Disease-Specific Survival of Type I and Type II Epithelial Ovarian Cancers—Stage Challenges Categorical Assignments of Indolence & Aggressiveness
Abstract
1. Introduction
2. Materials and Methods
3. Results
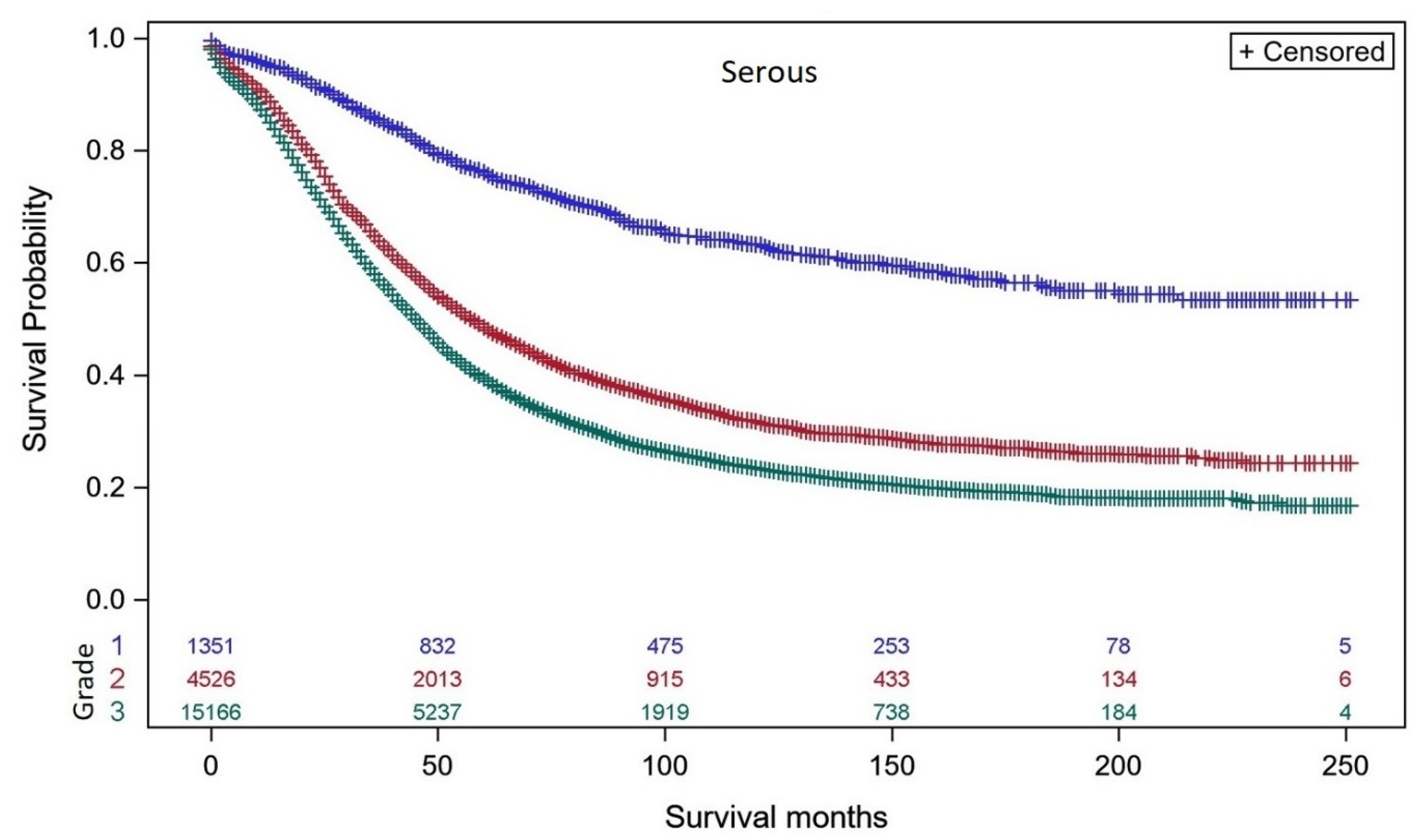
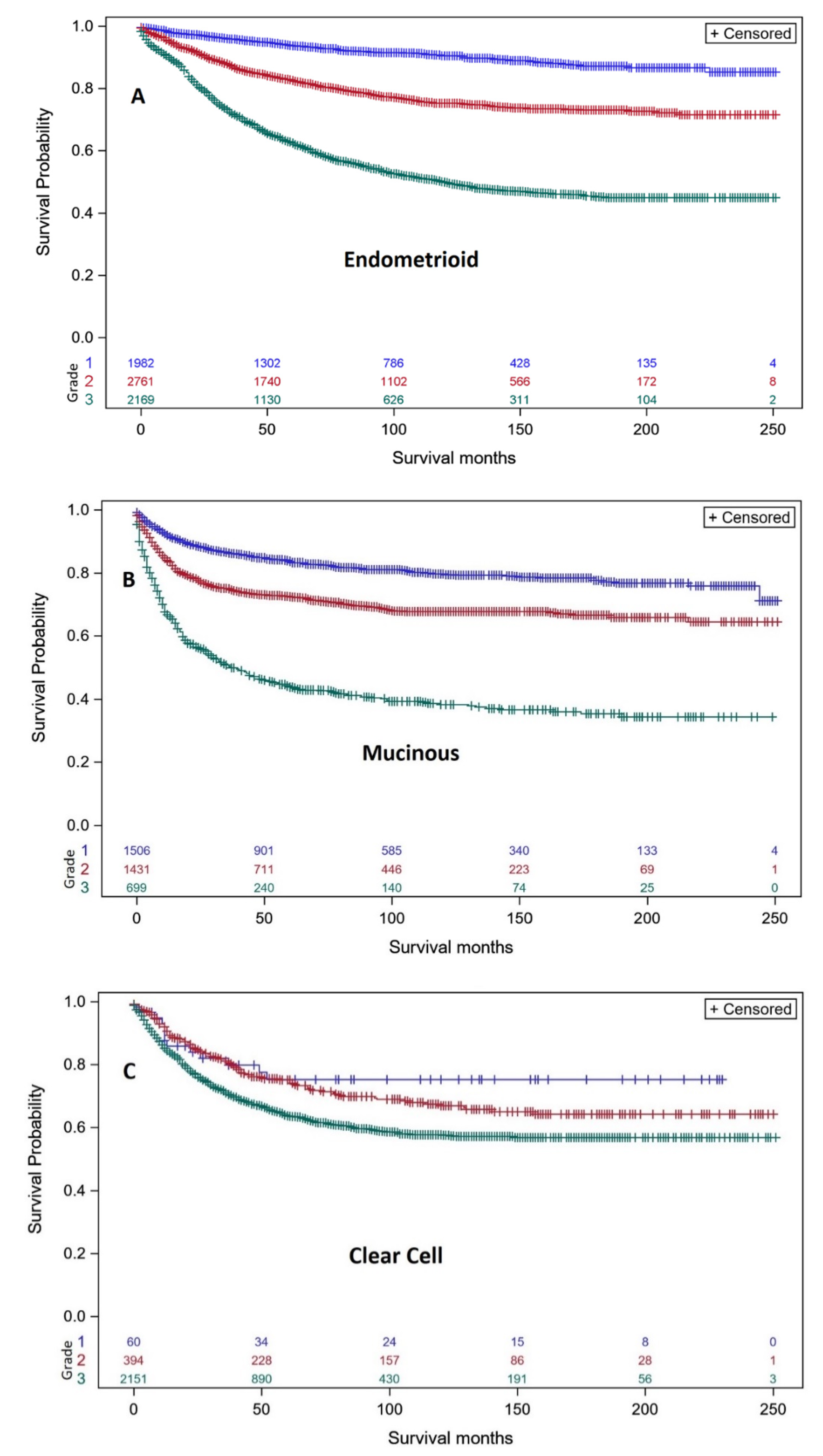
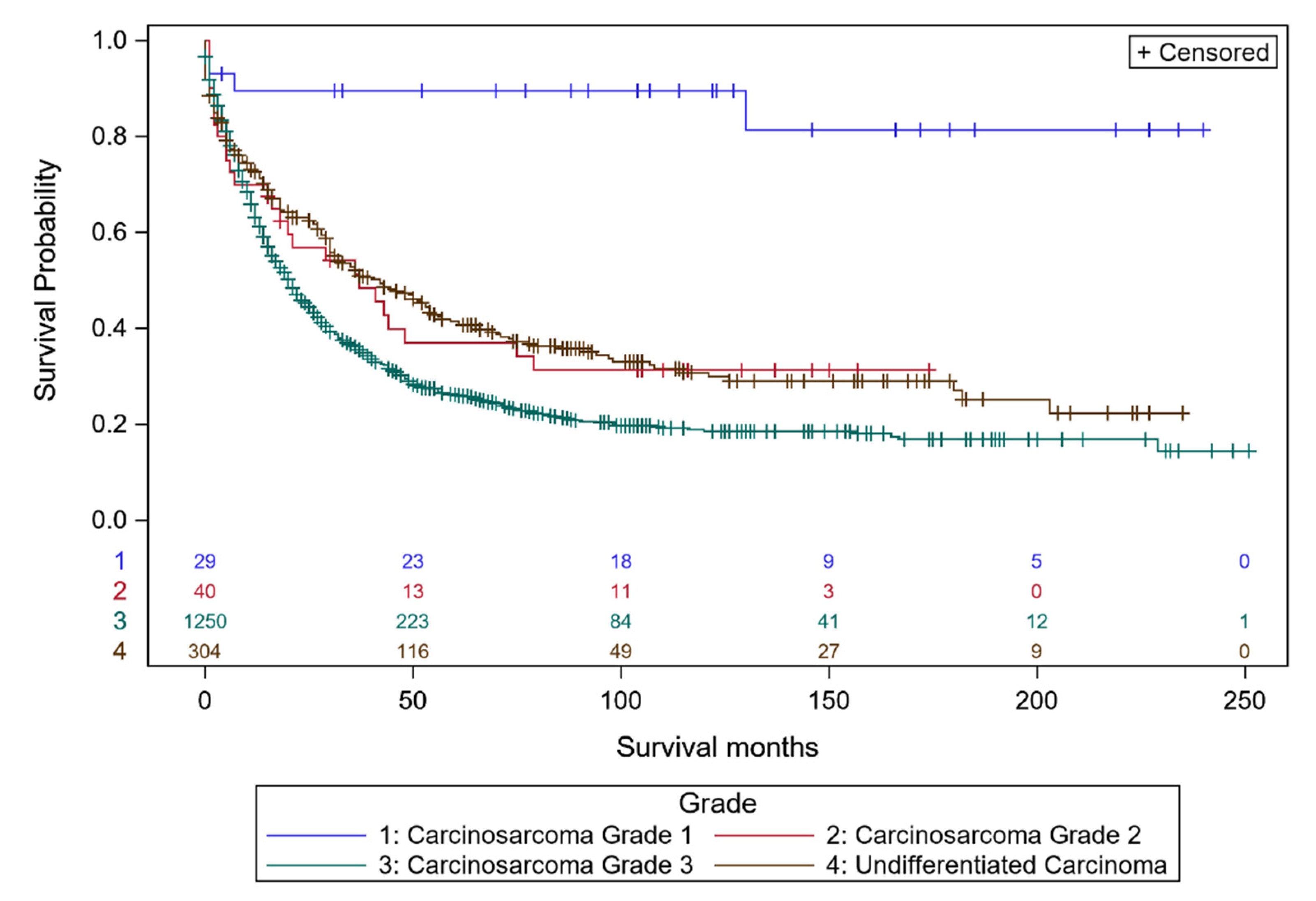
3.1. Survival Analyses of Individual Ovarian Cancer Histo-Types Related to Intrinsic Differentiation
3.2. Survival Analyses of Individual Ovarian Cancer Histo-Types Related to Differentiation and Extent of Disease
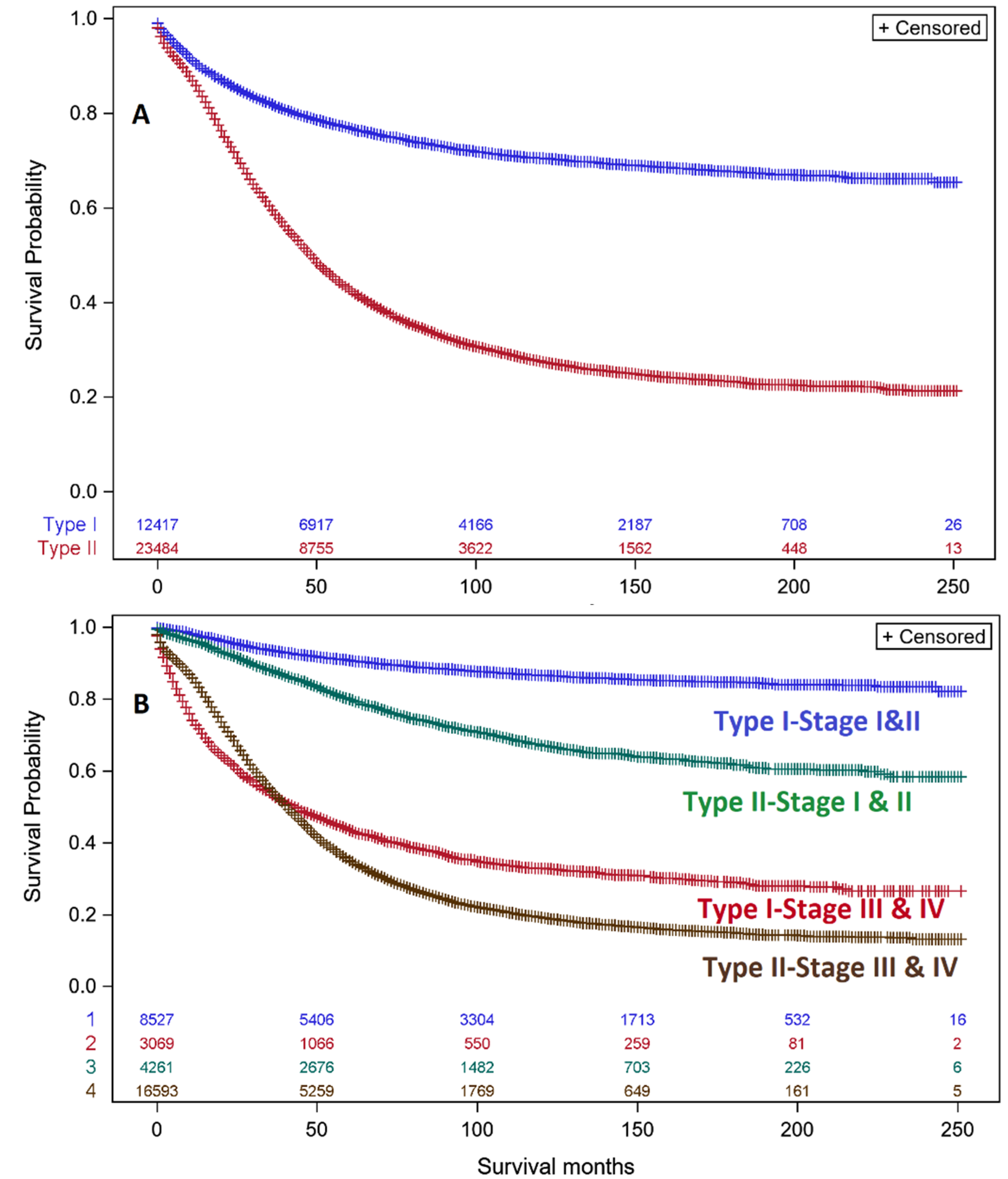
3.3. Analyses of Type I & II Ovarian Cancers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Garshell, J.; Miller, D.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. Seer Cancer Statistics Review; National Cancer Institute: Bethesda, MD, USA, 2015; pp. 1975–2016. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 20 December 2019).
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics. CA A Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.T.; Yurii, B.; Shvetsov, Y.B. Incidence of Ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995–2004. Cancer Epidemiol. Biomark. Prev. 2009, 18, 132–139. [Google Scholar] [CrossRef]
- Pavlik, E.J.; van Nagell, J.R., Jr. Early detection of ovarian tumors using ultrasound. Womens Health Lond 2013, 9, 39–55. [Google Scholar] [CrossRef]
- Committee on the State of the Science in Ovarian Cancer Research; Board on Health Care Services; Institute of Medicine; National Academies of Sciences, Engineering, and Medicine. Ovarian Cancers: Evolving Paradigms in Research and Care; National Academies Press: Washington, DC, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK367618/ (accessed on 31 December 2019). [CrossRef]
- Crum, C.P. Intercepting pelvic cancer in the distal fallopian tube: Theories and realities. Mol. Oncol. 2009, 3, 165–170. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Tortolero-Luna, G.; Malpica, A.; Baker, V.V.; Whittaker, L.; Johnson, E.; Follen, M.M. Ovarian intraepithelial neoplasia and ovarian cancer. Obstet. Gynecol. Clin. N. Am. 1996, 23, 475–543. [Google Scholar]
- Kindelberger, D.W.; Lee, Y.; Miron, A.; Hirsch, M.S.; Feltmate, C.; Medeiros, F.; Callahan, M.J.; Garner, E.O.; Gordon, R.W.; Birch, C.; et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am. J. Surg. Pathol. 2007, 31, 161–169. [Google Scholar] [CrossRef]
- Crum, C.P.; McKeon, F.D.; Xian, W. The oviduct and ovarian cancer: Causality, clinical implications, and “targeted prevention”. Clin. Obstet. Gynecol. 2012, 55, 24–35. [Google Scholar] [CrossRef]
- SEER Explorer: An Interactive Website for SEER Cancer Statistics [Internet]. Surveillance Research Program, National Cancer Institute; [Cited 2019 Apr 15]. Available online: https://seer.cancer.gov/explorer/ (accessed on 18 December 2019).
- Seidman, J.D.; Kurman, R.J.; Ronnett, B.M. Primary and metastatic mucinous adenocarcinomas in the ovaries: Incidence in routine practice with a new approach to improve intraoperative diagnosis. Am. J. Surg. Pathol. 2003, 27, 985–993. [Google Scholar] [CrossRef]
- Seidman, J.D.; Horkayne-Szakaly, I.; Haiba, M.; Boice, C.R.; Kurman, R.J.; Ronnett, B.M. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int. J. Gynecol. Pathol. 2004, 23, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Gilks, C.B.; Ionescu, D.N.; Kalloger, S.E.; Kobel, M.; Irving, J.; Clarke, B.; Santos, J.; Le, N.; Moravan, V.; Swenerton, K. Ovarian Cancer Outcomes Unit of the British Columbia Cancer Agency. Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum. Pathol. 2008, 39, 1239–1251. [Google Scholar] [CrossRef]
- Meinhold-Heerlein, I.; Fotopoulou, C.; Harter, P.; Kurzeder, C.; Mustea, A.; Wimberger, P.; Hauptmann, S.; Sehouli, J. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch. Gynecol. Obstet. 2016, 293, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—Shifting the paradigm. Hum. Pathol. 2001, 42, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.-M.; Kurman, R.J. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Recent concepts of ovarian carcinogenesis: Type I and Type II. Biomed Res. Int. 2014, 2014, 11. [Google Scholar] [CrossRef]
- Meinhold-Heerlein, I.; Hauptmann, S. The heterogeneity of ovarian cancer. Arch. Gynecol. Obstet. 2014, 289, 237–239. [Google Scholar] [CrossRef]
- Nezhat, F.R.; Apostol, R.; Nezhat, C.; Pejovic, T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am. J. Obstet. Gynecol. 2015, 213, 262–267. [Google Scholar] [CrossRef]
- Soong, T.R.; Howitt, B.E.; Horowitz, N.; Nucci, M.R.; Crum, C.P. The fallopian tube, “precursor escape” and narrowing the knowledge gap to the origins of high-grade serous carcinoma. Gynecol. Oncol. 2019, 152, 426–433. [Google Scholar] [CrossRef]
- Duggan, M.A.; Anderson, W.F.; Altekruse, S.; Penberthy, L.; Sherman, M.E. The surveillance, epidemiology, and end results (SEER) program and pathology: Toward strengthening the critical relationship. Am. J. Surg. Pathol. 2016, 40, e94–e102. [Google Scholar] [CrossRef]
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med. 1996, 334, 1–6. [Google Scholar] [CrossRef]
- Malpica, A.; Deavers, M.T.; Lu, K.; Bodurka, D.C.; Atkinson, E.N.; Gershenson, D.M.; Silva, E.G. Grading ovarian serous carcinoma using a two-tier system. Am. J. Surg. Pathol. 2004, 28, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Vang, R.; Shih, I.M.; Kurman, R.J. Ovarian low-grade and high-grade serouw carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv. Anat. Pathol. 2009, 16, 267–282. [Google Scholar] [CrossRef]
- Bodurka, D.C.; Deavers, M.T.; Tian, C.; Sun, C.C.; Malpica, A.; Coleman, R.L.; Lu, K.H.; Sood, A.K.; Birrer, M.J.; Ozols, R.; et al. Reclassification of serous ovarian carcinoma by a 2-tier system: A Gynecologic Oncology Group Study. Cancer 2012, 118, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.C.; Cushing-Haugen, K.L.; Köbel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive epithelial ovarian cancer survival by histotype and disease stage. J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef]
- Lan, A.; Yang, G. Clinicopathological parameters and survival of invasive epithelial ovarian cancer by histotype and disease stage. Future Oncol. 2019, 15, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Dinkelspiel, H.E.; Champer, M.; Hou, J.; Tergas, A.; Burke, W.M.; Huang, Y.; Neugut, A.I.; Ananth, C.V.; Hershman, D.L.; Wright, J.D. Long-term mortality among women with epithelial ovarian cancer. Gynecol. Oncol. 2015, 138, 421–428. [Google Scholar] [CrossRef]
- Arora, N.; Talhouk, A.; McAlpine, J.N.; Law, M.R.; Hanley, G.E. Long-term mortality among women with epithelial ovarian cancer: A population-based study in british columbia, Canada. BMC Cancer 2018, 18, 1039. [Google Scholar] [CrossRef]
- Arora, N.; Talhouk, A.; McAlpine, J.N.; Law, M.R.; Hanley, G.E. Causes of death among women with epithelial ovarian cancer by length of survival post-diagnosis: A population-based study in British Columbia, Canada. Int. J. Gynecol. Cancer 2019, 29, 593–598. [Google Scholar] [CrossRef]
- Kurman, R.J.; Visvanathan, K.; Roden, R.; Wu, T.C.; Shih, I.-M. Early detection and treatment of ovarian cancer: Shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am. J. Obstet. Gynecol. 2008, 198, 351–356. [Google Scholar] [CrossRef]
- Clinical Trials.gov. Available online: https://www.clinicaltrials.gov/ct2/results?cond=ovarian+cancer&term=&cntry=&state=&city=&dist= (accessed on 20 December 2019).
- Percy, C.; Stanek, E.; Gloeckler, L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am. J. Public Health 1981, 71, 242–250. [Google Scholar] [CrossRef]
- German, R.R.; Fink, A.K.; Heron, M.; Stewart, S.L.; Johnson, C.J.; Finch, J.L.; Yin, D. Accuracy of Cancer Mortality Study Group. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011, 35, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Powell, M.A.; Al-Hammadi, N.; Chen, L.; Miller, J.P.; Roland, P.Y.; Mutch, D.G.; Cliby, W.A. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. JNCI J. Natl. Cancer Instit. 2013, 105, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, R.K.; Sherman, M.E.; Visvanathan, K.; Goodman, M.T.; Hernandez, B.Y.; Lynch, C.F.; Ioffe, O.B.; Horio, D.; Platz, C.; Altekruse, S.F.; et al. Agreement for tumor grade of ovarian carcinoma: Analysis of archival tissues from the surveillance, epidemiology, and end results residual tissue repository. Cancer Causes Control. 2013, 24, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.R.; Deavers, M.T.; Silva, E.G.; Malpica, A. Ovarian involvement by metastatic colorectal adenocarcinoma: Still a diagnostic challenge. Am. J. Surg. Pathol. 2006, 30, 177–184. [Google Scholar] [CrossRef]
- Perrin, T.J. Mucinous epithelial ovarian carcinoma. Ann. Oncol. 2016, 27, i53–i57. [Google Scholar] [CrossRef]
- Differentiation of Primary Ovarian Tumours from Metastatic Colorectal Carcinoma. Available online: http://e-immunohistochemistry.info/web/Differentiation_of_primary_ovarian_tumours_from_metastatic_colorectal_carcinoma.htm (accessed on 20 December 2019).
- College of American Pathologists Protocol. Protocol for the Examination of Specimens from Patients with Primary Tumors of the Ovary, Fallopian Tube, or Peritoneum. Posted August 2018. Available online: https://documents.cap.org/protocols/cp-femalereproductive-ovary-fallopian-18protocol-1100.pdf (accessed on 20 December 2019).
| Stage I–II | Stage I–II | Stage III–IV | Stage III–IV | |
|---|---|---|---|---|
| Serous | n | 10 yr Survival | n | 10 yr Survival |
| Grade 1 | 530 | 85.2% (81.3–88.4%) | 697 | 51.0% (46.8–55.0%) |
| Grade 2 | 1022 | 73.1% (69.8–76.0%) | 2983 | 24.6% (23.0–26.3%) |
| Grade 3 | 2032 | 64.8% (62.3–67.1%) | 11,801 | 23.9% (23.1–24.7%) |
| Endometrioid | n | 10 yr Survival | n | 10 yr Survival |
| Grade 1 | 1759 | 93.4% (91.8–94.6%) | 142 | 63.9% (54.1–72.1%) |
| Grade 2 | 2033 | 87.2% (85.4–88.8%) | 564 | 38.1% (33.8–42.5%) |
| Grade 3 | 951 | 74.2% (70.9–77.3%) | 1026 | 32.9% (29.8–36.0%) |
| Clear Cell | n = | 10 yr Survival | n | 10 yr Survival |
| Grade 1 | 48 | 81.1% (64.4–90.5%) | 7 | 16.7% (0.8–51.7%) |
| Grade 2 | 284 | 81.4% (75.5–86.0%) | 88 | 26.8% (17.5–37.0%) |
| Grade 3 | 902 | 78.3% (75.5–80.9%) | 478 | 25.3% (21.9–28.9%) |
| Mucinous | n | 10 yr Survival | n | 10 yr Survival |
| Grade 1 | 1180 | 89.6% (87.3–91.5%) | 197 | 32.9% (26.1–40.0%) |
| Grade 2 | 1000 | 84.8% (82.0–87.3%) | 328 | 22.1% (17.6–27.0%) |
| Grade 3 | 235 | 72.8% (66.4–78.2%) | 286 | 14.9% (11.2–19.2%) |
| Carcinosarcoma | n | 10 yr Survival | n | 10 yr Survival |
| Grade 1 | 24 | 100% (100–100%) | 2 | 0.0% (0.0–0.0%) |
| Grade 2 | 13 | 68.4% (30.6–88.6%) | 19 | 15.8% (3.9–34.9%) |
| Grade 3 | 107 | 44.2% (35.4–52.7%) | 342 | 17.6% (14.4–21.1%) |
| Undifferentiated carcinoma | n | 10 yr Survival | n | 10 yr Survival |
| Grade 3 | 63 | 54.6% (39.7–67.3%) | 213 | 23.7% (17.9–30.0%) |
| Early Stage | |||||
|---|---|---|---|---|---|
| Stage I-II | |||||
| Endometrioid grade 1= | Mucinous grade 1> | ||||
| Carcinosarcoma grade 1 > | 89.6% 10 yr survival | Serous grade 1 = | |||
| 93+% 10 yr survival | Endometrioid grade 2= | Serous grade 2 = | |||
| Clear cell grade 1 = | Endometrioid grade 3 = | Serous grade 3 | |||
| Mucinous grade 2 > | Clear cell grade 2&3 = | Undifferentiated carcinoma > | Carcinosarcoma grade 3 | ||
| 80–87% 10 yr survival | Mucinous grade 3= | 54–65% 10 yr survival | 44% 10 yr survival | ||
| Carcinosarcoma grade 2> | |||||
| 68–79% 10 yr survival |
| Late Stage | ||||
|---|---|---|---|---|
| Stage III–IV | ||||
| Endometrioid grade 1 = Serous grade 1> | ||||
| 51–64% 10 yr survival | Endometrioid grade 2> | |||
| 38% 10 yr survival | Endometrioid grade 3= | Mucinous grade 2= | ||
| Clear cell grade 2= | Serous grade 3 = | |||
| Mucinous grade 1= Serous grade 2> | Clear cell grade 3= | Clear cell grade 3 = | ||
| 27–33% 10 yr survival | Undifferentiated carcinoma > | Mucinous grade 3 = | ||
| 22–25% 10 yr survival | Carcinosarcoma grade 2 &3 > | |||
| 14–18% 10 yr survival |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlik, E.J.; Smith, C.; Dennis, T.S.; Harvey, E.; Huang, B.; Chen, Q.; West Piecoro, D.; Burgess, B.T.; McDowell, A.; Gorski, J.; et al. Disease-Specific Survival of Type I and Type II Epithelial Ovarian Cancers—Stage Challenges Categorical Assignments of Indolence & Aggressiveness. Diagnostics 2020, 10, 56. https://doi.org/10.3390/diagnostics10020056
Pavlik EJ, Smith C, Dennis TS, Harvey E, Huang B, Chen Q, West Piecoro D, Burgess BT, McDowell A, Gorski J, et al. Disease-Specific Survival of Type I and Type II Epithelial Ovarian Cancers—Stage Challenges Categorical Assignments of Indolence & Aggressiveness. Diagnostics. 2020; 10(2):56. https://doi.org/10.3390/diagnostics10020056
Chicago/Turabian StylePavlik, Edward J., Christopher Smith, Taylor S. Dennis, Elizabeth Harvey, Bin Huang, Quan Chen, Dava West Piecoro, Brian T. Burgess, Anthony McDowell, Justin Gorski, and et al. 2020. "Disease-Specific Survival of Type I and Type II Epithelial Ovarian Cancers—Stage Challenges Categorical Assignments of Indolence & Aggressiveness" Diagnostics 10, no. 2: 56. https://doi.org/10.3390/diagnostics10020056
APA StylePavlik, E. J., Smith, C., Dennis, T. S., Harvey, E., Huang, B., Chen, Q., West Piecoro, D., Burgess, B. T., McDowell, A., Gorski, J., Baldwin, L. A., Miller, R. W., DeSimone, C. P., Dietrich, C., III, Gallion, H. H., Ueland, F. R., & van Nagell, J. R., Jr. (2020). Disease-Specific Survival of Type I and Type II Epithelial Ovarian Cancers—Stage Challenges Categorical Assignments of Indolence & Aggressiveness. Diagnostics, 10(2), 56. https://doi.org/10.3390/diagnostics10020056






