Positive Association of Serum Alkaline Phosphatase Level with Severe Knee Osteoarthritis: A Nationwide Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
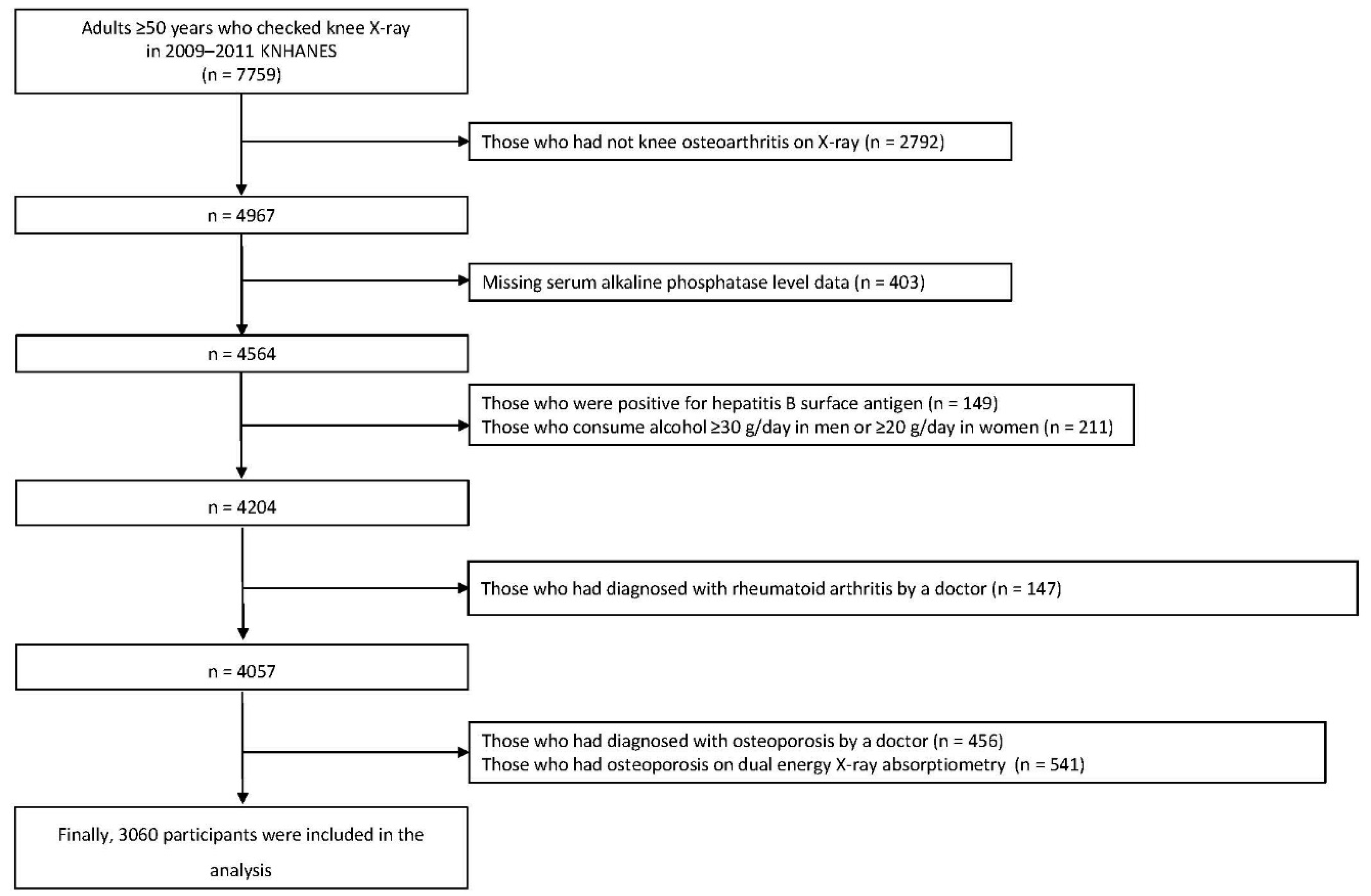
2.1. Study Population
2.2. Biochemical Measurements
2.3. Assessment of Knee Osteoarthritis
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Clinical Characteristics of the Study Population
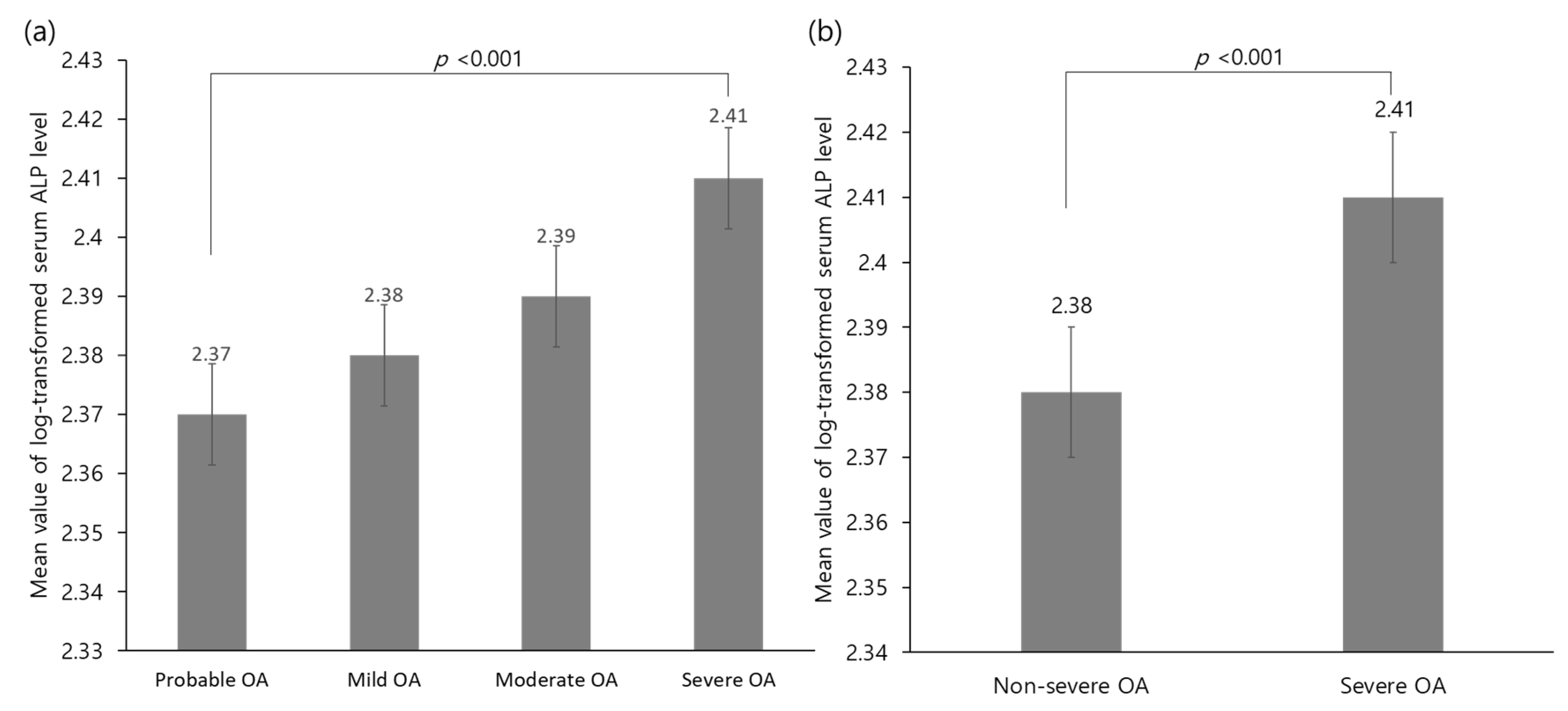
3.2. Relationship between Serum ALP Level and Severe Osteoarthritis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, L.; Kapoor, D.; Issa, S. Epidemiology of osteoarthritis: An update. Curr. Opin. Rheumatol. 2006, 18, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Garstang, S.V.; Stitik, T.P. Osteoarthritis: Epidemiology, risk factors, and pathophysiology. Am. J. Phys. Med. Rehabil. 2006, 85, S2–S11. [Google Scholar] [CrossRef]
- Nelson, A.E. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Chronic Conditions (UK); National Institute for Clinical Excellence. Osteoarthritis: National Clinical Guidelines for Care and Management in Adults; Royal College of Physicians: London, UK, 2008. [Google Scholar]
- Park, H.M.; Kwon, Y.J.; Kim, H.S.; Lee, Y.J. Relationship between sleep duration and osteoarthritis in middle-aged and older women: A nationwide population-based study. J. Clin. Med. 2019, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Kubo, M.; Yonemoto, K.; Ninomiya, T.; Iwase, M.; Tanizaki, Y.; Shikata, K.; Iida, M.; Kiyohara, Y. Liver enzymes as a predictor for incident diabetes in a japanese population: The hisayama study. Obesity (Silver Spring) 2007, 15, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, H.S.; Park, H.M.; Lee, Y.J. Serum alkaline phosphatase level is positively associated with metabolic syndrome: A nationwide population-based study. Clin. Chim. Acta 2020, 500, 189–194. [Google Scholar] [CrossRef]
- Seo, M.S.; Shim, J.Y.; Lee, Y.J. Relationship between serum alkaline phosphatase level, c-reactive protein and leukocyte counts in adults aged 60 years or older. Scand. J. Clin. Lab. Investig. 2019, 79, 233–237. [Google Scholar] [CrossRef]
- Spector, T.D.; Hart, D.J.; Nandra, D.; Doyle, D.V.; Mackillop, N.; Gallimore, J.R.; Pepys, M.B. Low-level increases in serum c-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheumatol. 1997, 40, 723–727. [Google Scholar] [CrossRef]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Loeser, R.F. Inflammatory activity in symptomatic knee osteoarthritis: Not all inflammation is local. Arthritis Rheumatol. (Hoboken, NJ) 2015, 67, 2797. [Google Scholar] [CrossRef]
- Wang, X.; Hunter, D.; Xu, J.; Ding, C. Metabolic triggered inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Katz, J.D. Osteoarthritis: Another component of metabolic syndrome? Metab. Syndr. Relat. Disord. 2010, 8, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The korea national health and nutrition examination survey (knhanes). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Creamer, M.R.; Wang, T.W.; Babb, S.; Cullen, K.A.; Day, H.; Willis, G.; Jamal, A.; Neff, L. Tobacco product use and cessation indicators among adults—United states, 2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Chun, M.Y. Validity and reliability of korean version of international physical activity questionnaire short form in the elderly. Korean J. Fam. Med 2012, 33, 144–151. [Google Scholar] [CrossRef]
- Hwang, J.; Shon, C. Relationship between socioeconomic status and type 2 diabetes: Results from korea national health and nutrition examination survey (knhanes) 2010–2012. BMJ Open 2014, 4, e005710. [Google Scholar] [CrossRef]
- The Korean National Health and Nutrition Examination Survey. Available online: https://knhanes.cdc.go.kr/knhanes (accessed on 15 October 2020).
- Fuller, C.J.; Barr, A.R.; Sharif, M.; Dieppe, P.A. Cross-sectional comparison of synovial fluid biochemical markers in equine osteoarthritis and the correlation of these markers with articular cartilage damage. Osteoarthr. Cartil. 2001, 9, 49–55. [Google Scholar] [CrossRef]
- Siede, W.H.; Seiffert, U.B.; Merle, S.; Goll, H.G.; Oremek, G. Alkaline phosphatase isoenzymes in rheumatic diseases. Clin. Biochem. 1989, 22, 121–124. [Google Scholar] [CrossRef]
- Niino-Nanke, Y.; Akama, H.; Hara, M.; Kashiwazaki, S. Alkaline phosphatase (alp) activity in rheumatoid arthritis (ra): Its clinical significance and synthesis of alp in ra synovium. Ryumachi 1998, 38, 581–588. [Google Scholar] [PubMed]
- Webber, M.; Krishnan, A.; Thomas, N.G.; Cheung, B.M. Association between serum alkaline phosphatase and c-reactive protein in the united states national health and nutrition examination survey 2005–2006. Clin. Chem. Lab. Med. 2010, 48, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Haarhaus, M.; Brandenburg, V.; Kalantar-Zadeh, K.; Stenvinkel, P.; Magnusson, P. Alkaline phosphatase: A novel treatment target for cardiovascular disease in ckd. Nat. Rev. Nephrol. 2017, 13, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.L.; Hunter, D.J. The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 5–15. [Google Scholar] [CrossRef]
- Rojas-Rodríguez, J.; Escobar-Linares, L.E.; Garcia-Carrasco, M.; Escárcega, R.O.; Fuentes-Alexandro, S.; Zamora-Ustaran, A. The relationship between the metabolic syndrome and energy-utilization deficit in the pathogenesis of obesity-induced osteoarthritis. Med. Hypotheses 2007, 69, 860–868. [Google Scholar] [CrossRef]
- Berenbaum, F. Diabetes-induced osteoarthritis: From a new paradigm to a new phenotype. Ann. Rheum. Dis. 2011, 70, 1354–1356. [Google Scholar] [CrossRef]
- Hiraiwa, H.; Sakai, T.; Mitsuyama, H.; Hamada, T.; Yamamoto, R.; Omachi, T.; Ohno, Y.; Nakashima, M.; Ishiguro, N. Inflammatory effect of advanced glycation end products on human meniscal cells from osteoarthritic knees. Inflamm. Res. 2011, 60, 1039–1048. [Google Scholar] [CrossRef]
- Kamimura, M.; Uchiyama, S.; Takahara, K.; Hashidate, H.; Kawaguchi, A.; Nakagawa, H. Urinary excretion of type i collagen cross-linked n-telopeptide and serum bone-specific alkaline phosphatase analysis to determine the correlation of age and back-pain related changes in elderly women. J. Bone Miner. Metab. 2005, 23, 495–500. [Google Scholar] [CrossRef]
- Frohnhofen, H. Pain and sleep: A bidirectional relationship. Z. Gerontol. Geriatr. 2018, 51, 871–874. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Depner, C.M.; Stothard, E.R.; Wright, K.P., Jr. Metabolic consequences of sleep and circadian disorders. Curr. Diabetes Rep. 2014, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.P., Jr.; Drake, A.L.; Frey, D.J.; Fleshner, M.; Desouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Meng, Q.J. The brain-joint axis in osteoarthritis: Nerves, circadian clocks and beyond. Nat. Rev. Rheumatol. 2016, 12, 508–516. [Google Scholar] [CrossRef]
| Log-Transformed Serum ALP Level | 2009–2011 KNHANES | ||||
|---|---|---|---|---|---|
| T1 (1.74–2.32) | T2 (2.33–2.43) | T3 (2.44–3.01) | Total | p | |
| Unweighted n | 1017 | 1001 | 1042 | 3060 | |
| Male sex, % (SE) | 52.8 (1.8) | 50.9 (1.8) | 44.0(1.9) | 49.2(1.1) | 0.003 |
| Age, years | 62.9 ± 0.4 | 63.2 ± 0.3 | 64.1± 0.4 | 63.4 ± 0.2 | 0.082 |
| Body mass index, kg/m2 | 24.6 ± 0.1 | 24.6 ± 0.1 | 24.5± 0.1 | 24.6 ± 0.1 | 0.911 |
| Dietary Ca intake, mg/day | 504.4 ± 12.6 | 479.3 ± 12.9 | 485.8 ± 17.0 | 489.8 ± 9.2 | 0.289 |
| Dietary P intake, mg/day | 1131.0 ± 19.3 | 1122.8 ± 20.6 | 1076.7 ± 21.5 | 1110.2 ± 13.3 | 0.124 |
| Dietary Ca/P ratio | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.375 |
| Mean blood pressure, mmHg | 92.8 ± 0.6 | 93.0 ± 0.5 | 94.2 ± 0.7 | 93.4 ± 0.3 | 0.218 |
| Fasting plasma glucose, mg/dL | 101.9 ± 0.7 | 102.6 ± 0.9 | 107.9 ± 1.2 | 104.1 ± 0.6 | <0.001 |
| Total cholesterol, mg/dL | 191.7 ± 1.3 | 192.7 ± 1.4 | 194.7 ± 1.5 | 193.1 ± 0.8 | 0.293 |
| Serum vitamin D, ng/mL | 20.3 ± 0.3 | 19.6 ± 0.3 | 19.1 ± 0.3 | 19.7 ± 0.2 | 0.007 |
| Serum AST, U/L | 23.1 ± 0.3 | 23.4 ± 0.3 | 25.4 ± 0.5 | 24.0 ± 0.2 | <0.001 |
| Serum ALT, U/L | 21.1 ± 0.5 | 21.7 ± 0.5 | 23.5 ± 0.6 | 22.1 ± 0.3 | 0.014 |
| Current smoker, % (SE) | 31.1 (2.4) | 28.2 (2.0) | 30.7 (2.1) | 30.1 (1.2) | 0.603 |
| Current drinker, % (SE) | 52.3 (2.0) | 43.3 (1.9) | 40.8 (1.7) | 45.6 (1.1) | <0.001 |
| Regular exercise, % (SE) | 22.9 (1.8) | 19.5 (1.7) | 19.9 (1.7) | 20.8 (1.1) | 0.256 |
| Employed status, % (SE) | 59.1 (2.0) | 57.2 (1.9) | 54.8 (2.0) | 57.1 (1.3) | 0.208 |
| Monthly household income, % (SE) | 0.001 | ||||
| Lowest | 29.5 (1.9) | 30.9 (1.8) | 33.9 (1.8) | 31.5 (1.2) | |
| Medium-lowest | 22.5 (1.8) | 28.5 (1.6) | 28.7 (1.9) | 26.5 (1.1) | |
| Medium-highest | 22.7 (1.7) | 21.8 (1.7) | 21.8 (1.9) | 22.1 (1.2) | |
| Highest | 25.2 (2.2) | 18.8 (1.7) | 15.6 (1.5) | 19.9 (1.1) | |
| Severity of OA, % (SE) | 0.002 | ||||
| Probable OA | 46.4 (1.9) | 47.8 (2.0) | 39.4 (2.1) | 44.5 (1.2) | |
| Mild OA | 26.9 (1.8) | 23.8 (1.7) | 25.5 (1.7) | 25.5 (1.1) | |
| Moderate OA | 19.2 (1.3) | 20.8 (1.4) | 23.0 (1.6) | 21.0 (0.9) | |
| Severe OA | 7.5 (1.0) | 7.6 (0.9) | 12.0 (1.3) | 9.1 (0.7) | |
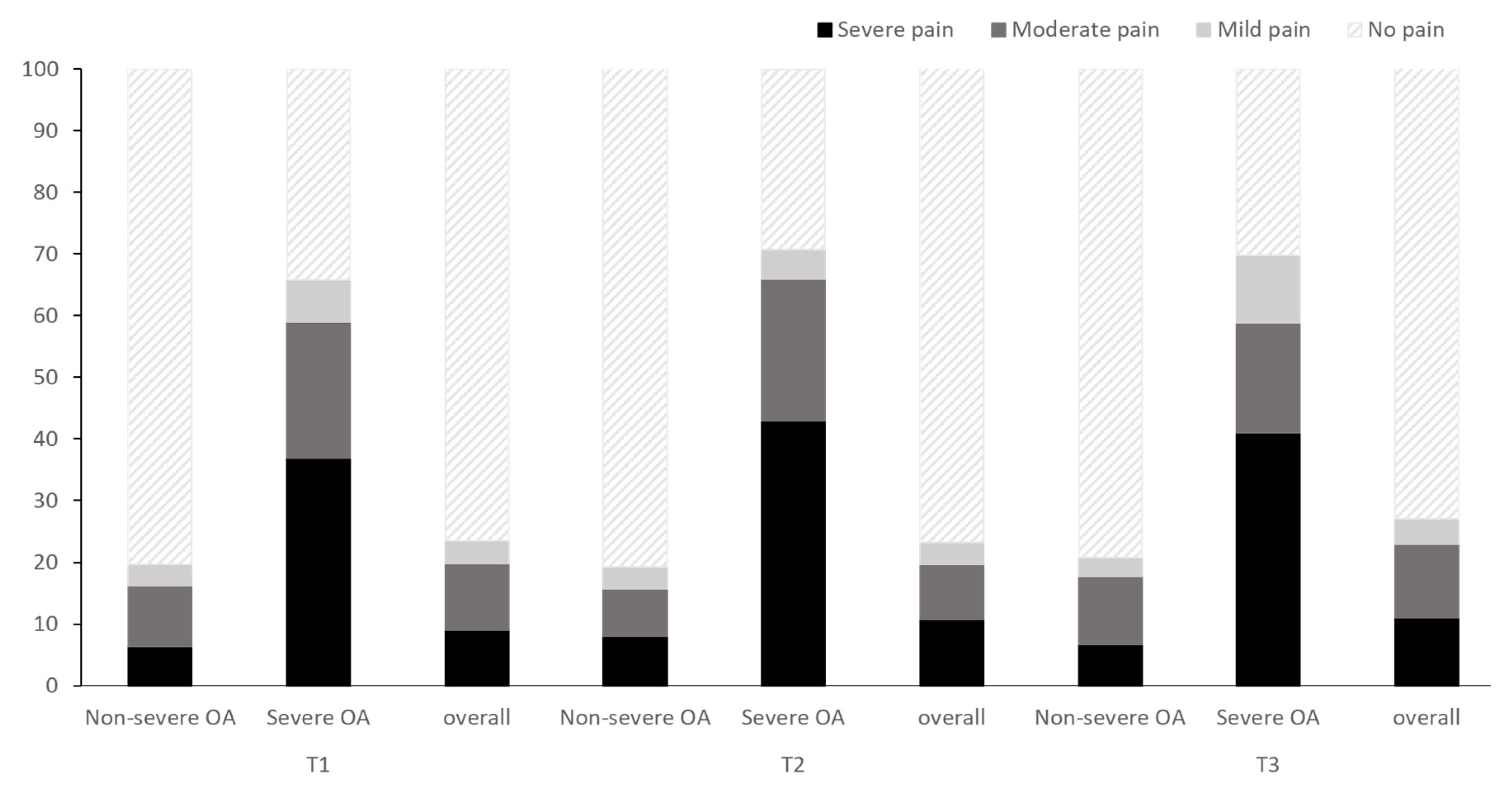
| Knee joint pain, % (SE) | 0.544 | ||||
| No pain | 76.5 (1.9) | 76.9 (1.9) | 73.1 (2.0) | 75.4 (1.2) | |
| Mild | 3.7 (0.7) | 3.6 (0.7) | 4.0 (0.8) | 3.8 (0.4) | |
| Moderate | 10.8 (1.4) | 8.9 (1.3) | 12.0 (1.4) | 10.6 (0.7) | |
| Severe | 9.0 (1.1) | 10.7 (1.4) | 11.0 (1.3) | 10.2 (0.8) | |
| T1 | T2 | T3 | |||||
|---|---|---|---|---|---|---|---|
| ORs (95% CIs) | p | ORs (95% CIs) | p | Overall p | p for Trend | ||
| Unadjusted | 1 (ref) | 1.025 (0.723–1.413) | 0.889 | 1.696 (1.182–2.433) | 0.004 | 0.004 | 0.004 |
| Model 1 | 1 (ref) | 1.036 (0.719–1.493) | 0.848 | 1.528 (1.050–2.223) | 0.027 | 0.045 | 0.025 |
| Model 2 | 1 (ref) | 1.077 (0.704–1.650) | 0.731 | 1.620 (1.097–2.393) | 0.016 | 0.035 | 0.015 |
| Model 3 | 1 (ref) | 1.079 (0.706–1.648) | 0.726 | 1.613 (1.087–2.394) | 0.018 | 0.039 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-M.; Lee, J.-H.; Lee, Y.-J. Positive Association of Serum Alkaline Phosphatase Level with Severe Knee Osteoarthritis: A Nationwide Population-Based Study. Diagnostics 2020, 10, 1016. https://doi.org/10.3390/diagnostics10121016
Park H-M, Lee J-H, Lee Y-J. Positive Association of Serum Alkaline Phosphatase Level with Severe Knee Osteoarthritis: A Nationwide Population-Based Study. Diagnostics. 2020; 10(12):1016. https://doi.org/10.3390/diagnostics10121016
Chicago/Turabian StylePark, Hye-Min, Jun-Hyuk Lee, and Yong-Jae Lee. 2020. "Positive Association of Serum Alkaline Phosphatase Level with Severe Knee Osteoarthritis: A Nationwide Population-Based Study" Diagnostics 10, no. 12: 1016. https://doi.org/10.3390/diagnostics10121016
APA StylePark, H.-M., Lee, J.-H., & Lee, Y.-J. (2020). Positive Association of Serum Alkaline Phosphatase Level with Severe Knee Osteoarthritis: A Nationwide Population-Based Study. Diagnostics, 10(12), 1016. https://doi.org/10.3390/diagnostics10121016





