Periostin Circulating Levels and Genetic Variants in Patients with Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.1.1. NAFLD Patients
2.1.2. HCV Control Patients
2.1.3. Healthy Controls
2.2. POSTN Genetic Studies
2.3. Plasma Periostin Concentration Dosage
2.4. Statistical Analysis
3. Results
3.1. Patients and Healthy Controls
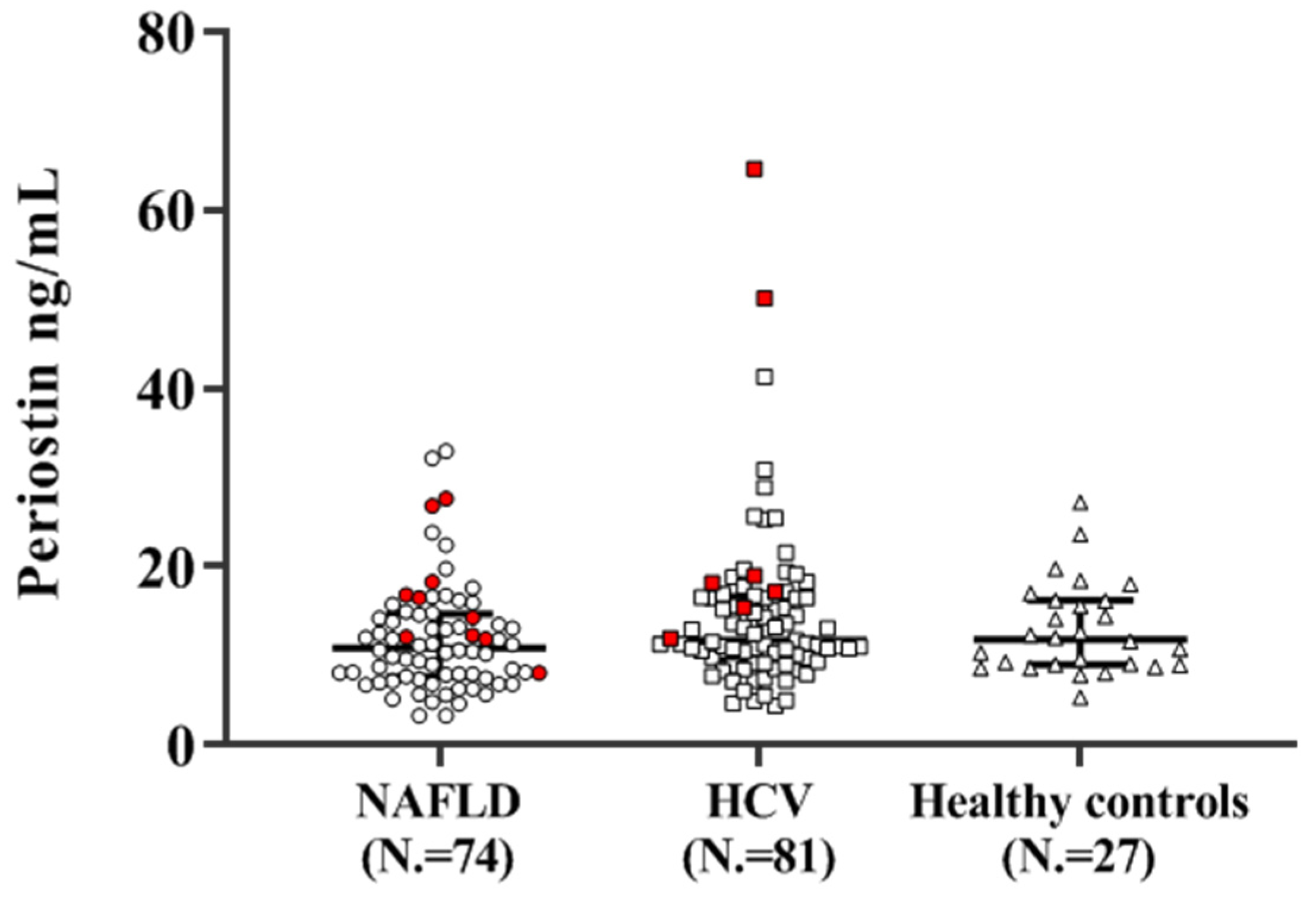
3.2. Plasma Periostin Concentrations
3.2.1. Periostin Concentrations and Disease Etiology
3.2.2. Periostin Concentrations and Hepatic Steatosis Degree
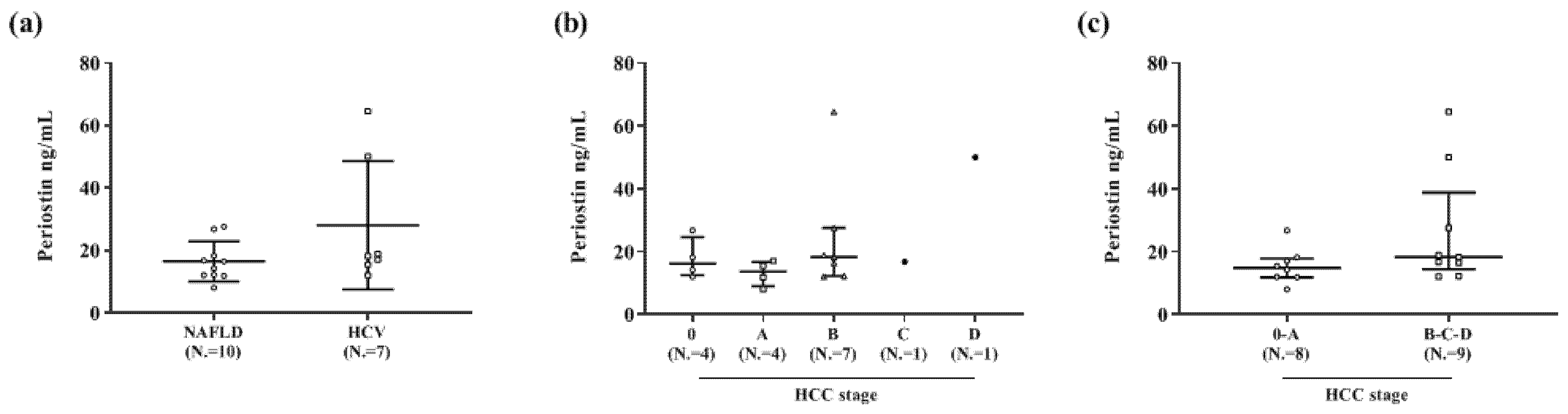
3.2.3. Periostin Concentrations and Hepatic Fibrosis
3.2.4. Periostin Concentrations and Main Demographic and Clinical Variables
3.3. POSTN Genotyping
3.3.1. Genotypic Frequencies
3.3.2. Periostin and POSTN Gene Polymorphisms
3.3.3. Periostin and POSTN Gene Diplotypes
3.4. Multivariate Analysis of Factors Associated with High Periostin
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NAFLD | Non-alcoholic fatty liver disease |
| SS | Simple steatosis |
| NASH | Non-alcoholic steatohepatitis |
| HCC | Hepatocellular carcinoma |
| TG | Triglyceride |
| PN | Periostin |
| OSF-2 | Osteoblast-specific factor 2 |
| ECM | Extracellular matrix |
| POSTN | Periostin |
| PPAR | Peroxisome proliferator-activated receptor |
| Rac | Ras-related C3 botulinum toxin substrate |
| ROR | RAR-related orphan receptor |
| ELISA | Enzyme Linked Immunosorbent Assay |
| VEGF | Vascular Endothelial Growth Factor |
| SNP | Single nucleotide polymorphism |
| HCV | Hepatitis C virus |
| EASL-EORTC | European Association For The Study Of The Liver and the European Organization for Research and Treatment of Cancer |
| CAP | Controlled attenuation parameter |
| NFS | NAFLD fibrosis score |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| N.A. | Not applicable |
| DAA | Direct antiviral agents |
| SD | Standard deviation |
| IQR | Interquartile range |
| MELD | Model for End-Stage Liver Disease |
| CHC | Chronic hepatitis C |
| BCLC | Barcelona Clinic Liver Cancer |
| BMI | Body mass index |
| M | Major allele |
| m | Minor allele |
| OR | Odds ratio |
| CI | Confidence interval |
| TGF | Transforming growth factor |
| LOX | Lysyl oxidases |
| LOXL | LOX like enzymes |
| PI3K | Phosphoinositide 3-kinase |
| SMAD | Small mother against decapentaplegic |
| WC | Waist circumference |
| HOMA-IR | Homeostasis model assessment-insulin resistance |
| GGT | γ-glutamyltranspeptidase |
| Stat | Signal transducer and activator of transcription |
| Akt | Ak strain transforming |
| HIF | Hypoxia-inducible factor |
| HBsAg | Australia antigen |
| TE | Transient elastography |
| NFS | NAFLD fibrosis score |
| PCR | Polymerase chain reaction |
Appendix A
| rs9603226 | rs3829365 | rs1028728 | ||
|---|---|---|---|---|
| Diplotype 2/2 | Haplotype 2 | G | C | A |
| Haplotype 2 | G | C | A | |
| Diplotype 2/4 | Haplotype 2 | G | C | A |
| Haplotype 4 | G | G | A | |
| Diplotype 2/5 | Haplotype 2 | G | C | A |
| Haplotype 5 | A | C | A | |
| Diplotype 2/6 | Haplotype 2 | G | C | A |
| Haplotype 6 | G | C | T | |
| Diplotype 4/2 | Haplotype 4 | G | G | A |
| Haplotype 2 | G | C | A | |
| Diplotype 4/4 | Haplotype 4 | G | G | A |
| Haplotype 4 | G | G | A | |
| Diplotype 4/5 | Haplotype 4 | G | G | A |
| Haplotype 5 | A | C | A | |
| Diplotype 4/6 | Haplotype 4 | G | G | A |
| Haplotype 6 | G | C | T | |
| Diplotype 5/2 | Haplotype 5 | A | C | A |
| Haplotype 2 | G | C | A | |
| Diplotype 5/4 | Haplotype 5 | A | C | A |
| Haplotype 4 | G | G | A | |
| Diplotype 5/5 | Haplotype 5 | A | C | A |
| Haplotype 5 | A | C | A | |
| Diplotype 5/6 | Haplotype 5 | A | C | A |
| Haplotype 6 | G | C | T | |
| Diplotype 6/2 | Haplotype 6 | G | C | T |
| Haplotype 2 | G | C | A | |
| Diplotype 6/3 | Haplotype 6 | G | C | T |
| Haplotype 3 | G | G | T | |
| Diplotype 6/5 | Haplotype 6 | G | C | T |
| Haplotype 5 | A | C | A | |
| Diplotype 6/6 | Haplotype 6 | G | C | T |
| Haplotype 6 | G | C | T |
| N. | % | Cumulative Frequency | |
|---|---|---|---|
| NAFLD Activity Score (NAS) | |||
| 0 | 1 | 1.56 | 1.56 |
| 1 | 8 | 12.50 | 14.06 |
| 2 | 8 | 12.50 | 26.56 |
| 3 | 11 | 17.19 | 43.75 |
| 4 | 16 | 25.00 | 68.75 |
| 5 | 16 | 25.00 | 93.75 |
| 6 | 1 | 1.56 | 95.31 |
| 7 | 3 | 4.69 | 100.00 |
| Fibrosis stage | |||
| 0 | 18 | 28.13 | 28.13 |
| 1 | 15 | 23.44 | 51.56 |
| 2 | 6 | 9.38 | 60.94 |
| 3 | 13 | 20.31 | 81.25 |
| 4 | 12 | 18.75 | 100.00 |
References
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Brunt, E.M.; Wong, V.W.S.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Prim. 2015, 1, 15080. [Google Scholar] [CrossRef]
- Arab, J.P.; Arrese, M.; Trauner, M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 321–350. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog. Lipid Res. 2009, 48, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Dowman, J.K.; Tomlinson, J.W.; Newsome, P.N. Pathogenesis of non-alcoholic fatty liver disease. QJM 2009, 103, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Kikuno, R.; Tezuka, K.; Amann, E. Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 1993, 294, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.J.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef]
- Jia, Y.; Zhong, F.; Jiang, S.; Guo, Q.; Jin, H.; Wang, F.; Li, M.; Wang, L.; Chen, A.; Zhang, F.; et al. Periostin in chronic liver diseases: Current research and future perspectives. Life Sci. 2019, 226, 91–97. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W.; Xiao, H.; Maitikabili, A.; Lin, Q.; Wu, T.; Huang, Z.; Liu, F.; Luo, Q.; Ouyang, G. Matricellular protein periostin contributes to hepatic inflammation and fibrosis. Am. J. Pathol. 2015, 185, 786–797. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, W.; Jia, W.D.; Sun, Q.K.; Li, J.S.; Ma, J.L.; Liu, W. Bin; Zhou, H.C.; Ge, Y.S.; Yu, J.H.; et al. High-level expression of periostin is closely related to metastatic potential and poor prognosis of hepatocellular carcinoma. Med. Oncol. 2013, 30, 385. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Wu, S.; Huang, Z.; Chen, X.; Liu, Y.; Cui, D.; Song, G.; Luo, Q.; Liu, F.; et al. Deficiency of periostin impairs liver regeneration in mice after partial hepatectomy. Matrix Biol. 2018, 66, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Graja, A.; Garcia-Carrizo, F.; Jank, A.M.; Gohlke, S.; Ambrosi, T.H.; Jonas, W.; Ussar, S.; Kern, M.; Schürmann, A.; Aleksandrova, K.; et al. Loss of periostin occurs in aging adipose tissue of mice and its genetic ablation impairs adipose tissue lipid metabolism. Aging Cell 2018, 17, e12810. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, X.; Jiao, Y.; Xiong, X.; Wang, E.; Wang, X.; Zhang, Z.; Zhang, H.; Pan, L.; Guan, Y.; et al. Periostin promotes liver steatosis and hypertriglyceridemia through downregulation of PPARα. J. Clin. Investig. 2014, 124, 3501–3513. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K.; Rao, M.S. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G852–G858. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S.; Xiong, S.; Ouyang, G. Deficiency of periostin protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis. J. Hepatol. 2015, 62, 495–497. [Google Scholar] [CrossRef][Green Version]
- Zhu, J.Z.; Zhu, H.T.; Dai, Y.N.; Li, C.X.; Fang, Z.Y.; Zhao, D.J.; Wan, X.Y.; Wang, Y.M.; Wang, F.; Yu, C.H.; et al. Serum periostin is a potential biomarker for non-alcoholic fatty liver disease: A case–control study. Endocrine 2016, 51, 91–100. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, H.; Niu, Y.; Zhang, W.; Zhu, L.; Li, X.; Lu, S.; Fan, J.; Li, X.; Ning, G.; et al. Circulating periostin in relation to insulin resistance and nonalcoholic fatty liver disease among overweight and obese subjects. Sci. Rep. 2016, 6, 37886. [Google Scholar] [CrossRef]
- Luo, Y.; Qu, H.; Wang, H.; Wei, H.; Wu, J.; Duan, Y.; Liu, D.; Deng, H. Plasma Periostin Levels Are Increased in Chinese Subjects with Obesity and Type 2 Diabetes and Are Positively Correlated with Glucose and Lipid Parameters. Mediat. Inflamm. 2016, 16, 6423637. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Anastasilakis, A.D.; Papatheodorou, A.; Kokkoris, P.; Terpos, E. Circulating periostin in patients with nonalcoholic fatty liver disease. Endocrine 2017, 56, 438–441. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Anastasilakis, A.D. Periostin on the road to nonalcoholic fatty liver disease. Endocrine 2016, 51, 4–6. [Google Scholar] [CrossRef]
- Lee, J. Il Role of periostin in hepatocellular carcinoma: The importance of tumor microenvironment. Gut Liver 2016, 10, 871–872. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lv, Y.; Wang, W.; Jia, W.D.; Sun, Q.K.; Huang, M.; Zhou, H.C.; Xia, H.H.; Liu, W.B.; Chen, H.; Sun, S.N.; et al. High preoparative levels of serum periostin are associated with poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Eur. J. Surg. Oncol. 2013, 39, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Matsumoto, H.; Izuhara, K.; Tohda, Y.; Kita, H.; Horiguchi, T.; Kuwabara, K.; Tomii, K.; Otsuka, K.; Fujimura, M.; et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J. Allergy Clin. Immunol. 2013, 132, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Duran, J.; Olavarría, P.S.; Mola, M.; Götzens, V.; Carballo, J.; Pelegrina, E.M.; Petit, M.; Abdul-Jawad, O.; Otaegui, I.; del Blanco, B.G.; et al. Genetic association study of coronary collateral circulation in patients with coronary artery disease using 22 single nucleotide polymorphisms corresponding to 10 genes involved in postischemic neovascularization. BMC Cardiovasc. Disord. 2015, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Hixson, J.E.; Shimmin, L.C.; Montasser, M.E.; Kim, D.K.; Zhong, Y.; Ibarguen, H.; Follis, J.; Malcom, G.; Strong, J.; Howard, T.; et al. Common variants in the periostin gene influence development of atherosclerosis in young persons. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition; December 2015. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 28 September 2020).
- Center for Substance Abuse Treatment (CSAT) and Substance Abuse and Mental Health Services Administration (SAMHSA). Substance Abuse Among Older Adults; Treatment Improvement Protocol (TIP) Series, No. 26; Report No.: (SMA) 98-3179; 1998. Available online: https://www.ncbi.nlm.nih.gov/books/NBK64419/ (accessed on 28 September 2020).
- Dufour, J.F.; Greten, T.F.; Raymond, E.; Roskams, T.; De, T.; Ducreux, M.; Mazzaferro, V.; Governing, E. Clinical Practice Guidelines EASL—EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma European Organisation for Research and Treatment of Cancer. J. Hepatol. 2012, 56, 908–943. [Google Scholar]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Poynard, T.; Castera, L. Critical comparison of elastography methods to assess chronic liver disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 402–411. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Orlic, L.; Franjic, N.; Hauser, G.; Stimac, D.; Milic, S. Transient elastography (FibroScan®) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease—Where do we stand? World J. Gastroenterol. 2016, 22, 7236–7251. [Google Scholar] [CrossRef] [PubMed]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.M.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Browning, S.R.; Browning, B.L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 2007, 81, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J. A wilcoxon-type test for trend. Stat. Med. 1985, 4, 87–90. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Regional Office for Europe. Body Mass Index—BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 28 September 2020).
- Horita, N.; Kaneko, T. Genetic model selection for a case-control study and a meta-analysis. Meta Gene 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Liang, W.; Lindeman, J.H.; Menke, A.L.; Koonen, D.P.; Morrison, M.; Havekes, L.M.; Van Den Hoek, A.M.; Kleemann, R. Metabolically induced liver inflammation leads to NASH and differs from LPS-or IL-1β-induced chronic inflammation. Lab. Investig. 2014, 94, 491–502. [Google Scholar] [CrossRef]
- Sugiyama, A.; Kanno, K.; Nishimichi, N.; Ohta, S.; Ono, J.; Conway, S.J.; Izuhara, K.; Yokosaki, Y.; Tazuma, S. Periostin promotes hepatic fibrosis in mice by modulating hepatic stellate cell activation via αv integrin interaction. J. Gastroenterol. 2016, 51, 1161–1174. [Google Scholar] [CrossRef]
- Chackelevicius, C.M.; Gambaro, S.E.; Tiribelli, C.; Rosso, N. Th17 involvement in nonalcoholic fatty liver disease progression to non-Alcoholic steatohepatitis. World J. Gastroenterol. 2016, 22, 9096–9103. [Google Scholar] [CrossRef]
- Amara, S.; Lopez, K.; Banan, B.; Brown, S.K.; Whalen, M.; Myles, E.; Ivy, M.T.; Johnson, T.; Schey, K.L.; Tiriveedhi, V. Synergistic effect of pro-inflammatory TNFα and IL-17 in periostin mediated collagen deposition: Potential role in liver fibrosis. Mol. Immunol. 2015, 64, 26–35. [Google Scholar] [CrossRef]
- Arteel, G.E.; Naba, A. The liver matrisome—Looking beyond collagens. JHEP Rep. 2020, 2, 100115. [Google Scholar] [CrossRef]
- Kumar, P.; Smith, T.; Raeman, R.; Chopyk, D.M.; Brink, H.; Liu, Y.; Sulchek, T.; Anania, F.A. Periostin promotes liver fibrogenesis by activating lysyl oxidase in hepatic stellate cells. J. Biol. Chem. 2018, 293, 12781–12792. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Jae, W.S.; Park, J.; Lee, S.Y.; Ohrr, H.; Guallar, E.; Samet, J.M. Body-mass index and mortality in Korean men and women. N. Engl. J. Med. 2006, 355, 779–787. [Google Scholar]
- Haffner, S.M.; Bowsher, R.R.; Mykkänen, L.; Hazuda, H.P.; Mitchell, B.D.; Valdez, R.A.; Gingerich, R.; Monterossa, A.; Stern, M.P. Proinsulin and specific insulin concentration in high-and low-risk populations for NIDDM. Diabetes 1994, 43, 1490–1493. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, J.S.; Noh, S.Y.; Rhee, E.J.; Kim, S.W.; Zimmet, P.Z. Prevalence of the metabolic syndrome among 40,698 Korean metropolitan subjects. Diabetes Res. Clin. Pract. 2004, 65, 143–149. [Google Scholar] [CrossRef]
- González-González, L.; Alonso, J. Periostin: A matricellular protein with multiple functions in cancer development and progression. Front. Oncol. 2018, 8, 225. [Google Scholar] [CrossRef]
- Zinn, P.O.; Singh, S.K.; Kotrotsou, A.; Hassan, I.; Thomas, G.; Luedi, M.M.; Elakkad, A.; Elshafeey, N.; Idris, T.; Mosley, J.; et al. A coclinical radiogenomic validation study: Conserved magnetic resonance radiomic appearance of periostin-expressing glioblastoma in patients and xenograft models. Clin. Cancer Res. 2018, 24, 6288–6299. [Google Scholar] [CrossRef]
- Keklikoglou, I.; Kadioglu, E.; Bissinger, S.; Langlois, B.; Bellotti, A.; Orend, G.; Ries, C.H.; De Palma, M. Periostin Limits Tumor Response to VEGFA Inhibition. Cell Rep. 2018, 22, 2530–2540. [Google Scholar] [CrossRef]
- Hu, W.; Jin, P.; Liu, W. Periostin contributes to cisplatin resistance in human non-small cell lung cancer A549 cells via activation of Stat3 and Akt and upregulation of survivin. Cell. Physiol. Biochem. 2016, 38, 1199–1208. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, F.; Song, W. Periostin contributes to arsenic trioxide resistance in hepatocellular carcinoma cells under hypoxia. Biomed. Pharmacother. 2017, 88, 342–348. [Google Scholar] [CrossRef]
- Jang, S.Y.; Park, S.Y.; Lee, H.W.; Choi, Y.K.; Park, K.G.; Yoon, G.S.; Tak, W.Y.; Kweon, Y.O.; Hur, K.; Lee, W.K. The combination of periostin overexpression and microvascular invasion is related to a poor prognosis for hepatocellular carcinoma. Gut Liver 2016, 10, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Song, Y.; Jiang, Y.; Yang, C.; Ding, Z. Associations among periostin gene polymorphisms, clinical parameters and heart failure: A case-control study in 1104 Chinese individuals. J. Cardiovasc. Med. 2011, 12, 469–474. [Google Scholar] [CrossRef] [PubMed]
- NIH/NCBI. dbSNP Short Genetic Variations. rs1028728. ALFA Allele Frequency (New). Available online: https://www.ncbi.nlm.nih.gov/snp/rs1028728#frequency_tab (accessed on 17 November 2020).
- NIH/NCBI. dbSNP Short Genetic Variations. rs3829365. ALFA Allele Frequency (New). Available online: https://www.ncbi.nlm.nih.gov/snp/rs3829365#frequency_tab (accessed on 17 November 2020).
- NIH/NCBI. dbSNP Short Genetic Variations. rs9603226. ALFA Allele Frequency (New). Available online: https://www.ncbi.nlm.nih.gov/snp/rs9603226#frequency_tab (accessed on 17 November 2020).
- Kudo, A. Clinical Applications Targeting Periostin. Adv. Exp. Med. Biol. 2019, 1132, 207–210. [Google Scholar] [PubMed]
| rs9603226 | rs3829365 | rs1028728 | |
|---|---|---|---|
| Haplotype 1 | A | G | A |
| Haplotype 2 | G | C | A |
| Haplotype 3 | G | G | T |
| Haplotype 4 | G | G | A |
| Haplotype 5 | A | C | A |
| Haplotype 6 | G | C | T |
| Parameter | NAFLD (N. = 74) | HCV (N. = 81) | p | All Patients (N. = 155) |
|---|---|---|---|---|
| Age, years | 56 (47–66) | 55 (45–64) | 0.532 | 56 (45–66) |
| Male/female sex, N. | 37 (50)/37 (50) | 40 (49)/41 (50) | 1.000 | 77 (50)/78 (50) |
| Body Mass Index, kg/m2 | 28.2 (26.8–33.2) | 23.1 (21.3–27.4) | <0.001 | 27.1 (24.4–30.9) |
| Obese subjects, N. | 31 (42) | 11 (14) | <0.001 | 42 (27) |
| Diabetic/prediabetic subjects, N. | 20 (27)/38 (51) | 14 (17) 28 (35) | 0.003 | 34 (22) |
| Liver stiffness, kPa 1 | 9.1 (7.2–13.3) | 8.4 (6.5–11.6) | 0.536 | 8.8 (6.2–12.0) |
| CAP 2, db/m | 300 (265–328) | 251 (214–289) | 0.032 | 280 (227–311) |
| Advanced histological fibrosis, N. 3 | 24 (38) | 24 (32) | 0.593 | 48 (31) |
| Severe histological steatosis, N. 4 | 13 (20) | 3 (4) 5 | 0.006 | 16 (10) |
| NFS, value | −0.625 (−1.534–0.468) | N.A. | N.A. | |
| NFS > 0.676 6, N. | 17 (23) | N.A. | N.A. | |
| HCC, N. | 10 (14) | 7 (9) | 0.442 | 17 (11) |
| Platelets, ×109/L | 182 (153–236) | 185 (144–233) | 0.617 | 183 (146–234) |
| AST, U/L | 36 (24–52) | 56 (35–84) | <0.001 | 41 (29–67) |
| ALT, U/L | 49 (30–75) | 77 (50–113) | <0.001 | 62 (36–98) |
| AST/ALT ratio | 0.75 (0.58–0.97) | 0.70 (0.58–0.93) | 0.878 | 0.73 (0.58–0.95) |
| Bilirubin, mg/dL | 0.7 (0.5–1.0) | 0.8 (0.6–1.1) | 0.113 | 0.8 (0.6–1.0) |
| Albumin, g/L | 43 (40–45) | 43 (41–46) | 0.904 | 43 (40–46) |
| Total cholesterol, mg/dL | 170 (145–199) | 158 (134–185) | 0.023 | 165 (137–189) |
| Triglycerides, mg/dL | 129 (87–170) | 98 (82–121) | 0.002 | 108 (83–139) |
| Glucose, mg/dL | 114 (100–140) | 100 (93–110) | <0.001 | 105 (94–117) |
| Creatinine, mg/dL | 0.8 (0.7–0.9) | 0.7 (0.7–0.9) | 0.050 | 0.77 (0.67–0.90) |
| Parameter | Parameter |
|---|---|
| Age, years | 25 (25–29) |
| Male/female sex, N. | 11 (41)/16 (59) |
| Body Mass Index, kg/m2 | 21.7 (19.4–23.4) |
| Obese subjects, N. | 0 (0) |
| Liver stiffness, kPa | 4.4 (3.9–5.4) |
| CAP, db/m | 196 (177–230) |
| (a) | Plasma Periostin (ng/mL) | |||||
| No/mild fibrosis (N. = 79) 1 | Moderate fibrosis (N. = 38) 2 | Severe fibrosis (N. = 48) 3 | HCC (N. = 17) | p | ||
| Total population (N. = 182) | 10.5 (8.1–15.0) | 12.0 (10.8–15.5) | 11.1 (8.4–15.7) | 16.8 (12.2–22.9) | 0.001 | |
| NAFLD (N. = 74) | 9.9 (6.8–13.1) | 10.9 (7.1–11.3) | 10.2 (7.7–14.7) | 15.4 (12.1–18.3) | 0.045 | |
| HCV (N. = 81) | 10.4 (7.8–14.4) | 13.1 (10.9–16.5) | 11.4 (8.9–18.2) | 18.2 (15.5–50.1) | 0.013 | |
| Healthy controls (N. = 27) | 12.0 (9.1–16.3) | - | - | - | N.A. | |
| (b) | Differences in plasma periostin concentrations, p value | |||||
| Moderate vs. no/mild fibrosis 1,2 | Severe vs. no/mild fibrosis 1,3 | Severe vs. moderate fibrosis 2,3 | Cirrhosis 4 vs. pre-cirrhosis 5 | |||
| Total non-HCC subjects (N. = 165) | 0.101 | 0.424 | 0.548 | 0.937 | ||
| Non-HCC NAFLD (N. = 64) | 0.952 | 0.535 | 0.696 | 0.238 | ||
| Non-HCC HCV (N. = 74) | 0.073 | 0.254 | 0.889 | 0.535 | ||
| (c) | Differences in plasma periostin concentrations, p value | |||||
| HCC vs. no/mild fibrosis 1 | HCC vs. moderate fibrosis 2 | HCC vs. severe fibrosis 3 | ||||
| Total patients (N. = 155) | <0.001 | 0.002 | 0.004 | |||
| NAFLD (N. = 74) | 0.008 | 0.01 | 0.04 | |||
| HCV(N. = 81) | 0.007 | 0.004 | 0.03 | |||
| SNPs | Genotype | p | ||
|---|---|---|---|---|
| rs9603226—chr13:37569449 (GRCh38.p12) | G/G | G/A | A/A | |
| Total (N. = 182) NAFLD (N. = 74) HCV (N. = 81) Healthy controls (N. = 27) | 132 (72) 53 (72) 58 (72 )21 (78) | 49 (27) 21 (28) 23 (28) 5 (18) | 1 (1) 0 (0) 0 (0) 1 (4) | 0.153 |
| rs3829365—chr13:37598759 (GRCh38.p12) | C/C | C/G | G/G | |
| Total (N. = 182) NAFLD (N. = 74) HCV (N. = 81) Healthy controls (N. = 27) | 168 (92) 70 (95) 71 (88) 27 (100) | 13 (7) 4 (5) 9 (11) 0 (0) | 1 (1) 0 (0) 1 (1) 0 (0) | 0.224 |
| rs1028728—chr13:37599679 (GRCh38.p12) | A/A | A/T | T/T | |
| Total (N. = 182) NAFLD (N. = 74) HCV (N. = 81) Healthy controls (N. = 27) | 106 (58) 44 (59) 46 (57) 16 (59) | 64 (35) 25 (34) 29 (36) 10 (37) | 12 (7) 5 (7) 6 (7) 1 (4) | 0.968 |
| SNPs | Genotype and Plasmatic Periostin (ng/mL) | |||
|---|---|---|---|---|
| rs9603226 | G/G | G/A | A/A | p |
| Total (N. = 182) NAFLD (N. = 74) HCV (N. = 81) Healthy controls (N. = 27) | 11.9 (9.0–16.3) 11.4 (7.7–15.8) 12.2 (9.8–16.5) 12.0 (9.3–16.2) | 11.1 (8.2–15.6) 9.7 (8.0–12.3) 11.2 (9.2–16.8) 15.6 (8.7–19.8) | 9.1 (9.1–9.1) – – 9.1 (9.1–9.1) | 0.491 0.232 0.742 0.710 |
| rs3829365 | C/C | C/G | G/G | p |
| Total (N. = 182) NAFLD (N. = 74) HCV (N. = 81) Healthy controls (N. = 27) | 11.8 (8.6–16.4) 11.2 (7.7–14.8) 12.5 (10.1–16.8) 12.0 (9.1–16.3) | 10.5 (8.7–13.0) 9.6 (7.2–11.7) 10.5 (9.2–13.2) – | 64.6 (64.6–64.6) – 64.6 (64.6–64.6) – | 0.121 0.430 0.119 NA |
| rs1028728 | A/A | A/T | T/T | p |
| Total (N. = 182) NAFLD (N. = 74) HCV (N. = 81) Healthy controls (N. = 27) | 11.4 (8.0–15.6) 9.8 (6.9–13.3) 12.3 (9.2–16.5) 13.3 (10.2–16.2) | 11.1 (9.11–15.9) 11.9 (9.4–15.8) 11.2 (10.3–16.5) 9.2 (8.7–12.6) | 15.1 (10.3–19.1) 11.8 (8.1–17.7) 16.0 (13.1–19.4) 17.1 (17.1–17.1) | 0.153 0.206 0.394 0.232 |
| POSTN SNPs | POSTN Genotypes 1 and Periostin Levels ng/mL | p | ||
|---|---|---|---|---|
| (a) | rs9603226 | G/G | A/* | 0.301 |
| 11.9 (9.0–16.3) | 11.1 (8.2–16.6) | |||
| rs3829365 | C/C | G/* | 0.521 | |
| 11.8 (8.6–16.4) | 10.5 (8.7–13.1) | |||
| rs1028728 | A/A | T/* | 0.153 | |
| 11.5 (8.0–15.6) | 11.9 (9.1–16.7) | |||
| (b) | rs9603226 | G/* | A/A | 0.714 |
| 11.7 (9.7–16.3) | 9.1 (9.1–9.1) | |||
| rs3829365 | C/* | G/G | 0.085 | |
| 11.5 (8.7–16.2) | 64.6 (64.6–64.6) | |||
| rs1028728 | A/* | T/T | 0.091 | |
| 11.3 (8.6–15.8) | 15.1 (10.3–19.1) | |||
| POSTN Gene Diplotypes and Periostin Levels ng/mL | p | |||
|---|---|---|---|---|
| Group | Diplotype 2/2 | Diplotype 2/* | Diplotype */* | |
| Total (N. = 182) | 11.7 (7.9–16.2) (N. = 61) | 11.1 (8.7–15.6) (N. = 95) | 11.9 (8.9–18.8) (N. = 26) | 0.253 |
| NAFLD (N. = 74) | 10.4 (6.8–15.0) (N. = 25) | 11.1 (8.1–14.2) (N. = 39) | 11.2 (8.1–17.7) (N. = 10) | 0.447 |
| HCV (N. = 81) | 11.7 (9.3–16.5) (N. = 25) | 11.2 (9.2–16.5) (N. = 43) | 13.2 (11.0–19.4) (N. = 13) | 0.233 |
| Healthy controls (N. = 27) | 12.4 (10.8–16.2) (N. = 11) | 10.4 (9.0–18.1) (N. = 13) | 9.1 (8.7–17.1) (N. = 3) | 0.460 |
| (a) All Patients (N. = 155) | (b) NAFLD (N. = 74) | (c) HCV (N. = 81) | ||||
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Diplotype 2/2 + 2/* | 0.25 (0.09–0.70) | 0.008 | 0.37 (0.07–2.00) | 0.219 | 0.25 (0.07–0.97) | 0.041 |
| Age, years | 0.98 (0.95–1.02) | 0.338 | 0.97 (0.92–1.03) | 0.342 | 0.99 (0.95–1.04) | 0.808 |
| Male/female sex | 1.98 (0.79–4.96) | 0.144 | 1.67 (0.39–7.11) | 0.519 | 2.74 (0.80–9.35) | 0.113 |
| HCC, N. | 6.76 (1.73–26.38) | 0.006 | 7.46 (1.19–56.02) | 0.022 | 15.64 (2.15–113.87) | 0.001 |
| Platelets, ×109/L | 0.99 (0.98–1.00) | 0.230 | 1.00 (0.99–1.01) | 0.399 | 1.00 (0.99–1.01) | 0.883 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirne, C.; Mulas, V.; Barbaglia, M.N.; Mallela, V.R.; Minisini, R.; Barizzone, N.; Burlone, M.E.; Pirisi, M.; Grossini, E. Periostin Circulating Levels and Genetic Variants in Patients with Non-Alcoholic Fatty Liver Disease. Diagnostics 2020, 10, 1003. https://doi.org/10.3390/diagnostics10121003
Smirne C, Mulas V, Barbaglia MN, Mallela VR, Minisini R, Barizzone N, Burlone ME, Pirisi M, Grossini E. Periostin Circulating Levels and Genetic Variants in Patients with Non-Alcoholic Fatty Liver Disease. Diagnostics. 2020; 10(12):1003. https://doi.org/10.3390/diagnostics10121003
Chicago/Turabian StyleSmirne, Carlo, Violante Mulas, Matteo Nazzareno Barbaglia, Venkata Ramana Mallela, Rosalba Minisini, Nadia Barizzone, Michela Emma Burlone, Mario Pirisi, and Elena Grossini. 2020. "Periostin Circulating Levels and Genetic Variants in Patients with Non-Alcoholic Fatty Liver Disease" Diagnostics 10, no. 12: 1003. https://doi.org/10.3390/diagnostics10121003
APA StyleSmirne, C., Mulas, V., Barbaglia, M. N., Mallela, V. R., Minisini, R., Barizzone, N., Burlone, M. E., Pirisi, M., & Grossini, E. (2020). Periostin Circulating Levels and Genetic Variants in Patients with Non-Alcoholic Fatty Liver Disease. Diagnostics, 10(12), 1003. https://doi.org/10.3390/diagnostics10121003









