In Situ Mass Spectrometry Diagnostics of Impaired Glucose Tolerance Using Label-Free Metabolomic Signature
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Plasma Samples
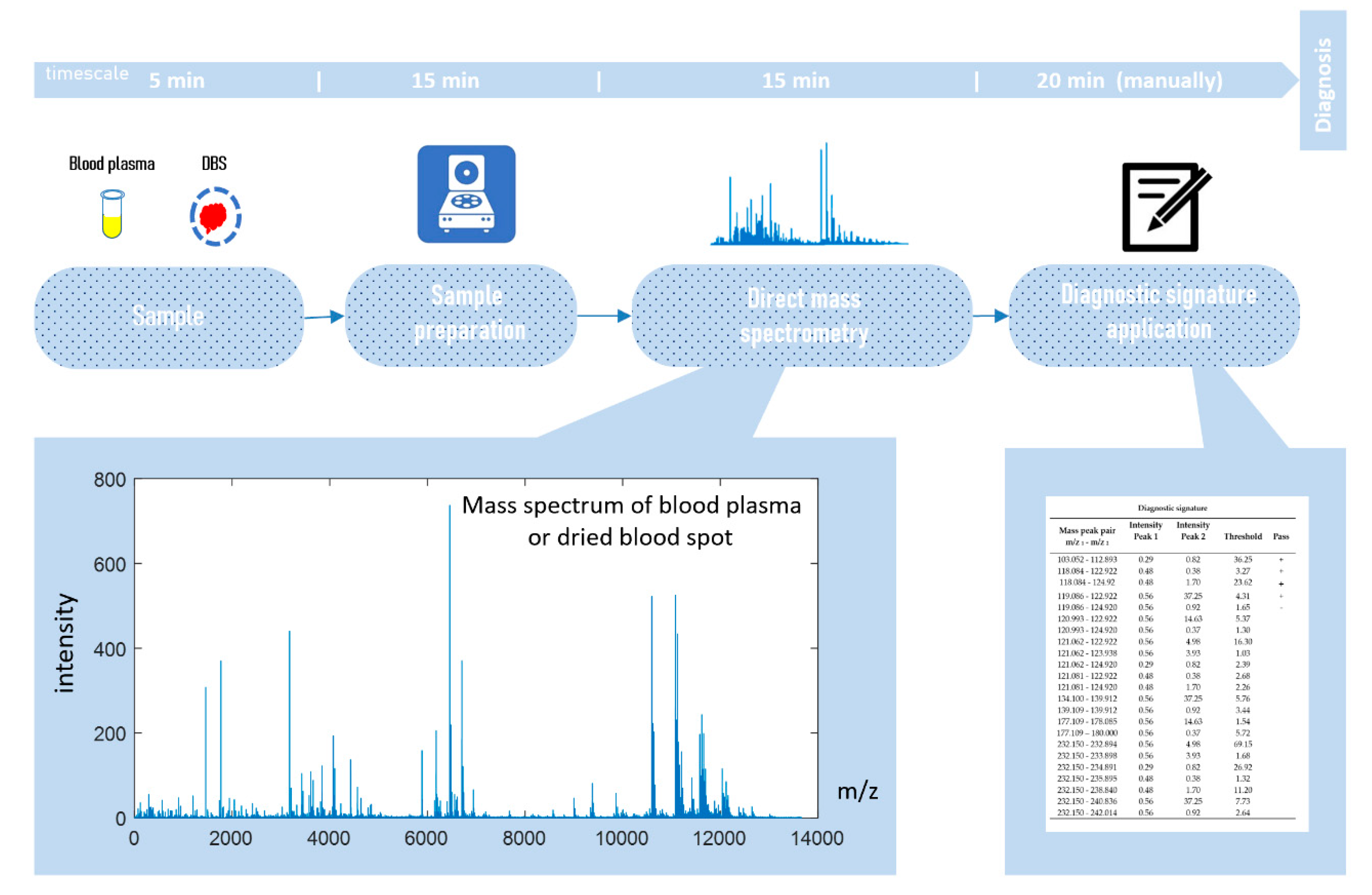
2.2. Mass Spectrometry Analysis
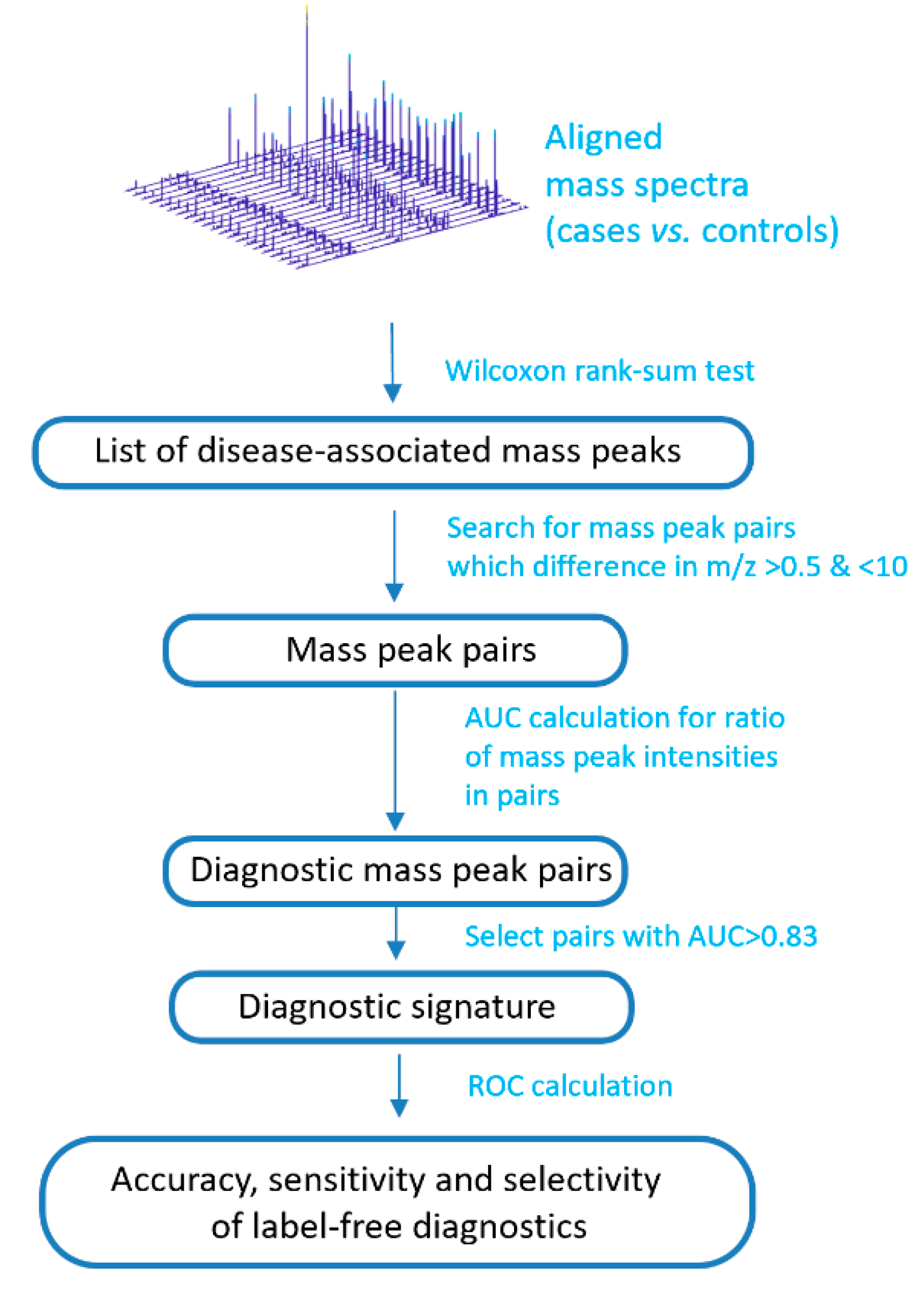
2.3. Compilation of Label-Free Diagnostic Signature
2.4. Diagnostic Score Calculation
2.5. Leave-One-Out Testing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bossuyt, P.M. Where are all the new omics-based tests? Clin. Chem. 2014, 60, 1256–1257. [Google Scholar] [CrossRef]
- McShane, L.M.; Cavenagh, M.M.; Lively, T.G.; Eberhard, D.A.; Bigbee, W.L.; Williams, P.M.; Mesirov, J.P.; Polley, M.Y.C.; Kim, K.Y.; Tricoli, J.V.; et al. Criteria for the use of omics-based predictors in clinical trials. Nature 2013, 502, 317–320. [Google Scholar] [CrossRef]
- Beger, R.D.; Dunn, W.; Schmidt, M.A.; Gross, S.S.; Kirwan, J.A.; Cascante, M.; Brennan, L.; Wishart, D.S.; Oresic, M.; Hankemeier, T.; et al. Metabolomics enables precision medicine: “A White Paper, Community Perspective”. Metabolomics 2016, 12, 149. [Google Scholar] [CrossRef]
- Keller, G.A.; Gago, M.L.F.; Diez, R.A.; Di Girolamo, G. In vivo Phenotyping Methods: Cytochrome P450 Probes with Emphasis on the Cocktail Approach. Curr. Pharm. Des. 2017, 23, 2035–2049. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Balashova, E.E.; Voskresenskaya, A.A.; Trifonova, O.P.; Maslov, D.L.; Archakov, A.I. Mass spectrometric signatures of blood plasma metabolome for disease diagnostics. Biomed. Rep. 2016, 4, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Julius, S.; Colwell, J.A. Shock During Oral Glucose Tolerance Testing. JAMA 1973, 226, 667–668. [Google Scholar]
- Lokhov, P.G.; Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Archakov, A.I.; Shestakova, E.A.; Shestakova, M.V.; Dedov, I.I. Diagnosing impaired glucose tolerance using direct infusion mass spectrometry of blood plasma. PLoS ONE 2014, 9, e105343. [Google Scholar] [CrossRef]
- Mellitus, D. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications Part 1: Diagnosis and Classification of. World Health 1999, 15, 539–553. [Google Scholar]
- Lokhov, P.G.; Kharybin, O.N.; Archakov, A.I. Diagnosis of lung cancer based on direct-infusion electrospray mass spectrometry of blood plasma metabolites. Int. J. Mass Spectrom. 2011, 309, 200–205. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Balashova, E.E.; Trifonova, O.P.; Maslov, D.L.; Ponomarenko, E.A.; Archakov, A.I. Mass spectrometry-based metabolomics analysis of obese patients’ blood plasma. Int. J. Mol. Sci. 2020, 21, 568. [Google Scholar] [CrossRef] [PubMed]
- Martens, H.A.; Dardenne, P. Validation and verification of regression in small data sets. Chemom. Intell. Lab. Syst. 1998, 44, 99–121. [Google Scholar] [CrossRef]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Committee on the Review of Omics-Based Tests for Predicting Patient Outcomes in Clinical Trials; Board on Health Care Services; Board on Health Sciences Policy; Institute of Medicine. Evolution of Translational Omics: Lessons Learned and the Path Forward; Micheel, C.M., Sharyl, N.J., Omenn, G.S., Eds.; National Academies Press (US): Washington, DC, USA, 2012; ISBN 9780309224185. [Google Scholar]
- Allegra, A.; Innao, V.; Gerace, D.; Bianco, O.; Musolino, C. The metabolomic signature of hematologic malignancies. Leuk. Res. 2016, 49, 22–35. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 93. [Google Scholar] [CrossRef]
- Troisi, J.; Sarno, L.; Landolfi, A.; Scala, G.; Martinelli, P.; Venturella, R.; Di Cello, A.; Zullo, F.; Guida, M. Metabolomic Signature of Endometrial Cancer. J. Proteome Res. 2018, 17, 804–812. [Google Scholar] [CrossRef]
- Pandey, R.; Caflisch, L.; Lodi, A.; Brenner, A.J.; Tiziani, S. Metabolomic signature of brain cancer. Mol. Carcinog. 2017, 56, 2355–2371. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Dashtiev, M.I.; Moshkovskii, S.A.; Archakov, A.I. Metabolite profiling of blood plasma of patients with prostate cancer. Metabolomics 2010, 6, 156–163. [Google Scholar] [CrossRef]
- Harshfield, E.L.; Koulman, A.; Ziemek, D.; Marney, L.; Fauman, E.B.; Paul, D.S.; Stacey, D.; Rasheed, A.; Lee, J.J.; Shah, N.; et al. An Unbiased Lipid Phenotyping Approach to Study the Genetic Determinants of Lipids and Their Association with Coronary Heart Disease Risk Factors. J. Proteome Res. 2019, 18, 2397–2410. [Google Scholar] [CrossRef]
- Lokhov, P.G.; Trifonova, O.P.; Maslov, D.L.; Lichtenberg, S.; Balashova, E.E. Diagnosis of Parkinson’s disease by a metabolomics-based laboratory-developed test (LDT). Diagnostics 2020, 10, 332. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Sayago, A.; Fernández-Recamales, Á. High-throughput direct mass spectrometry-based metabolomics to characterize metabolite fingerprints associated with Alzheimer’s disease pathogenesis. Metabolites 2018, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; González-Domínguez, Á.; Segundo, C.; Schwarz, M.; Sayago, A.; María Mateos, R.M.; Durán-Guerrero, E.; Lechuga-Sancho, A.M.; Fernández-Recamales, Á. High-Throughput Metabolomics Based on Direct Mass Spectrometry Analysis in Biomedical Research. Methods Mol. Biol. 2019, 1978, 27–38. [Google Scholar] [PubMed]
- González-Domínguez, R.; Sayago, A.; Fernández-Recamales, Á. Direct infusion mass spectrometry for metabolomic phenotyping of diseases. Bioanalysis 2017, 9, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Southam, A.D.; Weber, R.J.M.; Engel, J.; Jones, M.R.; Viant, M.R. A complete workflow for high-resolution spectral-stitching nanoelectrospray direct-infusion mass-spectrometry-based metabolomics and lipidomics. Nat. Protoc. 2017, 12, 310–328. [Google Scholar] [CrossRef]


| Characteristics | Value | t-Test (p-Value) | AUC | |
|---|---|---|---|---|
| Control Subjects | Subjects with IGT | |||
| Number | 20 | 20 | N/A | N/A |
| Sex (male/female) | 10/10 | 10/10 | 1 | 0.50 |
| Age (years) | 56.1 ± 13.9 | 61.1 ± 10.2 | 0.21 | 0.59 |
| BMI (kg/m2) | 36.1 ± 9.1 | 33.7 ± 7.6 | 0.38 | 0.47 |
| Glucose in OGTT (mmol/L) | 6.4 ± 1.0 | 10.6 ± 1.7 | 0 | 1.00 1 |
| Fasting glucose (mmol/L) | 5.3 ± 0.3 | 5.5 ± 0.3 | 0.04 | 0.66 |
| HbA1c (%) | 5.7 ± 0.4 | 6.1 ± 0.4 | 0.0032 | 0.74 |
| Insulin (µU/mL) | 11.3 ± 7.9 | 12.8 ± 6.3 | 0.52 | 0.62 |
| Cholesterol (mmol/L) | 5.2 ± 0.8 | 4.9 ± 1.2 | 0.39 | 0.43 |
| Uric acid (µmol/L) | 389 ± 86 | 382 ± 84 | 0.81 | 0.48 |
| HDL (mmol/L) | 1.2 ± 0.4 | 1.1 ± 0.4 | 0.38 | 0.42 |
| LDL (mmol/L) | 3.5 ± 0.8 | 3.0 ± 0.9 | 0.1 | 0.35 |
| Triglycerides (mmol/L) | 1.3 ± 0.5 | 2.2 ± 2.7 | 0.15 | 0.66 |
| HOMA-β | 126 ± 87 | 129 ± 73 | 0.8 | 0.58 |
| HOMA-IR | 2.7 ± 1.9 | 3.1 ± 1.7 | 0.43 | 0.61 |
| Mass Peak Pair m/z 1–m/z 2 | Mean | Wilcoxon Rank-Sum Test (p-Value) | Threshold | AUC | Specificity | Sensitivity | |
|---|---|---|---|---|---|---|---|
| For m/z 1 | For m/z 2 | ||||||
| 103.052–112.893 | 1.75 | 59.39 | 0.0002 | 36.25 | 0.84 | 0.90 | 0.75 |
| 118.084–122.922 | 20.40 | 7.53 | 0.0002 | 3.27 | 0.85 | 0.85 | 0.75 |
| 118.084–124.92 | 20.40 | 1.13 | 0.0001 | 23.62 | 0.86 | 0.95 | 0.65 |
| 119.086–122.922 | 1.37 | 7.53 | 0.0001 | 4.31 | 0.86 | 0.70 | 0.90 |
| 119.086–124.920 | 1.37 | 1.13 | 0.0002 | 1.65 | 0.85 | 1.00 | 0.60 |
| 120.993–122.922 | 1.60 | 7.53 | 0.0003 | 5.37 | 0.83 | 0.80 | 0.80 |
| 120.993–124.920 | 1.60 | 1.13 | 0.0002 | 1.30 | 0.84 | 0.80 | 0.80 |
| 121.062–122.922 | 0.44 | 7.53 | 0.0004 | 16.30 | 0.83 | 0.75 | 0.90 |
| 121.062–123.938 | 0.44 | 0.47 | 0.0002 | 1.03 | 0.85 | 0.80 | 0.90 |
| 121.062–124.920 | 0.44 | 1.13 | 0.0004 | 2.39 | 0.83 | 0.75 | 0.90 |
| 121.081–122.922 | 2.15 | 7.53 | 0.0002 | 2.68 | 0.85 | 0.70 | 0.95 |
| 121.081–124.920 | 2.15 | 1.13 | 0.0002 | 2.26 | 0.85 | 0.80 | 0.80 |
| 134.100–139.912 | 0.14 | 0.82 | 0.0002 | 5.76 | 0.84 | 0.70 | 0.90 |
| 139.109–139.912 | 0.29 | 0.82 | 0.0002 | 3.44 | 0.85 | 0.90 | 0.75 |
| 177.109–178.085 | 0.48 | 0.38 | 0.0002 | 1.54 | 0.85 | 0.85 | 0.90 |
| 177.109–180.000 | 0.48 | 1.70 | 0.0001 | 5.72 | 0.86 | 0.90 | 0.80 |
| 232.150–232.894 | 0.56 | 37.25 | 0.0002 | 69.15 | 0.85 | 0.80 | 0.75 |
| 232.150–233.898 | 0.56 | 0.92 | 0.0001 | 1.68 | 0.86 | 0.80 | 0.75 |
| 232.150–234.891 | 0.56 | 14.63 | 0.0002 | 26.92 | 0.85 | 0.80 | 0.80 |
| 232.150–235.895 | 0.56 | 0.37 | 0.0002 | 1.32 | 0.84 | 0.65 | 0.90 |
| 232.150–238.840 | 0.56 | 4.98 | 0.0001 | 11.20 | 0.86 | 0.90 | 0.65 |
| 232.150–240.836 | 0.56 | 3.93 | 0.0001 | 7.73 | 0.86 | 0.80 | 0.75 |
| 232.150–242.014 | 0.56 | 1.07 | 0.00004 | 2.64 | 0.88 | 1.00 | 0.60 |
| 233.148–242.923 | 1.67 | 22.86 | 0.0002 | 14.18 | 0.84 | 0.80 | 0.80 |
| 234.079–242.923 | 0.21 | 22.86 | 0.0003 | 135.09 | 0.83 | 1.00 | 0.55 |
| 234.079–243.927 | 0.21 | 0.87 | 0.0003 | 4.04 | 0.83 | 0.70 | 0.85 |
| 234.151–242.923 | 0.27 | 22.86 | 0.0002 | 95.66 | 0.85 | 0.90 | 0.700 |
| 234.151–243.927 | 0.27 | 0.87 | 0.0001 | 3.13 | 0.86 | 0.70 | 0.90 |
| 239.013–240.836 | 0.28 | 3.93 | 0.0003 | 12.74 | 0.84 | 0.75 | 0.85 |
| 241.965–244.921 | 0.22 | 1.84 | 0.0002 | 10.37 | 0.85 | 1.00 | 0.50 |
| 246.047–248.868 | 0.22 | 16.81 | 0.0003 | 78.80 | 0.83 | 0.85 | 0.80 |
| 247.165–248.868 | 0.50 | 16.81 | 0.0003 | 30.40 | 0.84 | 0.75 | 0.90 |
| 247.165–249.872 | 0.50 | 0.42 | 0.0003 | 1.31 | 0.83 | 0.90 | 0.80 |
| 255.116–258.896 | 1.37 | 6.26 | 0.0003 | 5.41 | 0.84 | 0.90 | 0.75 |
| 256.153–261.920 | 1.70 | 0.37 | 0.0003 | 4.73 | 0.83 | 0.80 | 0.75 |
| 262.012–264.844 | 0.31 | 7.14 | 0.0002 | 20.91 | 0.84 | 0.75 | 0.90 |
| 262.012–266.968 | 0.31 | 0.32 | 0.0001 | 1.08 | 0.88 | 0.85 | 0.80 |
| 295.935–301.958 | 0.50 | 0.59 | 0.0003 | 1.11 | 0.84 | 0.65 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lokhov, P.G.; Trifonova, O.P.; Maslov, D.L.; Balashova, E.E. In Situ Mass Spectrometry Diagnostics of Impaired Glucose Tolerance Using Label-Free Metabolomic Signature. Diagnostics 2020, 10, 1052. https://doi.org/10.3390/diagnostics10121052
Lokhov PG, Trifonova OP, Maslov DL, Balashova EE. In Situ Mass Spectrometry Diagnostics of Impaired Glucose Tolerance Using Label-Free Metabolomic Signature. Diagnostics. 2020; 10(12):1052. https://doi.org/10.3390/diagnostics10121052
Chicago/Turabian StyleLokhov, Petr G., Oxana P. Trifonova, Dmitry L. Maslov, and Elena E. Balashova. 2020. "In Situ Mass Spectrometry Diagnostics of Impaired Glucose Tolerance Using Label-Free Metabolomic Signature" Diagnostics 10, no. 12: 1052. https://doi.org/10.3390/diagnostics10121052
APA StyleLokhov, P. G., Trifonova, O. P., Maslov, D. L., & Balashova, E. E. (2020). In Situ Mass Spectrometry Diagnostics of Impaired Glucose Tolerance Using Label-Free Metabolomic Signature. Diagnostics, 10(12), 1052. https://doi.org/10.3390/diagnostics10121052







