Circulating Anti-Sorting Nexins 16 Antibodies as an Emerging Biomarker of Coronary Artery Disease in Patients with Obstructive Sleep Apnea
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Research Subjects
2.3. Clinical Data
2.4. Blood Sample Collection and Experimental Method
2.5. Statistical Analysis
2.6. Ethics Approval and Consent to Participate
2.7. Availability of Data and Material
3. Results
3.1. Clinical Characteristics
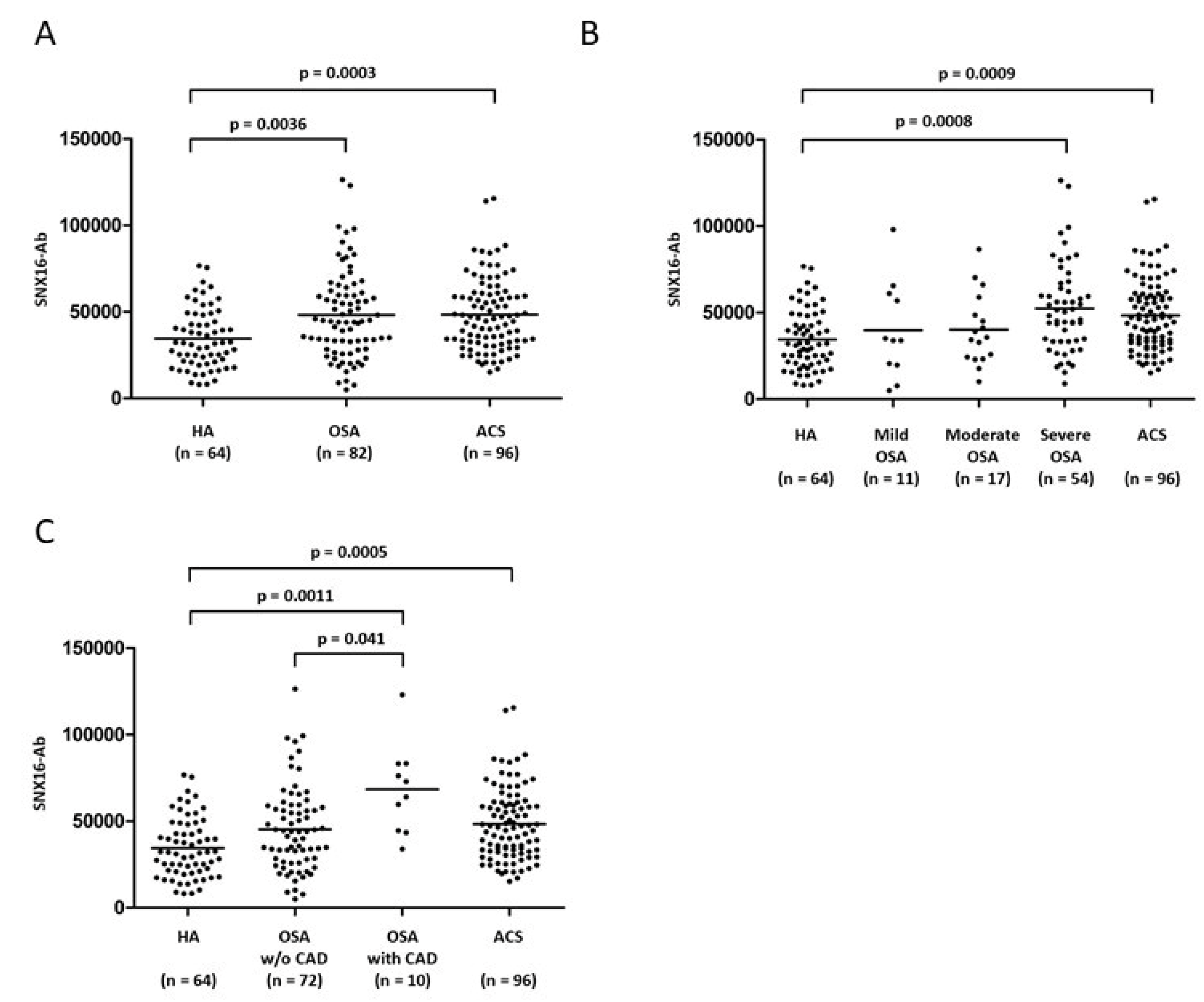
3.2. Difference in SNX16-Ab Level for Each Group
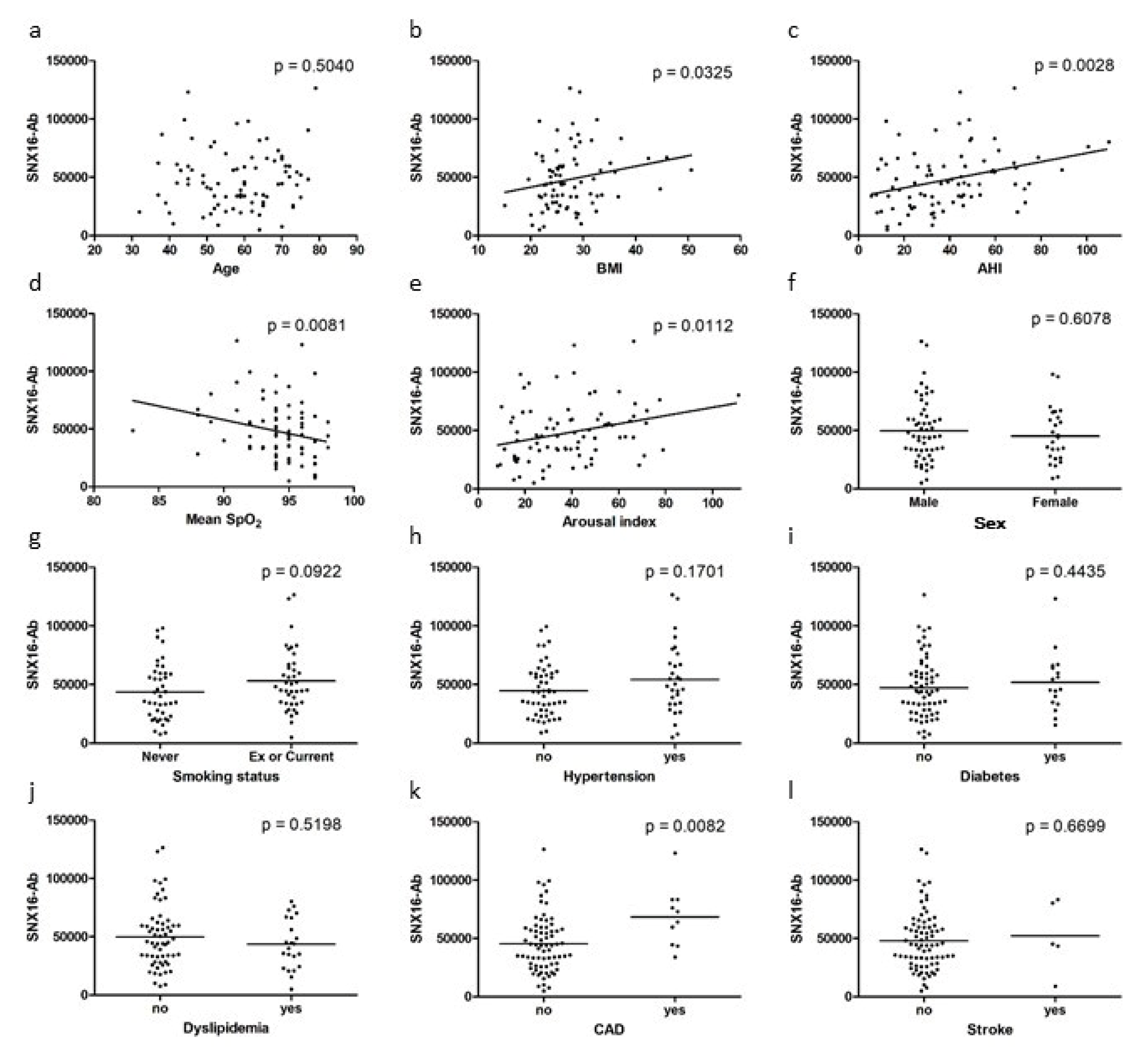
3.3. Correlation of SNX16-Ab Level and Clinical Data of OSA Group
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| AHI | Apnea-Hypopnea Index |
| AlphaLISA | Amplified Luminescence proximity homogenous Assay |
| AMI | Acute Myocardial Infarction |
| BMI | Body Mass Index |
| CAD | Coronary Artery Disease |
| CPAP | Continuous Positive Airway Pressure |
| EGFR | Epidermal Growth Factor Receptor |
| GST | Glutathione S-transferase |
| HA | Healthy Adults |
| LDL | Low-density lipoproteins |
| OSA | Obstructive Sleep Apnea |
| oxLDL | Oxidized low-density lipoproteins |
| PSG | Polysomnography |
| PIGF | placental growth factor |
| ROC | Receiver Operating Characteristic |
| SNXs | Sorting nexins |
| SNX16-Ab | Antibody against SNX16 |
| UAP | Unstable Angina Pectoris |
References
- Mandal, S.; Kent, B.D. Obstructive sleep apnoea and coronary artery disease. J. Thorac. Dis. 2018, 10, S4212–S4220. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, C.; Dematteis, M.; Pepin, J.L.; Baguet, J.P.; Lévy, P. Obstructive sleep apnea, immuno-inflammation, and atherosclerosis. Semin. Immunopathol. 2009, 31, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Randerath, W.; Bonsignore, M.R.; Herkenrath, S. Obstructive sleep apnoea in acute coronary syndrome. Eur. Respir. Rev. 2019, 152, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: An emerging risk factor for atherosclerosis. Chest 2011, 140, 534–542. [Google Scholar] [CrossRef]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, W.M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [CrossRef]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J. Am. Coll. Cardiol. 2008, 52, 686–717. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.K.H.; Liu, P.Y.; Cullen, S.R.; Knuiman, M.W.; Grunstein, R.R. Sleep Apnea as an Independent Risk Factor for All-Cause Mortality: The Busselton Health Study. Sleep 2008, 31, 1079–1085. [Google Scholar]
- Chan, A.; Antonio, N. Mechanism of sudden cardiac death in obstructive sleep apnea, revisited. Sleep Med. 2013, 14, e95. [Google Scholar] [CrossRef]
- Young, T.; Finn, L.; Peppard, P.E.; Szklo-Coxe, M.; Austin, D.; Nieto, F.J.; Stubbs, R.; Hla, K.M. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008, 31, 1071–1078. [Google Scholar]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Anandam, A.; Patil, M.; Akinnusi, M.; Jaoude, P.; El-Solh, A.A. Cardiovascular mortality in obstructive sleep apnoea treated with continuous positive airway pressure or oral appliance: An observational study. Respirology 2013, 18, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Fava, C.; Dorigoni, S.; Dalle Vedove, F.; Danese, E.; Montagnana, M.; Guidi, G.C.; Narkiewicz, K.; Minuz, P. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest 2014, 145, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, R.; Singh, M.; Nida, M.; Kwon, S.; Sajid, H.; Witkowski, J.; Pahomov, E.; Shah, K.; Park, W.; Champeau, D. Effect of CPAP Treatment for Obstructive Sleep Apnea Hypopnea Syndrome on Lipid Profile: A Meta-Regression Analysis. J. Clin. Sleep Med. 2014, 10, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, B.Y.; Light, M.; Malhotra, A. Strategies to augment adherence in the management of sleep-disordered breathing. Respirology 2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lamendola, C.; Ariel, D.; Abbasi, F.; Kim, S.H.; Cardell, J.; Tomasso, V.; Xu, S.; Patel, S.; Mojaddidi, H.; et al. Usefulness of Fetuin-A to Predict Risk for Cardiovascular Disease among Patients with Obstructive Sleep Apnea. Am. J. Cardiol. 2015, 116, 219–224. [Google Scholar] [CrossRef]
- Matsumura, T.; Terada, J.; Kinoshita, T.; Sakurai, Y.; Yahaba, M.; Tsushima, K.; Sakao, S.; Nagashima, K.; Ozaki, T.; Kobayashi, Y.; et al. Circulating autoantibodies against neuroblastoma suppressor of tumorigenicity 1 (NBL1): A potential biomarker for coronary artery disease in patients with obstructive sleep apnea. PLoS ONE 2018, 13, e0195015. [Google Scholar] [CrossRef]
- Goto, K.; Sugiyama, T.; Matsumura, R.; Zhang, X.; Kimura, R.; Taira, A.; Arita, E.; Iwase, K.; Kobayashi, E.; Iwadate, Y.; et al. Identification of Cerebral Infarction-Specific Antibody Markers from Autoantibodies Detected in Patients with Systemic Lupus Erythematosus. J. Mol. Biomark. Diagn. 2015, 6, 2. [Google Scholar] [CrossRef]
- Machida, T.; Kubota, M.; Kobayashi, E.; Iwadate, Y.; Saeki, N.; Yamaura, A.; Nomura, F.; Takiguchi, M.; Hiwasa, T. Identification of stroke-associated-antigens via screening of recombinant proteins from the human expression cDNA library (SEREX). J. Transl. Med. 2015, 13, 71. [Google Scholar] [CrossRef]
- Matsumura, T.; Terada, J.; Kinoshita, T.; Sakurai, Y.; Yahaba, M.; Ema, R.; Amata, A.; Sakao, S.; Nagashima, K.; Tatsumi, K.; et al. Circulating Anti-Coatomer Protein Complex Subunit Epsilon (COPE) Autoantibodies as a Potential Biomarker for Cardiovascular and Cerebrovascular Events in Patients with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2017, 13, 393–400. [Google Scholar] [CrossRef]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.L.; Quan, S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Hiwasa, T.; Machida, T.; Zhang, X.; Kimura, R.; Wang, H.; Iwase, K.; Ashino, H.; Taira, A.; Arita, E.; Mine, S.; et al. Elevated Levels of Autoantibodies against ATP2B4 and BMP-1 in Sera of Patients with Atherosclerosis-related Diseases. Immunome Res. 2015, 11. [Google Scholar] [CrossRef]
- Hiwasa, T.; Zhang, X.-M.; Kimura, R.; Machida, T.; Kitamura, K.; Yama, R.; Kunimatsu, M.; Kobayashi, E.; Kobayashi, K.; Kawamura, H.; et al. Association of Serum Antibody Levels against TUBB2C with Diabetes and Cerebral Infarction. Integ. Biomed. Sci. 2015, 1, 49–63. [Google Scholar] [CrossRef][Green Version]
- Worby, C.A.; Dixon, J.E. Sorting out the cellular functions of sorting nexins. Nat. Rev. Mol. Cell Biol. 2002, 3, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, Z.; Huang, W.; Chen, X.; Shan, P.; Zhong, P.; Khan, Z.; Wang, J.; Fang, Q.; Liang, G.; et al. Inhibition of epidermal growth factor receptor attenuates atherosclerosis via decreasing inflammation and oxidative stress. Sci. Rep. 2017, 8, 45917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chalothorn, D.; Jackson, L.F.; Lee, D.C.; Faber, J.E. Transactivation of epidermal growth factor receptor mediates catecholamine-induced growth of vascular smooth muscle. Circ. Res. 2004, 95, 989–997. [Google Scholar] [CrossRef]
- Choi, J.H.; Hong, W.-P.; Kim, M.J.; Kim, J.H.; Ryu, S.H.; Suh, P.G. Sorting nexin 16 regulates EGF receptor trafficking by phosphatidylinositol-3-phosphate interaction with the Phox domain. J. Cell Sci. 2004, 117, 4209–4218. [Google Scholar] [CrossRef]
- Brankatschk, B.; Pons, V.; Parton, R.G.; Gruenberg, J. Role of SNX16 in the dynamics of tubulo-cisternal membrane domains of late endosomes. PLoS ONE 2011, 6–7, e21771. [Google Scholar] [CrossRef]
- Trpkovic, A.; Resanovic, I.; Stanimirovic, J.; Radak, D.; Mousa, S.A.; Cenic-Milosevic, D.; Jevremovic, D.; Isenovic, E.R. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab. Sci. 2015, 52, 70–85. [Google Scholar] [CrossRef]
- Hartley, A.; Haskard, D.; Khamis, R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis - Novel insights and future directions in diagnosis and therapy. Trends Cardiovasc. Med. 2019, 29, 22–26. [Google Scholar] [CrossRef]
- Galovic, R.; Flegar-Mestric, Z.; Vidjak, V.; Matokanović, M.; Barišić, K. Heat shock protein 70 and antibodies to heat shock protein 60 are associated with cerebrovascular atherosclerosis. Clin. Biochem. 2016, 49, 66–69. [Google Scholar] [CrossRef]
- Iseme, R.A.; McEvoy, M.; Kelly, B.; Agnew, L.; Walker, F.R.; Handley, T.; Oldmeadow, C.; Attia, J.; Boyle, M. A role for autoantibodies in atherogenesis. Cardiovasc. Res. 2017, 113, 1102–1112. [Google Scholar] [CrossRef]
- Sun, H.; Fang, F.; Li, K.; Zhang, H.; Zhang, M.; Zhang, L.; Li, J.; Qin, Y.; Wei, Y. Circulating ESM-1 levels are correlated with the presence of coronary artery disease in patients with obstructive sleep apnea. Respir. Res. 2019, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, A.; Bauça, J.M.; Yañez, A.; Fueyo, L.; Gomez, C.; de la Peña, M.; Pierola, J.; Rodriguez, A.; Sanchez-de-la-Torre, M.; Abad, J.; et al. Spanish Sleep Group. Impact of OSA on biological markers in morbid obesity and metabolic syndrome. PLoS ONE 2016, 1, 3–11. [Google Scholar] [CrossRef][Green Version]
| HA (n = 64) | OSA (n = 82) | ACS (n = 96) | |
|---|---|---|---|
| Age | 42.5 (35.3–55.8) | 59.0 *** (49.8–66.5) | 67.0 *** (60.0–73.0) |
| Male (%) | 59.4 | 68.3 | 84.4 *** |
| BMI (kg/m2) | 23.1 (20.6–25.5) | 25.9 *** (23.9–29.4) | 23.4 (21.3–25.2) |
| OSA severity (%) | |||
| mild | 13.4 | ||
| moderate | 20.7 | ||
| severe | 65.9 | ||
| AHI (/h) | 36.7 (22.6–50.4) | ||
| Mean SpO2 (%) | 94 (93.0–96.0) | ||
| Mean SpO2 (%) | 78 (69.0–83.0) | ||
| Arousal Index (/h) | 37.3 (22.2–50.3) | ||
| Smoking status (%) | |||
| Never | 70 | 51.2 | 31.3 |
| Ex | 16.7 | 41.5 | 39.6 |
| Current | 13.3 | 7.3 ** | 29.2 *** |
| Hypertension (%) | 12.5 | 36.6 ** | 46.9 *** |
| Diabetes mellitus (%) | 1.6 | 20.7 ** | 17.7 ** |
| Dyslipidemia (%) | 3.1 | 26.8 * | 14.6 * |
| CAD (%) | 0 | 12.2 ** | 14.6 *** |
| Stroke (%) | 0 | 6.1 | 5.2 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age (per year) | 1.04 | 0.97–1.11 | 0.26 | |||
| BMI (≥25) | 0.80 | 0.21–3.36 | 0.75 | |||
| Smoking | 2.76 | 0.71–13.6 | 0.15 | |||
| Hypertension | 0.71 | 0.14–2.81 | 0.64 | |||
| Diabetes | 3.02 | 0.69–12.2 | 0.13 | |||
| Dyslipidemia | 1.20 | 0.24–4.79 | 0.81 | |||
| Severe SAS | 5.4 | 0.94–102.3 | 0.06 | 5.12 | 0.81–100.8 | 0.088 |
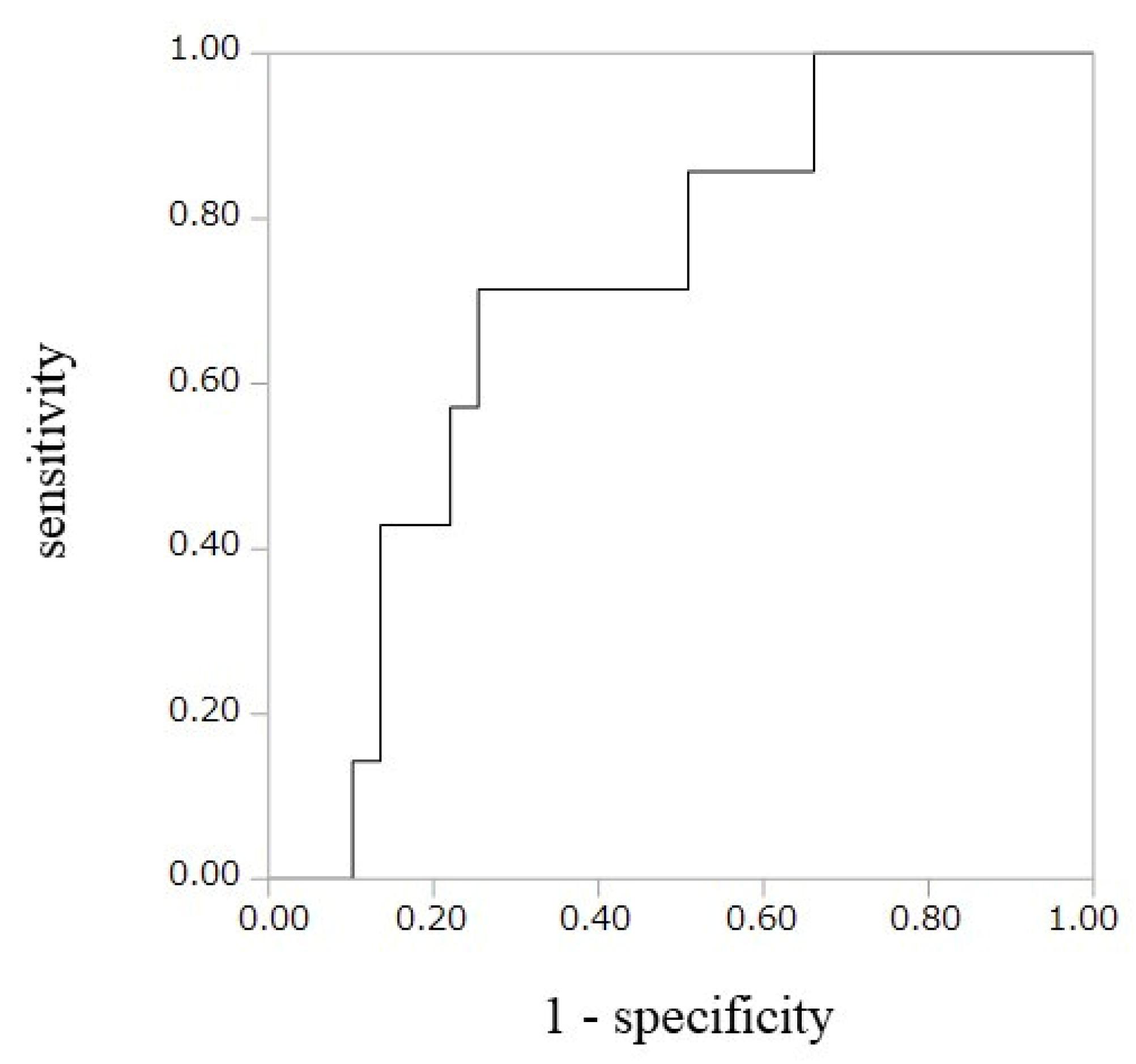
| SNX16-Ab (≥ 59735)† | 8.87 | 2.19–45.1 | 0.0021 | 8.61 | 2.07–45.0 | 0.0029 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsumata, Y.; Terada, J.; Matsumura, T.; Koshikawa, K.; Sakao, S.; Tomiyoshi, G.; Shinmen, N.; Nakamura, R.; Kuroda, H.; Nagashima, K.; et al. Circulating Anti-Sorting Nexins 16 Antibodies as an Emerging Biomarker of Coronary Artery Disease in Patients with Obstructive Sleep Apnea. Diagnostics 2020, 10, 71. https://doi.org/10.3390/diagnostics10020071
Katsumata Y, Terada J, Matsumura T, Koshikawa K, Sakao S, Tomiyoshi G, Shinmen N, Nakamura R, Kuroda H, Nagashima K, et al. Circulating Anti-Sorting Nexins 16 Antibodies as an Emerging Biomarker of Coronary Artery Disease in Patients with Obstructive Sleep Apnea. Diagnostics. 2020; 10(2):71. https://doi.org/10.3390/diagnostics10020071
Chicago/Turabian StyleKatsumata, Yusuke, Jiro Terada, Takuma Matsumura, Ken Koshikawa, Seiichiro Sakao, Go Tomiyoshi, Natsuko Shinmen, Rika Nakamura, Hideyuki Kuroda, Kengo Nagashima, and et al. 2020. "Circulating Anti-Sorting Nexins 16 Antibodies as an Emerging Biomarker of Coronary Artery Disease in Patients with Obstructive Sleep Apnea" Diagnostics 10, no. 2: 71. https://doi.org/10.3390/diagnostics10020071
APA StyleKatsumata, Y., Terada, J., Matsumura, T., Koshikawa, K., Sakao, S., Tomiyoshi, G., Shinmen, N., Nakamura, R., Kuroda, H., Nagashima, K., Kobayashi, Y., Kobayashi, E., Iwadate, Y., Zhang, X.-M., Hiwasa, T., & Tatsumi, K. (2020). Circulating Anti-Sorting Nexins 16 Antibodies as an Emerging Biomarker of Coronary Artery Disease in Patients with Obstructive Sleep Apnea. Diagnostics, 10(2), 71. https://doi.org/10.3390/diagnostics10020071






