Diagnostic Performance of 18F-FDG PET or PET/CT for Detection of Post-Transplant Lymphoproliferative Disorder: A Systematic Review and a Bivariate Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
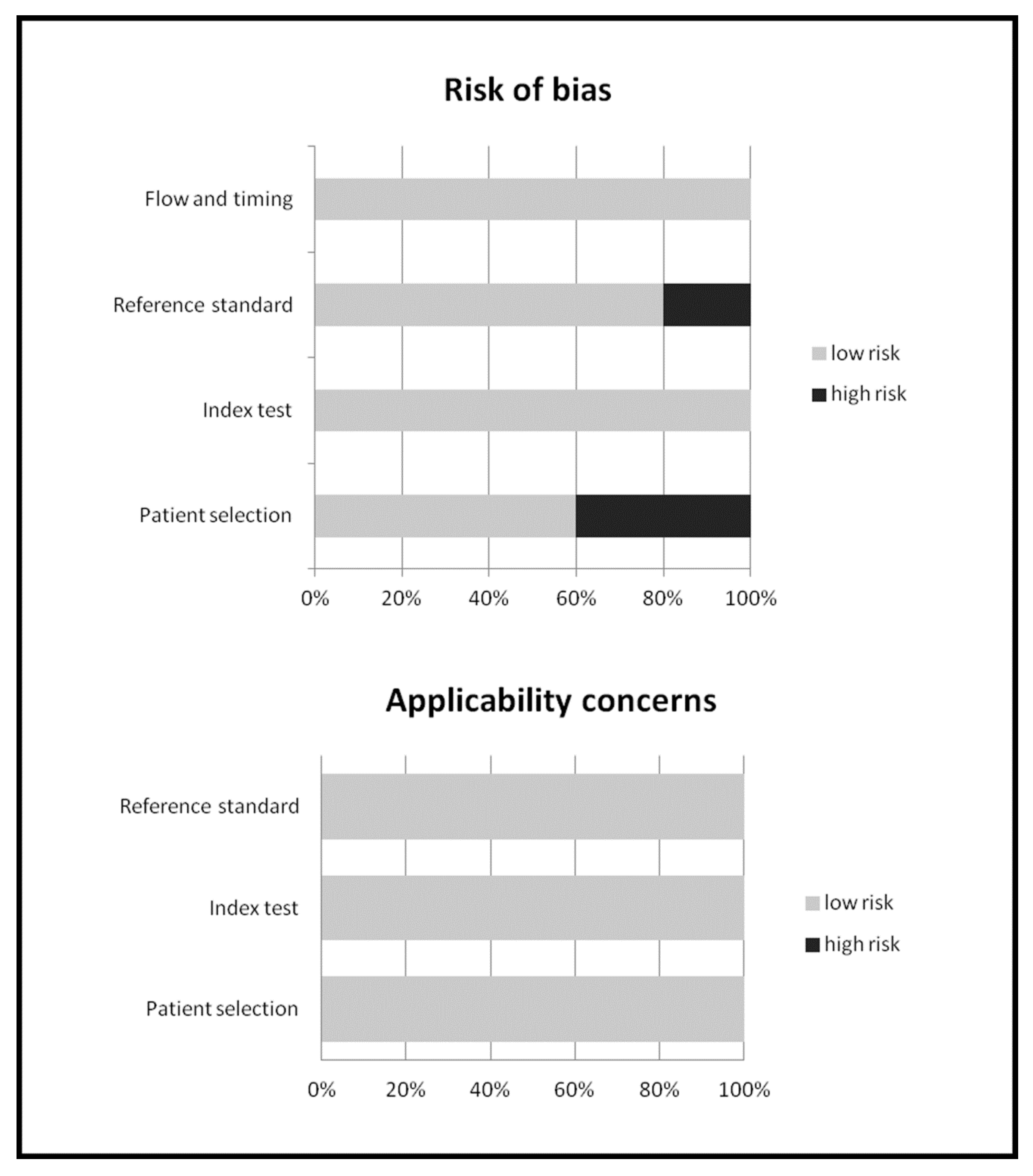
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
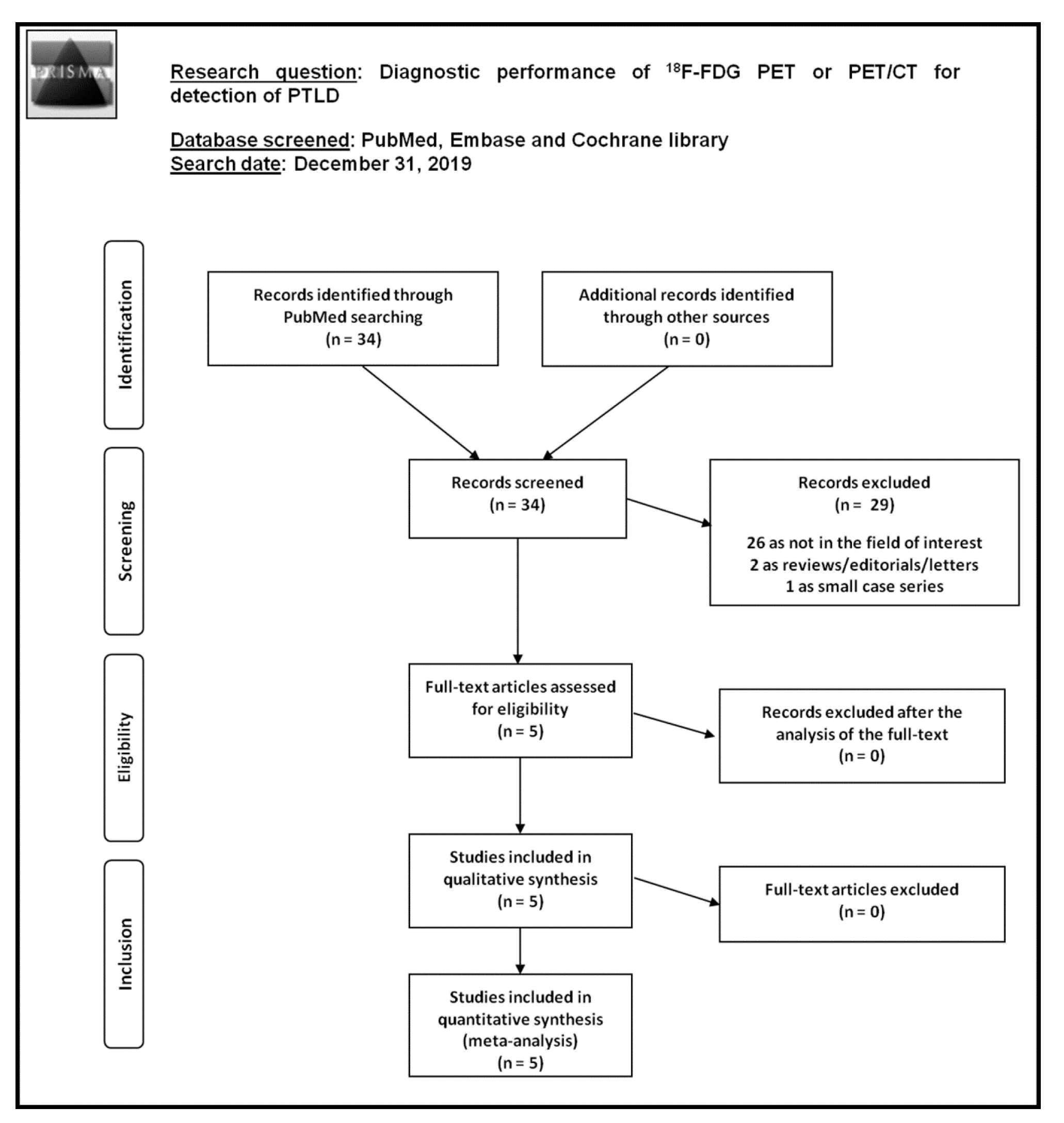
3.1. Literature Search
3.2. Qualitative Analysis (Systematic Review)
3.2.1. Basic Study and Patient Characteristics
3.2.2. Technical Aspects
3.2.3. Main Findings
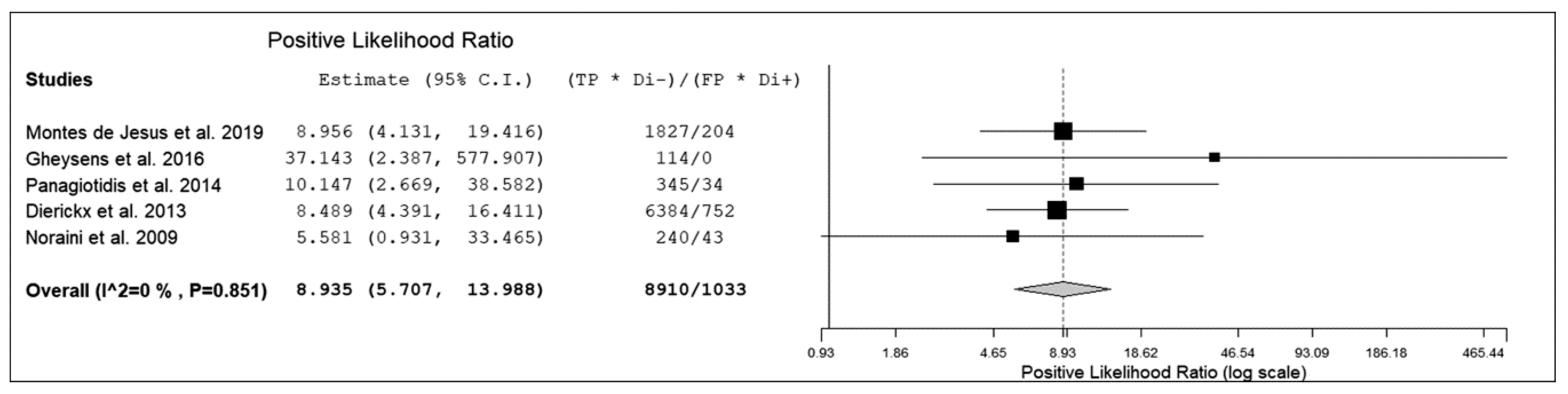
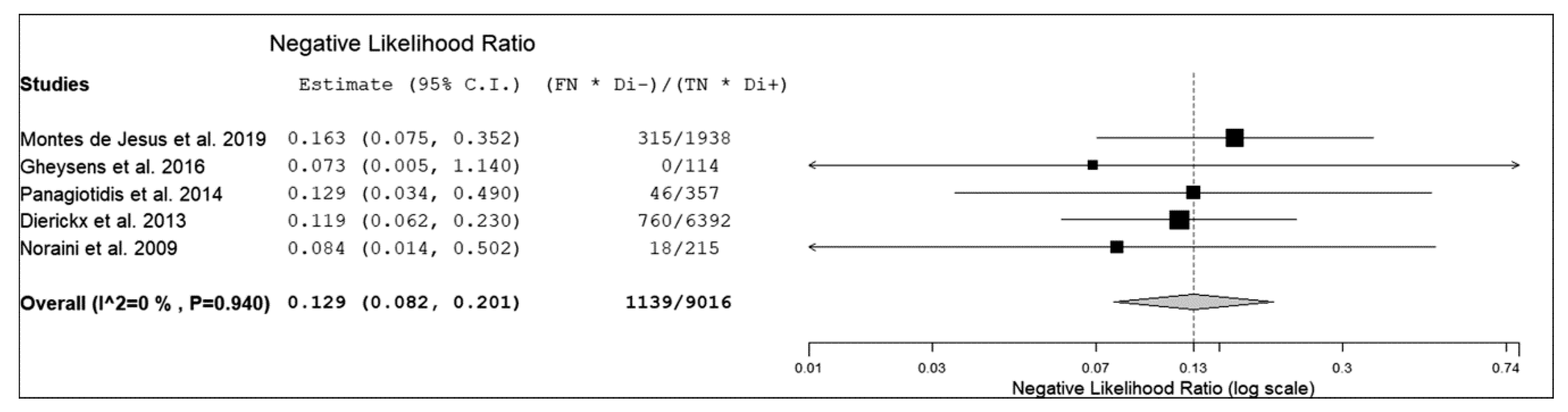
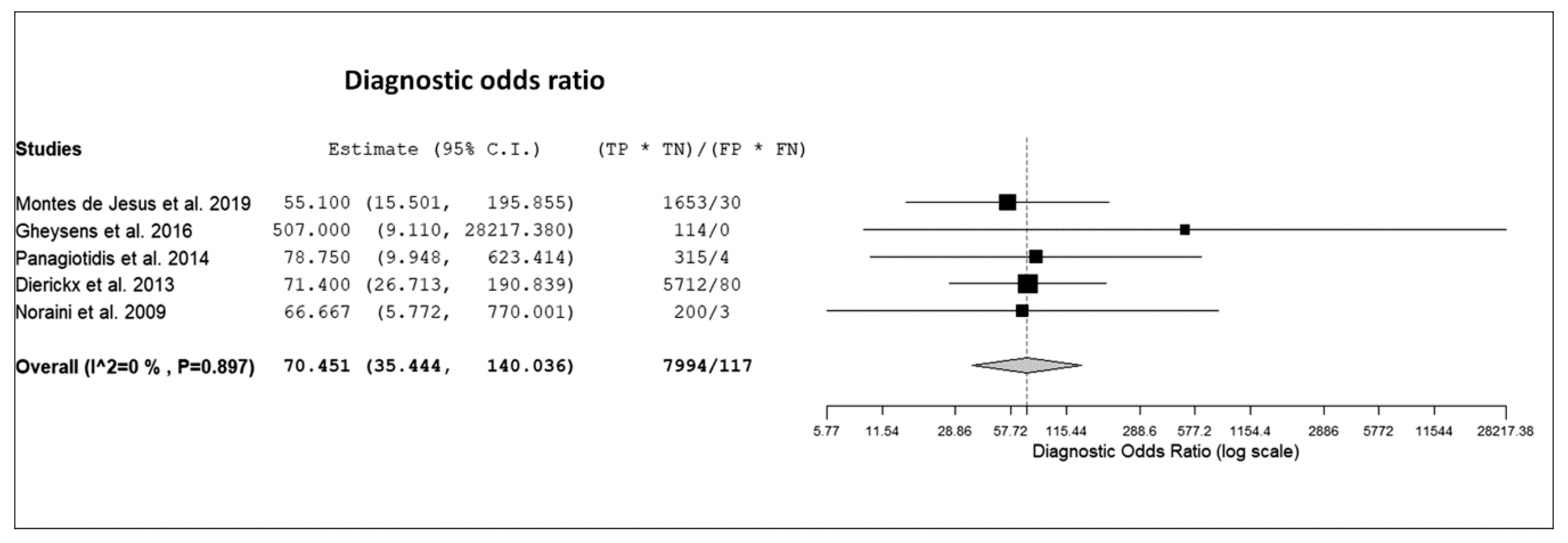
3.3. Quantitative Analysis (Meta-Analysis)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DeStefano, C.B.; Desai, S.H.; Shenoy, A.G.; Catlett, J.P. Management of post-transplant lymphoproliferative disorders. Br. J. Haematol. 2018, 182, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. B-Cell Lymphomas. Version 4.2019. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 12 February 2020).
- Treglia, G.; Ceriani, L. (18)F-FDG PET/CT in patients with post-transplant lymphoproliferative disorders: So far so good. Eur. J. Nucl. Med. Mol. Imaging. 2019, 47, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Allen, U.D.; Preiksaitis, J.K. AST Infectious Diseases Community of Practice. Post-transplant lymphoproliferative disorders, Epstein–Barr virus infection, and disease in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13652. [Google Scholar] [PubMed]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; the PRISMA-DTA Group; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Sadeghi, R. Meta-analyses and systematic reviews on PET and PET/CT in oncology: The state of the art. Clin. Transl. Imaging. 2013, 1, 73–75. [Google Scholar] [CrossRef][Green Version]
- Sadeghi, R.; Treglia, G. Systematic reviews and meta-analyses of diagnostic studies: A practical guideline. Clin. Transl. Imaging. 2017, 5, 83–87. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Int. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Harbord, R.M.; Egger, M.; Sterne, J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Montes de Jesus, F.M.; Kwee, T.C.; Kahle, X.U.; Nijland, M.; van Meerten, T.; Huls, G.; Dierckx, R.A.J.O.; Rosati, S.; Diepstra, A.; van der Bij, W.; et al. Diagnostic performance of FDG-PET/CT of post-transplant lymphoproliferative disorder and factors affecting diagnostic yield. Eur. J. Nucl. Med. Mol. Imaging. 2019, 47, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Gheysens, O.; Thielemans, S.; Morscio, J.; Boeckx, N.; Goffin, K.E.; Deroose, C.M.; Sagaert, X.; Wlodarska, I.; Verhoef, G.; Dierickx, D.; et al. Detection of bone marrow involvement in newly diagnosed post-transplant lymphoproliferative disorder: (18)F-fluorodeoxyglucose positron emission tomography/computed tomography versus bone marrow biopsy. Leuk. Lymphoma. 2016, 57, 2382–2388. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidis, E.; Quigley, A.M.; Pencharz, D.; Ardeshna, K.; Syed, R.; Sajjan, R.; Bomanji, J. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in diagnosis of post-transplant lymphoproliferative disorder. Leuk. Lymphoma. 2014, 55, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, D.; Tousseyn, T.; Requilé, A.; Verscuren, R.; Sagaert, X.; Morscio, J.; Wlodarska, I.; Herreman, A.; Kuypers, D.; Van Cleemput, J.; et al. The accuracy of positron emission tomography in the detection of posttransplant lymphoproliferative disorder. Haematologica. 2013, 98, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Noraini, A.R.; Gay, E.; Ferrara, C.; Ravelli, E.; Mancini, V.; Morra, E.; Muti, P.; Tahir, A.; Abdul Jalil, N.; Rossetti, C. PET-CT as an effective imaging modality in the staging and follow-up of post-transplant lymphoproliferative disorder following solid organ transplantation. Singapore. Med. J. 2009, 50, 1189–1195. [Google Scholar] [PubMed]
- Montes de Jesus, F.M.; Kwee, T.C.; Nijland, M.; Kahle, X.U.; Huls, G.; Dierckx, R.A.J.O.; van Meerten, T.; Gheysens, O.; Dierickx, D.; Vergote, V.; et al. Performance of advanced imaging modalities at diagnosis and treatment response evaluation of patients with post-transplant lymphoproliferative disorder: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 132, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Muller, N.; Kessler, R.; Caillard, S.; Epailly, E.; Hubelé, F.; Heimburger, C.; Namer, I.J.; Herbrecht, R.; Blondet, C.; Imperiale, A. 18F-FDG PET/CT for the Diagnosis of Malignant and Infectious Complications After Solid Organ Transplantation. Nucl. Med. Mol. Imaging. 2017, 51, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Wareham, N.E.; Lundgren, J.D.; Da Cunha-Bang, C.; Gustafsson, F.; Iversen, M.; Johannesen, H.H.; Kjær, A.; Rasmussen, A.; Sengeløv, H.; Sørensen, S.S.; et al. The clinical utility of FDG PET/CT among solid organ transplant recipients suspected of malignancy or infection. Eur. J. Nucl. Med. Mol. Imaging. 2017, 44, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Van Keerberghen, C.A.; Goffin, K.; Vergote, V.; Tousseyn, T.; Verhoef, G.; Laenen, A.; Vandenberghe, P.; Dierickx, D.; Gheysens, O. Role of interim and end of treatment positron emission tomography for response assessment and prediction of relapse in posttransplant lymphoproliferative disorder. Acta. Oncol. 2019, 58, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.; Denecke, T.; Dreyling, M.H.; Franzius, C.; Reinke, P.; Subklewe, M.; Amthauer, H.; Kneba, M.; Riess, H.; Trappe, R.U. End-of-Treatment Positron Emission Tomography After Uniform First-Line Therapy of B-Cell Posttransplant Lymphoproliferative Disorder Identifies Patients at Low Risk of Relapse in the Prospective German PTLD Registry. Transplantation. 2018, 102, 868–875. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Country | Study design | Number of Patients Performing 18F-FDG PET or PET/CT | Median Age (Years) | % Male | Percentage of EBV Positivity | Transplanted Organ | Median Time Between Transplant and PTLD Diagnosis or PET Acquisition (Months) |
|---|---|---|---|---|---|---|---|---|---|
| Montes de Jesus et al. [12] | 2019 | Netherlands | retrospective | 91 | 54 | 55% | 62% | lung (44%), kidney (34%), liver (12%), HSC (4%), other (6%) | 60 |
| Gheysens et al. [13] | 2016 | Belgium | retrospective | 25 | 42 | 60% | NR | kidney (40%), lung (40%), heart (8%), liver (8%), other (4%) | 76.5 |
| Panagiotidis et al. [14] | 2014 | United Kingdom | retrospective | 40 | 52 | 55% | NR | liver (40%), kidney (40%), heart (10%), other (10%) | 112 |
| Dierickx et al. [15] | 2013 | Belgium | retrospective | 150 | NR | 60% | 56% | kidney (34%), liver (15%), lung (15%), heart (15%), HSC (15%), other (6%) | 69 |
| Noraini et al. [16] | 2009 | Malaysia, Italy | retrospective | 30 | 49.5 | 87% | NR | liver (47%), heart (40%), kidney (10%), lung (3%) | NR |
| Authors | PET Modality | Mean Injected Activity of 18F-FDG | Time between 18F-FDG Injection and PET Acquisition | PET Protocol | Image Analysis | Reference Standard |
|---|---|---|---|---|---|---|
| Montes de Jesus et al. [12] | PET/CT | 3 MBq/kg | 60 min | Images from the skull base to mid-thigh | visual | Histology or clinical/biochemical/imaging data |
| Gheysens et al. [13] | PET/CT | NR | 60 min | Images from the skull to mid-thigh | visual | Histology or clinical/biochemical/imaging data |
| Panagiotidis et al. [14] | PET/CT | 370 MBq | 60 min | Images from the skull base to mid-thigh | visual | Histology or clinical/biochemical/imaging data |
| Dierickx et al. [15] | PET or PET/CT | 4 x body weight (kg) + 20 MBq | 60 min | Images from the skull to mid-thigh | visual and semiquantitative | Histology or clinical/biochemical/imaging data |
| Noraini et al. [16] | PET/CT | 259-333 MBq | 60 min | Images from the skull base to mid-thigh | visual and semiquantitative | Histology or clinical/biochemical/imaging data |
| Authors | True Positive | False Negative | False Positive | True Negative | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|---|---|
| Montes de Jesus et al. [12] | 29 | 5 | 6 | 57 | 85% | 90% | 83% | 92% | 89% |
| Gheysens et al. [13] * | 6 | 0 | 0 | 19 | 100% | 100% | 100% | 100% | 100% |
| Panagiotidis et al. [14] | 15 | 2 | 2 | 21 | 88% | 91% | 88% | 91% | 90% |
| Dierickx et al. [15] | 84 | 10 | 8 | 68 | 89% | 89% | 91% | 87% | 89% |
| Noraini et al. [16] | 40 | 3 | 1 | 5 | 93% | 83% | 98% | 63% | 92% |
| Authors | Monomorphic PTLD | Polymorphic PTLD | Early lesions(nondestructive PTLD) | Hodgkin-like PTLD | Unclear histology |
|---|---|---|---|---|---|
| Montes de Jesus et al. [12] | 70% | 18% | 6% | 3% | 3% |
| Gheysens et al. [13] | 84% | 8% | 8% | 0% | 0% |
| Panagiotidis et al. [14] | 93% | 0% | 0% | 7% | 0% |
| Dierickx et al. [15] | 82% | 3% | 12% | 3% | 0% |
| Noraini et al. [16] | 76% | 21% | 0% | 3% | 0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballova, V.; Muoio, B.; Albano, D.; Bertagna, F.; Canziani, L.; Ghielmini, M.; Ceriani, L.; Treglia, G. Diagnostic Performance of 18F-FDG PET or PET/CT for Detection of Post-Transplant Lymphoproliferative Disorder: A Systematic Review and a Bivariate Meta-Analysis. Diagnostics 2020, 10, 101. https://doi.org/10.3390/diagnostics10020101
Ballova V, Muoio B, Albano D, Bertagna F, Canziani L, Ghielmini M, Ceriani L, Treglia G. Diagnostic Performance of 18F-FDG PET or PET/CT for Detection of Post-Transplant Lymphoproliferative Disorder: A Systematic Review and a Bivariate Meta-Analysis. Diagnostics. 2020; 10(2):101. https://doi.org/10.3390/diagnostics10020101
Chicago/Turabian StyleBallova, Veronika, Barbara Muoio, Domenico Albano, Francesco Bertagna, Luca Canziani, Michele Ghielmini, Luca Ceriani, and Giorgio Treglia. 2020. "Diagnostic Performance of 18F-FDG PET or PET/CT for Detection of Post-Transplant Lymphoproliferative Disorder: A Systematic Review and a Bivariate Meta-Analysis" Diagnostics 10, no. 2: 101. https://doi.org/10.3390/diagnostics10020101
APA StyleBallova, V., Muoio, B., Albano, D., Bertagna, F., Canziani, L., Ghielmini, M., Ceriani, L., & Treglia, G. (2020). Diagnostic Performance of 18F-FDG PET or PET/CT for Detection of Post-Transplant Lymphoproliferative Disorder: A Systematic Review and a Bivariate Meta-Analysis. Diagnostics, 10(2), 101. https://doi.org/10.3390/diagnostics10020101







