Type I Kounis Syndrome after Protracted Anaphylaxis and Myocardial Bridge—Brief Literature Review and Case Report
Abstract
1. Introduction
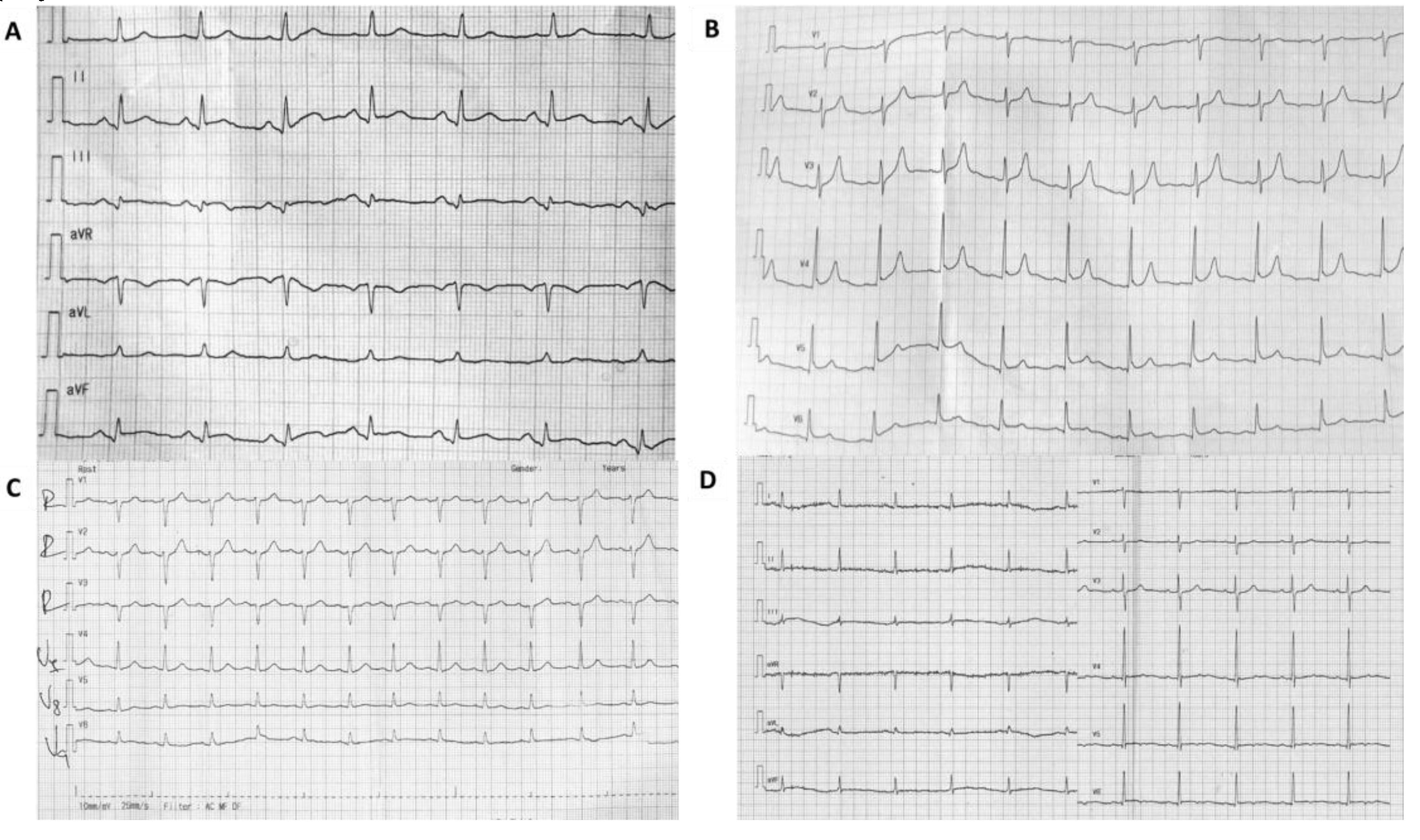
2. Case Presentation
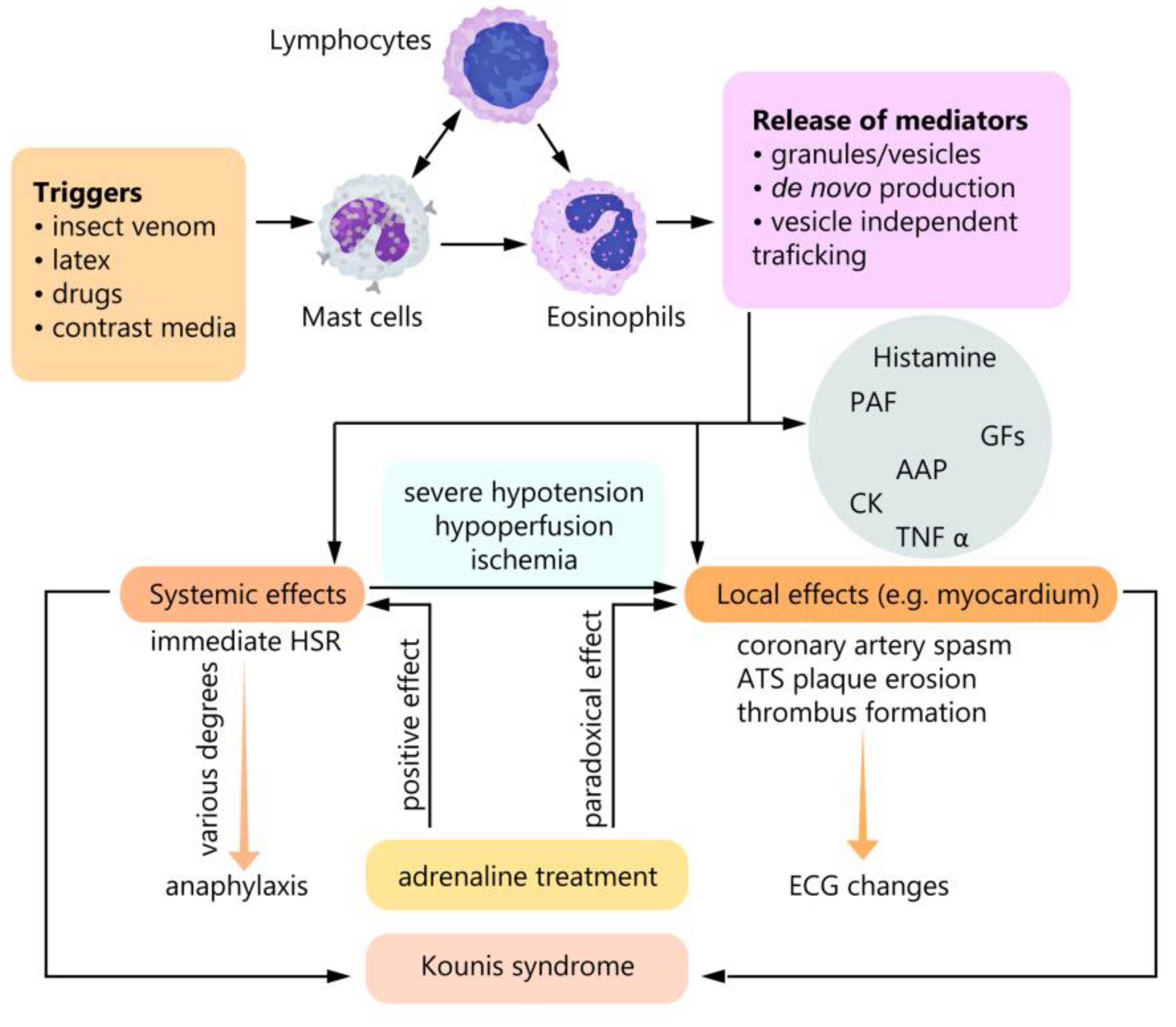
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, E. Serum carditis: The morphologic cardiac alterations in man associated with serum disease. J. Am. Med. Assoc. 1938, 110, 1098. [Google Scholar] [CrossRef]
- Pfister, C.W.; Plice, S.G. Acute myocardial infarction during a prolonged allergic reaction to penicillin. Am. Hear. J. 1950, 40, 945–947. [Google Scholar] [CrossRef]
- Kounis, N.G.; Zavras, G.M. Histamine-induced coronary artery spasm: The concept of allergic angina. Br. J. Clin. Pr. 1991, 45, 121–128. [Google Scholar]
- Li, J.; Zheng, J.; Zhou, Y.; Liu, X.; Peng, W. Acute coronary syndrome secondary to allergic coronary vasospasm (Kounis Syndrome): A case series, follow-up and literature review. BMC Cardiovasc. Disord. 2018, 18, 42. [Google Scholar] [CrossRef]
- Kounis, N.G. Coronary Hypersensitivity Disorder: The Kounis Syndrome. Clin. Ther. 2013, 35, 563–571. [Google Scholar] [CrossRef]
- Ozben, B.; Erdogan, O. The role of inflammation and allergy in acute coronary syndromes. Inflamm. Allergy - Drug Targets 2008, 7, 136–144. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Kounis, N.G. Kounis syndrome: An update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin. Chem. Lab. Med. 2016, 54, 1545–1559. [Google Scholar] [CrossRef]
- Abdelghany, M.; Subedi, R.; Shah, S.; Kozman, H. Kounis syndrome: A review article on epidemiology, diagnostic findings, management and complications of allergic acute coronary syndrome. Int. J. Cardiol. 2017, 232, 1–4. [Google Scholar] [CrossRef]
- Ravi, V.; Ayub, M.T.; Suboc, T.; Alyousef, T.; Gomez, J. A Curious Case of Coronary Vasospasm with Cardiogenic Shock: Type 1 Kounis Syndrome Complicated by Eosinophilic Myocarditis. Cureus 2019, 11, e4522. [Google Scholar] [CrossRef]
- Marcoux, V.; Nosib, S.; Bi, H.; Brownbridge, B. Intraoperative myocardial infarction: Kounis syndrome provoked by latex allergy. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Koniari, I.; Velissaris, D.; Tzanis, G.; Hahalis, G. Kounis Syndrome—not a Single-organ Arterial Disorder but a Multisystem and Multidisciplinary Disease. Balk. Med. J. 2019, 36, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Juste, J.F.M.; Garces, T.R.; Enguita, R.G.; Blasco, P.C.; Trallero, J.A. Cardiac complications in a metamizole-induced type I Kounis syndrome. Braz. J. Anesthesiol. 2016, 66, 194–196. [Google Scholar] [CrossRef][Green Version]
- Sciatti, E.; Vizzardi, E.; Cani, D.S.; Castiello, A.; Bonadei, I.; Savoldi, D.; Metra, M.; D’Aloia, A. Kounis syndrome, a disease to know: Case report and review of the literature. Monaldi Arch. Chest Dis. 2018, 88, 898. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I. Ampicillin/sulbactam-induced Kounis syndrome with cardiogenic shock. Anatol. J. Cardiol. 2017, 17, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Mazarakis, A.; Koutsojannis, C.M.; Kounis, N.G.; Alexopoulos, D. Cefuroxime-induced coronary artery spasm manifesting as Kounis syndrome. Acta Cardiol. 2005, 60, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Mitsis, A.; Christodoulou, E.; Georgiou, P. Coronary spasm secondary to cefuroxime injection, complicated with cardiogenic shock—A manifestation of Kounis syndrome: Case report and literature review. Eur. Hear. J. 2017, 7, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gao, Y.; Ma, J. Cefuroxime-associated Kounis syndrome with unique peculiarity in perioperative prophylaxis. J. Infect. Public Heal. 2018, 11, 889–892. [Google Scholar] [CrossRef]
- Maragkoudakis, S.; Hamilos, M.; Kallergis, E.; Vardas, P. Type 2 Kounis syndrome in an allergic woman: An uncommon presentation of acute coronary syndrome. J. Cardiol. Cases 2013, 8, 176–178. [Google Scholar] [CrossRef][Green Version]
- Abusnina, W.; Shehata, M.; Abouzid, M.; Price, M.; Zeid, F. Kounis syndrome secondary to gadolinium contrast agent. Bayl. Univ. Med. Cent. Proc. 2019, 32, 253–255. [Google Scholar] [CrossRef]
- Shibuya, K.; Kasama, S.; Funada, R.; Katoh, H.; Tsushima, Y. Kounis syndrome induced by contrast media: A case report and review of literature. Eur. J. Radiol. Open 2019, 6, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Priyankara, W.D.D.; Manoj, E.M.; Gunapala, A.; Ranaweera, A.G.R.M.A.; Vithanage, K.S.; Sivasubramanium, M.; Snajeeva, E. Cardiogenic Shock due to Kounis Syndrome following Cobra Bite. Case Rep. Crit. Care 2019, 2019, 5185716. [Google Scholar] [CrossRef] [PubMed]
- Sinkiewicz, W.; Sobański, P.; Bartuzi, Z. Allergic myocardial infarction. Cardiol. J. 2008, 15, 220–225. [Google Scholar] [PubMed]
- Dogan, V.; Çelik, O.; Özlek, B.; Özlek, E.; Çil, C.; Başaran, Ö.; Biteker, M. Allergic myocardial infarction: Type I Kounis syndrome following blue crab consumption. Acta Clin. Belg. 2018, 74, 375–377. [Google Scholar] [CrossRef]
- Kogias, J.S.; Sideris, S.K.; Anifadis, S.K. Kounis syndrome associated with hypersensitivity to hymenoptera stings. Int. J. Cardiol. 2007, 114, 252–255. [Google Scholar] [CrossRef]
- De Gennaro, L.; Brunetti, N.D.; Locuratolo, N.; Ruggiero, M.; Resta, M.; Diaferia, G.; Rana, M.; Caldarola, P. Kounis syndrome following canned tuna fish ingestion. Acta Clin. Belg. 2016, 72, 1–4. [Google Scholar] [CrossRef]
- Venturini, E.; Marabotti, C.; Magni, L.; Testa, R.; Kounis, N.G. Myocardial bridge as a trigger of Kounis syndrome. Int. J. Cardiol. 2016, 202, 87–89. [Google Scholar] [CrossRef]
- Lee, M.S.; Chen, C.-H. Myocardial Bridging: An Up-to-Date Review. J. Invasive Cardiol. 2015, 27, 521–528. [Google Scholar]
- Yanagawa, Y.; Nishi, K.; Tomiharu, N.; Kawaguchi, T. A case of takotsubo cardiomyopathy associated with Kounis syndrome. Int. J. Cardiol. 2009, 132, e65–e67. [Google Scholar] [CrossRef]
- Kounis, N. Serum tryptase levels and Kounis syndrome. Int. J. Cardiol. 2006, 114, 407–408. [Google Scholar] [CrossRef]
- Sainte-Laudy, J.; Cado, S. Comparison of the levels of histamine, tryptase, and interleukin-6 for the investigation of anaphylactoid drug reactions. Allerg. et Immunol. 1998, 30, 209–211. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghilencea, L.; Popescu, M.R.; Ghiordanescu, I.M.; Conea, C.; Melnic, M.; Popescu, A.C. Type I Kounis Syndrome after Protracted Anaphylaxis and Myocardial Bridge—Brief Literature Review and Case Report. Diagnostics 2020, 10, 59. https://doi.org/10.3390/diagnostics10020059
Ghilencea L, Popescu MR, Ghiordanescu IM, Conea C, Melnic M, Popescu AC. Type I Kounis Syndrome after Protracted Anaphylaxis and Myocardial Bridge—Brief Literature Review and Case Report. Diagnostics. 2020; 10(2):59. https://doi.org/10.3390/diagnostics10020059
Chicago/Turabian StyleGhilencea, Liviu, Mihaela Roxana Popescu, Ileana Maria Ghiordanescu, Cristina Conea, Mihai Melnic, and Andreea Catarina Popescu. 2020. "Type I Kounis Syndrome after Protracted Anaphylaxis and Myocardial Bridge—Brief Literature Review and Case Report" Diagnostics 10, no. 2: 59. https://doi.org/10.3390/diagnostics10020059
APA StyleGhilencea, L., Popescu, M. R., Ghiordanescu, I. M., Conea, C., Melnic, M., & Popescu, A. C. (2020). Type I Kounis Syndrome after Protracted Anaphylaxis and Myocardial Bridge—Brief Literature Review and Case Report. Diagnostics, 10(2), 59. https://doi.org/10.3390/diagnostics10020059





