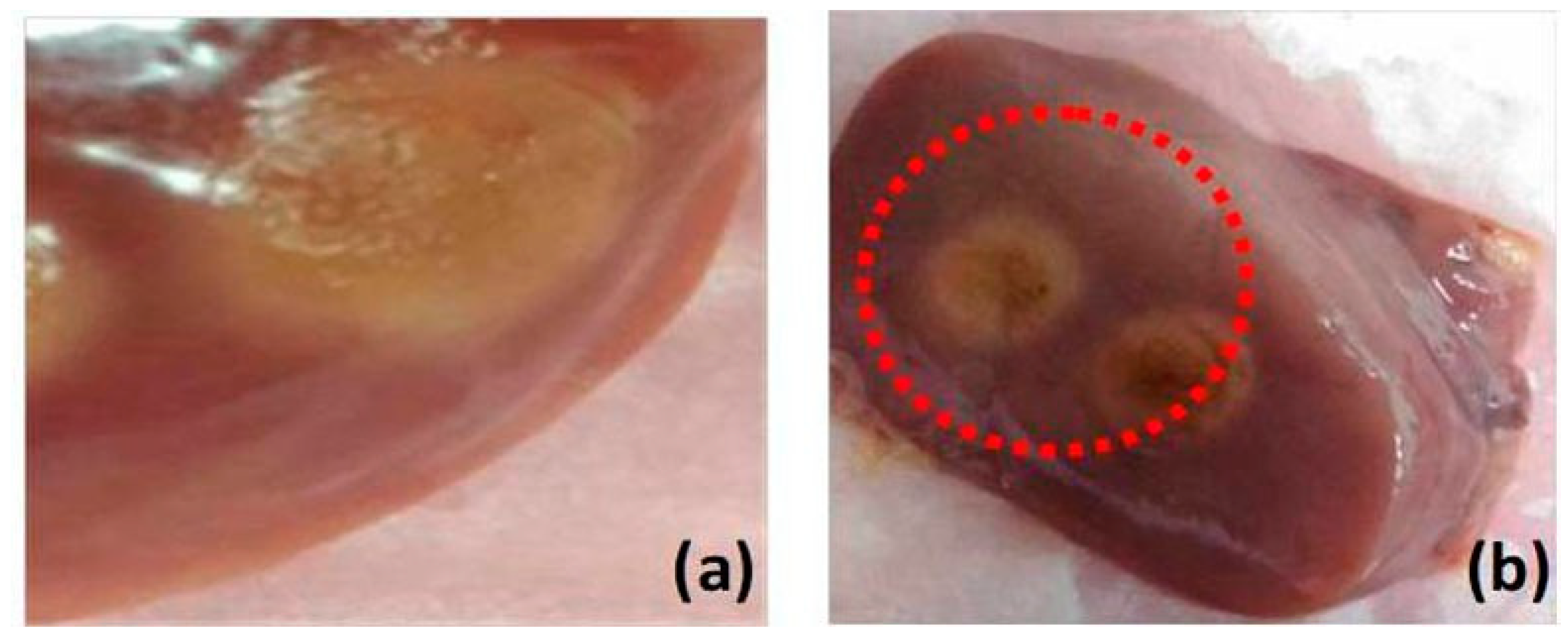
2.1. Sample Preparation for Coagulation Study
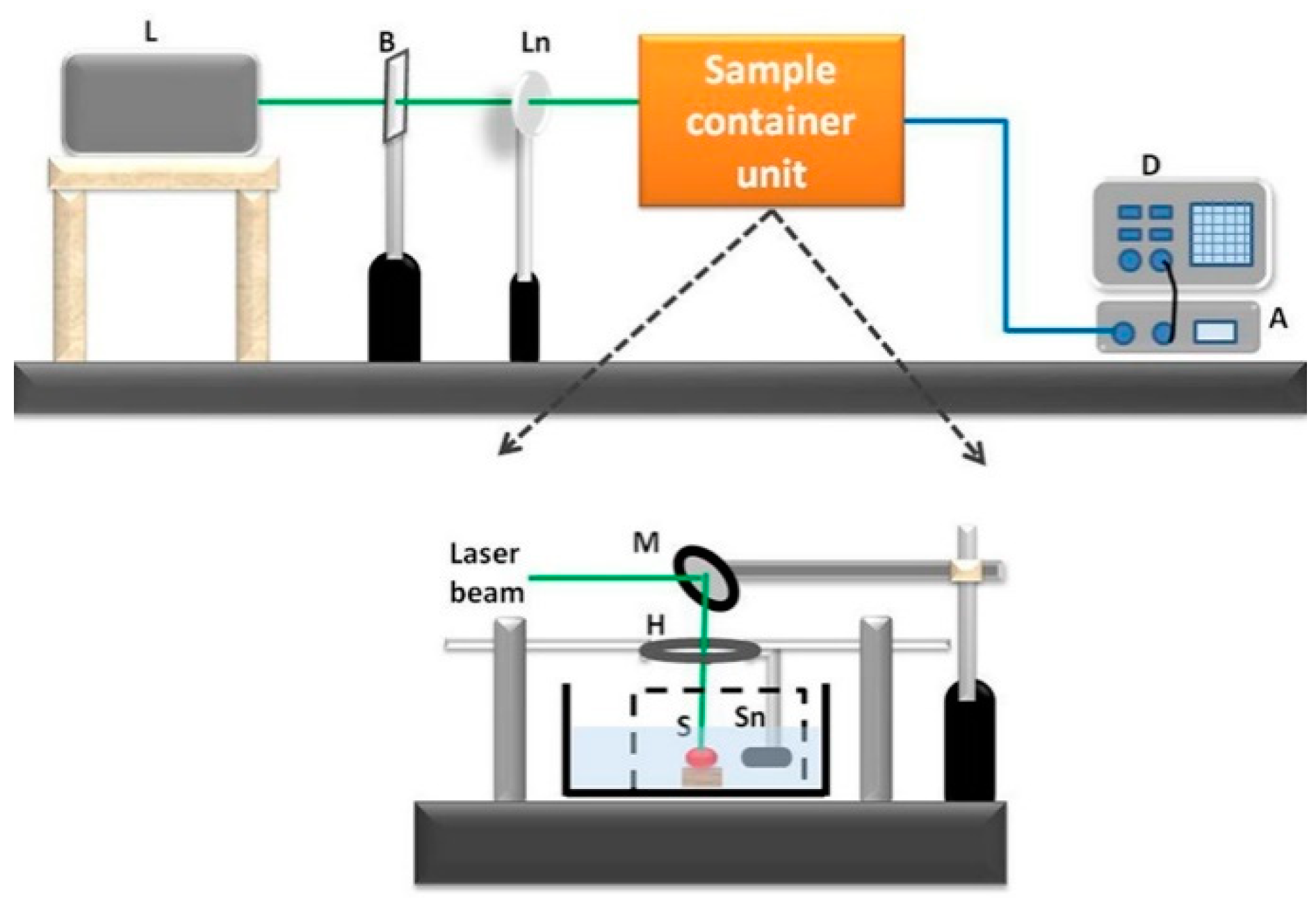
2.2. Experimental Setup
2.3. Frequency Spectral Analysis of PA Response
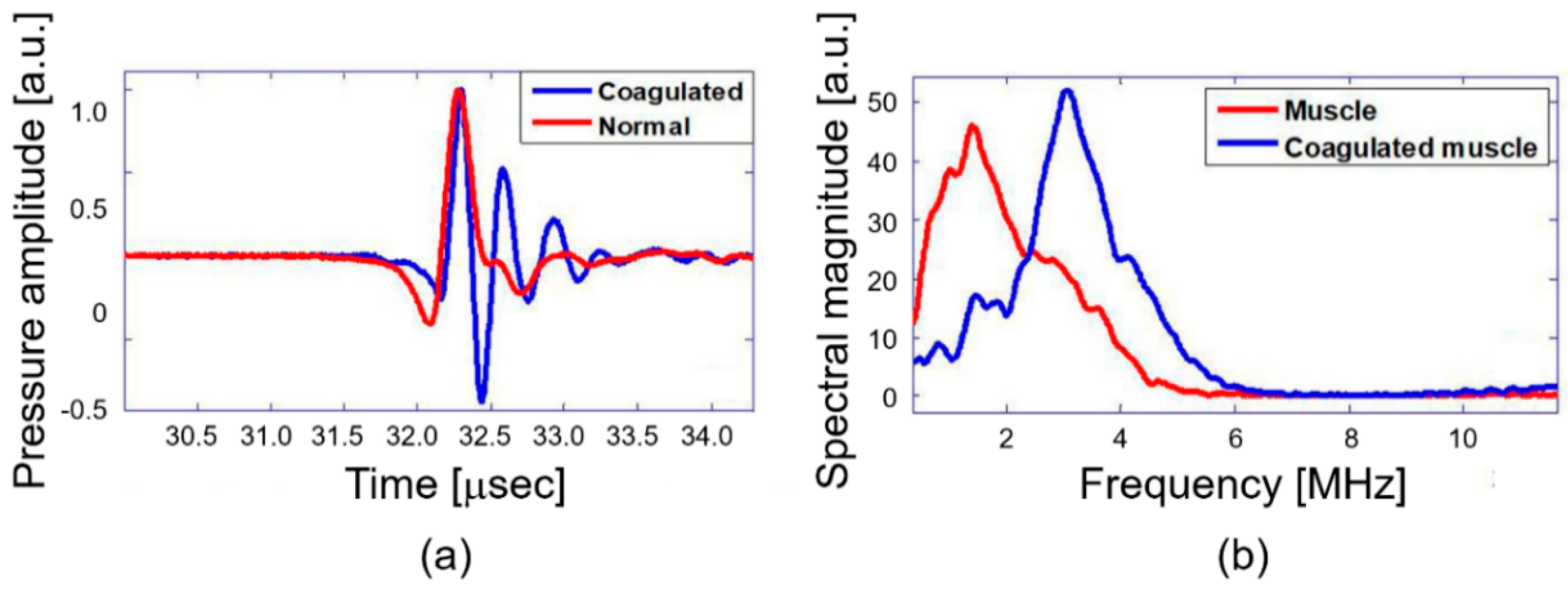
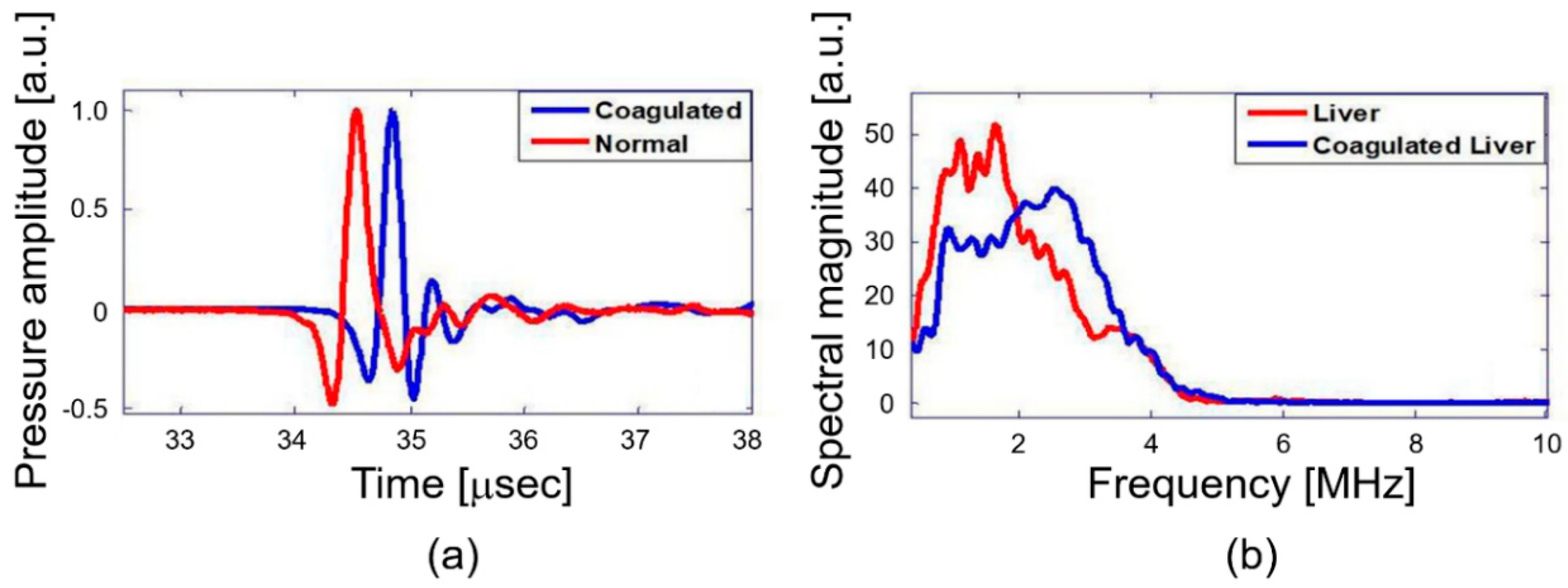
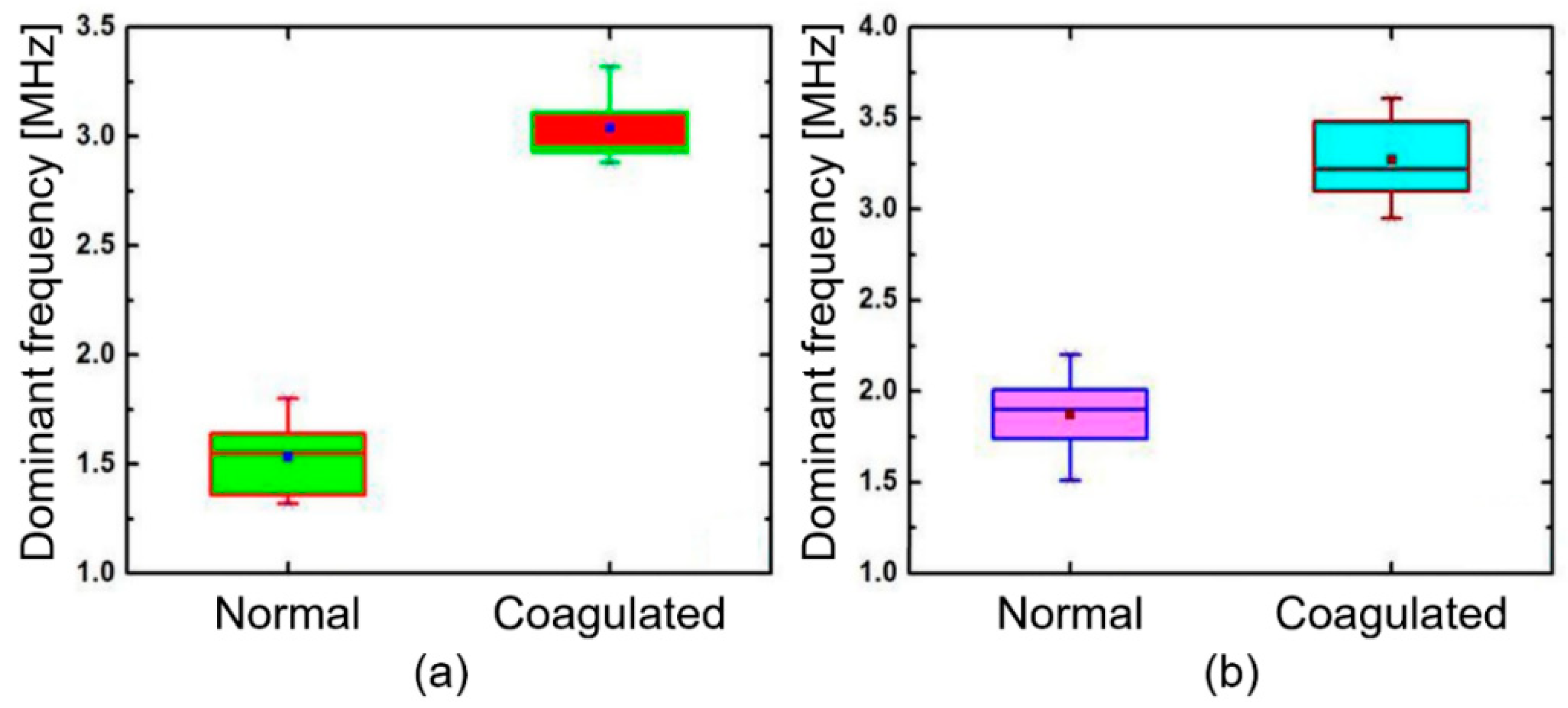
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stauffer, P.R. Evolving technology for thermal therapy of cancer. Int. J. Hyperth. 2005, 21, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Niemz, M.H. Laser-Tissue Interactions: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 9783662047170. [Google Scholar]
- Livraghi, T.; Goldberg, S.N.; Lazzaroni, S.; Meloni, F.; Ierace, T.; Solbiati, L.; Gazelle, G.S. Hepatocellular Carcinoma: Radio-frequency Ablation of Medium and Large Lesions. Radiology 2000, 214, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Rouvière, O.O.; Lyonnet, D.; Raudrant, A.; Colin-Pangaud, C.; Chapelon, J.Y.; Bouvier, R.; Dubernard, J.M.; Gelet, A. MRI Appearance of Prostate following Transrectal HIFU Ablation of Localized Cancer. Eur. Urol. 2001, 40, 265–274. [Google Scholar] [PubMed]
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 2012, 33, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.J.; Bronskill, M.J.; Henkelman, R.M. Time and temperature dependence of MR parameters during thermal coagulation ofex vivo rabbit muscle. Magn. Reson. Med. 1998, 39, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.; Vahala, E.; Ehnholm, G.J.; Vaara, T.; Ylihautala, M.; Savart, M.; Laurent, A.; Tanttu, J.; Saint-Jalmes, H. MR temperature measurement in liver tissue at 0.23 T with a steady-state free precession sequence. Magn. Reson. Med. 2002, 47, 940–947. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fife, S.; Andereck, C.D.; Rahal, S. Ultrasound thermometry in transparent and opaque fluids. Exp. Fluids 2003, 35, 152–158. [Google Scholar] [CrossRef]
- Kurokawa, K.; Makita, S.; Hong, Y.J.; Yasuno, Y. Two-dimensional micro-displacement measurement for laser coagulation using optical coherence tomography. Biomed. Opt. Express 2015, 6, 170–190. [Google Scholar] [CrossRef]
- Anderson, C.D.; Lin, W.C.; Buttemere, C.R.; Washington, M.K.; Mahadevan-Jansen, A.; Pierce, J.; Nicoud, I.B.; Pinson, C.W.; Chari, R.S. Real-time spectroscopic assessment of thermal damage: Implications for radiofrequency ablation. J. Gastrointest. Surg. 2004, 8, 660–669. [Google Scholar] [CrossRef]
- Wang, S.; Larin, K.V. Optical coherence elastography for tissue characterization: A review. J. Biophotonics 2015, 8, 279–302. [Google Scholar] [CrossRef]
- Ibne Mokbul, M. Optical coherence tomography: Basic concepts and applications in neuroscience research. J. Med. Eng. 2017, 2017, 3409327. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Hoelen, C.G.A.; de Mul, F.F.M. A new theoretical approach to photoacoustic signal generation. J. Acoust. Soc. Am. 1999, 106, 695–706. [Google Scholar] [CrossRef]
- Biswas, D.; Vasudevan, S.; Chen, G.C.K.; Sharma, N. Quantitative photoacoustic characterization of blood clot in blood: A mechanobiological assessment through spectral information. Rev. Sci. Instrum. 2017, 88, 024301. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Vasudevan, S.; Chen, G.C.K.; Bhagat, P.; Sharma, N.; Phatak, S. Time–frequency based photoacoustic spectral response technique for differentiating human breast masses. Biomed. Phys. Eng. Express 2017, 3, 035002. [Google Scholar] [CrossRef]
- Muratikov, K.L.; Glazov, A.L.; Rose, D.N.; Dumar, J.E. Photoacoustic effect in stressed elastic solids. J. Appl. Phys. 2000, 88, 2948–2955. [Google Scholar] [CrossRef]
- Wang, L.V. Prospects of photoacoustic tomography. Med. Phys. 2008, 35, 5758–5767. [Google Scholar] [CrossRef] [PubMed]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Z. Multi-spectral photoacoustic elasticity tomography. Biomed. Opt. Express 2016, 7, 3323–3334. [Google Scholar] [CrossRef]
- Zell, K.; Sperl, J.I.; Vogel, M.W.; Niessner, R.; Haisch, C. Acoustical properties of selected tissue phantom materials for ultrasound imaging. Phys. Med. Biol. 2007, 52, N475–N484. [Google Scholar] [CrossRef]
- Diebold, G.J.; Sun, T. Properties of Photoacoustic Waves in One, Two, and Three Dimensions. Acta Acust. United Acust. 1994, 80, 339–351. [Google Scholar]
- Zhao, L.; Zhuo, C.; Song, C.; Li, X.; Zhou, Y.; Shi, D. Histological characteristics of collagen denaturation and injuries in bipolar radiofrequency-induced colonic anastomoses. Pathol. Res. Pract. 2015, 211, 214–218. [Google Scholar] [CrossRef]
- Nagarajan, V.K.; Ward, J.M.; Yu, B. Association of Liver Tissue Optical Properties and Thermal Damage. In Lasers in Surgery and Medicine; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Nguyen, T.H.; Rhee, Y.; Ahn, J.; Kang, H.W. Circumferential irradiation for interstitial coagulation of urethral stricture. Opt. Express 2015, 23, 20829. [Google Scholar] [CrossRef]
- Bischof, J.C.; He, X. Thermal Stability of Proteins. Ann. N. Y. Acad. Sci. 2005, 1066, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Venugopalan, V. Mechanisms of Pulsed Laser Ablation of Biological Tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Kwiecinski, W.; Pernot, M.; Balvay, D.; Tanter, M.; Clement, O.; Cuenod, C.A.; Zinzindohoue, F. Real time shear waves elastography monitoring of thermal ablation: In vivo evaluation in pig livers. J. Surg. Res. 2014, 188, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, M.; Manduca, A.; Greenleaf, J.F. Imaging elastic properties of biological tissues by low-frequency harmonic vibration. Proc. IEEE 2003, 91, 1503–1519. [Google Scholar] [CrossRef]
- Varghese, T.; Zagzebski, J.; Lee, F. Elastographic imaging of thermal lesions in the liver in vivo following radiofrequency ablation: Preliminary results. Ultrasound Med. Biol. 2002, 28, 1467–1473. [Google Scholar] [CrossRef]
- Savukov, I.M.; Zotev, V.S.; Volegov, P.L.; Espy, M.A.; Matlashov, A.N.; Gomez, J.J.; Kraus, R.H. MRI with an atomic magnetometer suitable for practical imaging applications. J. Magn. Reson. 2009, 199, 188–191. [Google Scholar] [CrossRef]
- Larin, K.V.; Larina, I.V.; Esenaliev, R.O. Monitoring of tissue coagulation during thermotherapy using optoacoustic technique. J. Phys. D Appl. Phys. 2005, 38, 2645–2653. [Google Scholar] [CrossRef]
- Biswas, D.; Kumari, A.; Chen, G.C.K.; Vasudevan, S.; Gupta, S.; Shukla, S.; Garg, U.K. Quantitative Differentiation of Pneumonia from Normal Lungs: Diagnostic Assessment Using Photoacoustic Spectral Response. Appl. Spectrosc. 2017, 71, 2532–2537. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, D.; Chen, G.C.K.; Baac, H.W.; Vasudevan, S. Photoacoustic Spectral Sensing Technique for Diagnosis of Biological Tissue Coagulation: In-Vitro Study. Diagnostics 2020, 10, 133. https://doi.org/10.3390/diagnostics10030133
Biswas D, Chen GCK, Baac HW, Vasudevan S. Photoacoustic Spectral Sensing Technique for Diagnosis of Biological Tissue Coagulation: In-Vitro Study. Diagnostics. 2020; 10(3):133. https://doi.org/10.3390/diagnostics10030133
Chicago/Turabian StyleBiswas, Deblina, George C. K. Chen, Hyoung Won Baac, and Srivathsan Vasudevan. 2020. "Photoacoustic Spectral Sensing Technique for Diagnosis of Biological Tissue Coagulation: In-Vitro Study" Diagnostics 10, no. 3: 133. https://doi.org/10.3390/diagnostics10030133
APA StyleBiswas, D., Chen, G. C. K., Baac, H. W., & Vasudevan, S. (2020). Photoacoustic Spectral Sensing Technique for Diagnosis of Biological Tissue Coagulation: In-Vitro Study. Diagnostics, 10(3), 133. https://doi.org/10.3390/diagnostics10030133




