Consistent Cerebral Blood Flow Covariance Networks across Healthy Individuals and Their Similarity with Resting State Networks and Vascular Territories
Abstract
1. Introduction
2. Materials and Methods
2.1. MRI Acquisition
2.2. MRI Preprocessing
2.3. Consistency
2.4. Similarity
3. Results
3.1. Consistency
3.2. Similarity
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.J.; Rosas, H.D.; Salat, D.H. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 2011, 55, 468–478. [Google Scholar] [PubMed]
- Melzer, T.R.; Watts, R.; MacAskill, M.R.; Pearson, J.F.; Rüeger, S.; Pitcher, T.L.; Livingston, L.; Graham, C.; Keenan, R.; Shankaranarayanan, A.; et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson’s disease. Brain 2011, 134 Pt 3, 845–855. [Google Scholar]
- Fernández-Seara, M.A.; Mengual, E.; Vidorreta, M.; Aznárez-Sanado, M.; Loayza, F.R.; Villagra, F.; Irigoyen, J.; Pastor, M.A. Cortical hypoperfusion in Parkinson’s disease assessed using arterial spin labeled perfusion MRI. Neuroimage 2012, 59, 2743–2750. [Google Scholar] [PubMed]
- Johnson, N.A.; Jahng, G.H.; Weiner, M.W.; Miller, B.L.; Chui, H.C.; Jagust, W.J.; Gorno-Tempini, M.L.; Schuff, N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: Initial experience. Radiology 2005, 234, 851–859. [Google Scholar] [PubMed]
- Du, A.T.; Jahng, G.H.; Hayasaka, S.; Kramer, J.H.; Rosen, H.J.; Gorno-Tempini, M.L.; Rankin, K.P.; Miller, B.L.; Weiner, M.W.; Schuff, N. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology 2006, 67, 1215–1220. [Google Scholar]
- Steketee, R.M.; Bron, E.E.; Meijboom, R.; Houston, G.C.; Klein, S.; Mutsaerts, H.J.; Mendez Orellana, C.P.; de Jong, F.J.; van Swieten, J.C.; van der Lugt, A.; et al. Early-stage differentiation between presenile Alzheimer’s disease and frontotemporal dementia using arterial spin labeling MRI. Eur Radiol 2016, 26, 244–253. [Google Scholar]
- de la Peña, M.J.; Peña, I.C.; García, P.G.; Gavilán, M.L.; Malpica, N.; Rubio, M.; González, R.A.; de Vega, V.M. Early perfusion changes in multiple sclerosis patients as assessed by MRI using arterial spin labeling. Acta Radiol. Open 2019, 8. [Google Scholar] [CrossRef]
- Marshall, O.; Chawla, S.; Lu, H.; Pape, L.; Ge, Y. Cerebral blood flow modulation insufficiency in brain networks in multiple sclerosis: A hypercapnia MRI study. J. Cereb. Blood Flow Metab. 2016, 36, 2087–2095. [Google Scholar]
- Pelizzari, L.; Laganà, M.M.; Rossetto, F.; Bergsland, N.; Galli, M.; Baselli, G.; Clerici, M.; Nemni, R.; Baglio, F. Cerebral blood flow and cerebrovascular reactivity correlate with severity of motor symptoms in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2019, 12. [Google Scholar] [CrossRef]
- Pelizzari, L.; Di Tella, S.; Rossetto, F.; Laganà, M.M.; Bergsland, N.; Pirastru, A.; Meloni, M.; Nemni, R.; Baglio, F. Parietal Perfusion Alterations in Parkinson’s Disease Patients Without Dementia. Front. Neurol. 2020, 11, 562. [Google Scholar]
- Zhang, X.; Guo, X.; Zhang, N.; Cai, H.; Sun, J.; Wang, Q.; Qi, Y.; Zhang, L.; Yang, L.; Shi, F.D.; et al. Cerebral Blood Flow Changes in Multiple Sclerosis and Neuromyelitis Optica and Their Correlations WITH Clinical Disability. Front. Neurol. 2018, 9, 305. [Google Scholar] [PubMed]
- Lagana, M.M.; Pelizzari, L.; Baglio, F. Relationship between MRI perfusion and clinical severity in multiple sclerosis. Neural Regen. Res. 2020, 15, 646–652. [Google Scholar] [PubMed]
- Sierra-Marcos, A. Regional Cerebral Blood Flow in Mild Cognitive Impairment and Alzheimer’s Disease Measured with Arterial Spin Labeling Magnetic Resonance Imaging. Int. J. Alzheimers Dis. 2017, 2017, 5479597. [Google Scholar] [PubMed]
- Beckmann, C.F.; Smith, S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 2004, 23, 137–152. [Google Scholar] [PubMed]
- Calhoun, V.D.; Adali, T.; Pearlson, G.D.; Pekar, J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001, 14, 140–151. [Google Scholar] [PubMed]
- Laganà, M.M.; Pirastru, A.; Pelizzari, L.; Rossetto, F.; Di Tella, S.; Bergsland, N.; Nemni, R.; Meloni, M.; Baglio, F. Multimodal Evaluation of Neurovascular Functionality in Early Parkinson’s Disease. Front. Neurol. 2020, 11, 831. [Google Scholar]
- Liang, X.; Tournier, J.-D.; Masterton, R.; Connelly, A.; Calamante, F. A k-space sharing 3D GRASE pseudocontinuous ASL method for whole-brain resting-state functional connectivity. Int. J. Imaging Syst. Technol. 2012, 22, 37–43. [Google Scholar]
- Jann, K.; Gee, D.G.; Kilroy, E.; Schwab, S.; Smith, R.X.; Cannon, T.D.; Wang, D.J. Functional connectivity in BOLD and CBF data: Similarity and reliability of resting brain networks. Neuroimage 2015, 106, 111–122. [Google Scholar]
- Dai, W.; Varma, G.; Scheidegger, R.; Alsop, D.C. Quantifying fluctuations of resting state networks using arterial spin labeling perfusion MRI. J. Cereb. Blood Flow Metab. 2016, 36, 463–473. [Google Scholar]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar]
- Avants, B.B.; Tustison, N.J.; Song, G.; Cook, P.A.; Klein, A.; Gee, J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011, 54, 2033–2044. [Google Scholar] [PubMed]
- Chappell, M.; Groves, A.; Whitcher, B.; Woolrich, M. Variational Bayesian Inference for a Nonlinear Forward Model. IEEE Trans. Signal Process. 2009, 57, 223–236. [Google Scholar]
- Wang, D.J.; Alger, J.R.; Qiao, J.X.; Gunther, M.; Pope, W.B.; Saver, J.L.; Salamon, N.; Liebeskind, D.S. Multi-delay multi-parametric arterial spin-labeled perfusion MRI in acute ischemic stroke—Comparison with dynamic susceptibility contrast enhanced perfusion imaging. Neuroimage Clin. 2013, 3, 1–7. [Google Scholar] [PubMed]
- Laganá, M.M.; Mendozzi, L.; Pelizzari, L.; Bergsland, N.P.; Pugnetti, L.; Cecconi, P.; Baselli, G.; Clerici, M.; Nemni, R.; Baglio, F. Are Cerebral Perfusion and Atrophy Linked in Multiple Sclerosis? Evidence for a Multifactorial Approach to Assess Neurodegeneration. Curr. Neurovasc. Res. 2018, 15, 282–291. [Google Scholar]
- Jenkinson, M. Improving the registration of B0-disorted EPI images using calculated cost function weights. In Proceedings of the Tenth 10th International Conference for Functional Mapping of the Human Brain, Budapest, Hungary, 13–17 June 2004. [Google Scholar]
- Woolrich, M.W.; Ripley, B.D.; Brady, M.; Smith, S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 2001, 14, 1370–1386. [Google Scholar]
- Kundu, P.; Inati, S.J.; Evans, J.W.; Luh, W.M.; Bandettini, P.A. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage 2012, 60, 1759–1770. [Google Scholar]
- Greve, D.N.; Fischl, B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009, 48, 63–72. [Google Scholar]
- Himberg, J.; Hyvärinen, A.; Esposito, F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 2004, 22, 1214–1222. [Google Scholar]
- Lr, D. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar]
- Zijdenbos, A.P.; Dawant, B.M.; Margolin, R.A.; Palmer, A.C. Morphometric analysis of white matter lesions in MR images: Method and validation. IEEE Trans. Med. Imaging 1994, 13, 716–724. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. (Zagreb.) 2012, 22, 276–282. [Google Scholar] [PubMed]
- Beckmann, C.F.; DeLuca, M.; Devlin, J.T.; Smith, S.M. Investigations into resting-state connectivity using independent component analysis. Philos Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1001–1013. [Google Scholar] [PubMed]
- Mutsaerts, H.J.; van Dalen, J.W.; Heijtel, D.F.; Groot, P.F.; Majoie, C.B.; Petersen, E.T.; Richard, E.; Nederveen, A.J. Cerebral Perfusion Measurements in Elderly with Hypertension Using Arterial Spin Labeling. PLoS ONE 2015, 10, e0133717. [Google Scholar]
- Riederer, I.; Bohn, K.P.; Preibisch, C.; Wiedemann, E.; Zimmer, C.; Alexopoulos, P.; Förster, S. Alzheimer Disease and Mild Cognitive Impairment: Integrated Pulsed Arterial Spin-Labeling MRI and (18)F-FDG PET. Radiology 2018, 288, 198–206. [Google Scholar] [PubMed]
- Matsui, H.; Nishinaka, K.; Oda, M.; Hara, N.; Komatsu, K.; Kubori, T.; Udaka, F. Hypoperfusion of the visual pathway in parkinsonian patients with visual hallucinations. Mov. Disord. 2006, 21, 2140–2144. [Google Scholar] [PubMed]
- Hu, W.T.; Wang, Z.; Lee, V.M.; Trojanowski, J.Q.; Detre, J.A.; Grossman, M. Distinct cerebral perfusion patterns in FTLD and AD. Neurology 2010, 75, 881–888. [Google Scholar] [PubMed]
- Lin, W.C.; Chen, P.C.; Huang, C.C.; Tsai, N.W.; Chen, H.L.; Wang, H.C.; Chou, K.H.; Chen, M.H.; Chen, Y.W.; Lu, C.H. Autonomic Function Impairment and Brain Perfusion Deficit in Parkinson’s Disease. Front. Neurol. 2017, 8, 246. [Google Scholar]
- Minagar, A.; Barnett, M.H.; Benedict, R.H.; Pelletier, D.; Pirko, I.; Sahraian, M.A.; Frohman, E.; Zivadinov, R. The thalamus and multiple sclerosis: Modern views on pathologic, imaging, and clinical aspects. Neurology 2013, 80, 210–219. [Google Scholar] [PubMed]
- Doche, E.; Lecocq, A.; Maarouf, A.; Duhamel, G.; Soulier, E.; Confort-Gouny, S.; Rico, A.; Guye, M.; Audoin, B.; Pelletier, J.; et al. Hypoperfusion of the thalamus is associated with disability in relapsing remitting multiple sclerosis. J. Neuroradiol. 2017, 44, 158–164. [Google Scholar]
- Jakimovski, D.; Bergsland, N.; Dwyer, M.G.; Traversone, J.; Hagemeier, J.; Fuchs, T.A.; Ramasamy, D.P.; Weinstock-Guttman, B.; Benedict, R.H.B.; Zivadinov, R. Cortical and Deep Gray Matter Perfusion Associations With Physical and Cognitive Performance in Multiple Sclerosis Patients. Front. Neurol. 2020, 11, 700. [Google Scholar]
- Chaves, C.; Piemonte, T.; Lee, G. Cerebellar hypoperfusion in a patient with spells of imbalance. Arch. Neurol. 2008, 65, 1540–1541. [Google Scholar] [PubMed]
- Kellner-Weldon, F.; El-Koussy, M.; Jung, S.; Jossen, M.; Klinger-Gratz, P.P.; Wiest, R. Cerebellar Hypoperfusion in Migraine Attack: Incidence and Significance. AJNR Am. J. Neuroradiol. 2018, 39, 435–440. [Google Scholar] [PubMed]
- Amen, D.G.; Egan, S.; Meysami, S.; Raji, C.A.; George, N. Patterns of Regional Cerebral Blood Flow as a Function of Age Throughout the Lifespan. J. Alzheimers Dis. 2018, 65, 1087–1092. [Google Scholar] [PubMed]
- Langkilde, A.R.; Frederiksen, J.L.; Rostrup, E.; Larsson, H.B. Functional MRI of the visual cortex and visual testing in patients with previous optic neuritis. Eur. J. Neurol. 2002, 9, 277–286. [Google Scholar]
- Ganis, G.; Thompson, W.L.; Kosslyn, S.M. Brain areas underlying visual mental imagery and visual perception: An fMRI study. Brain Res. Cogn. Brain. Res. 2004, 20, 226–241. [Google Scholar]
- Paiva, F.F.; Tannús, A.; Silva, A.C. Measurement of cerebral perfusion territories using arterial spin labelling. NMR Biomed. 2007, 20, 633–642. [Google Scholar]
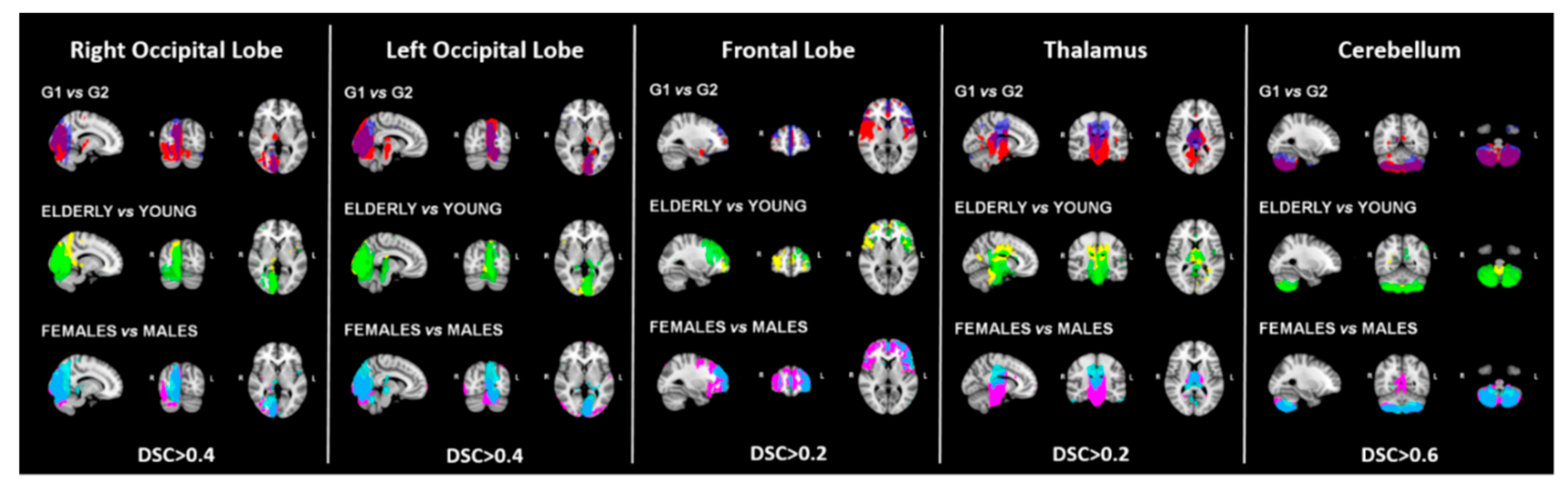
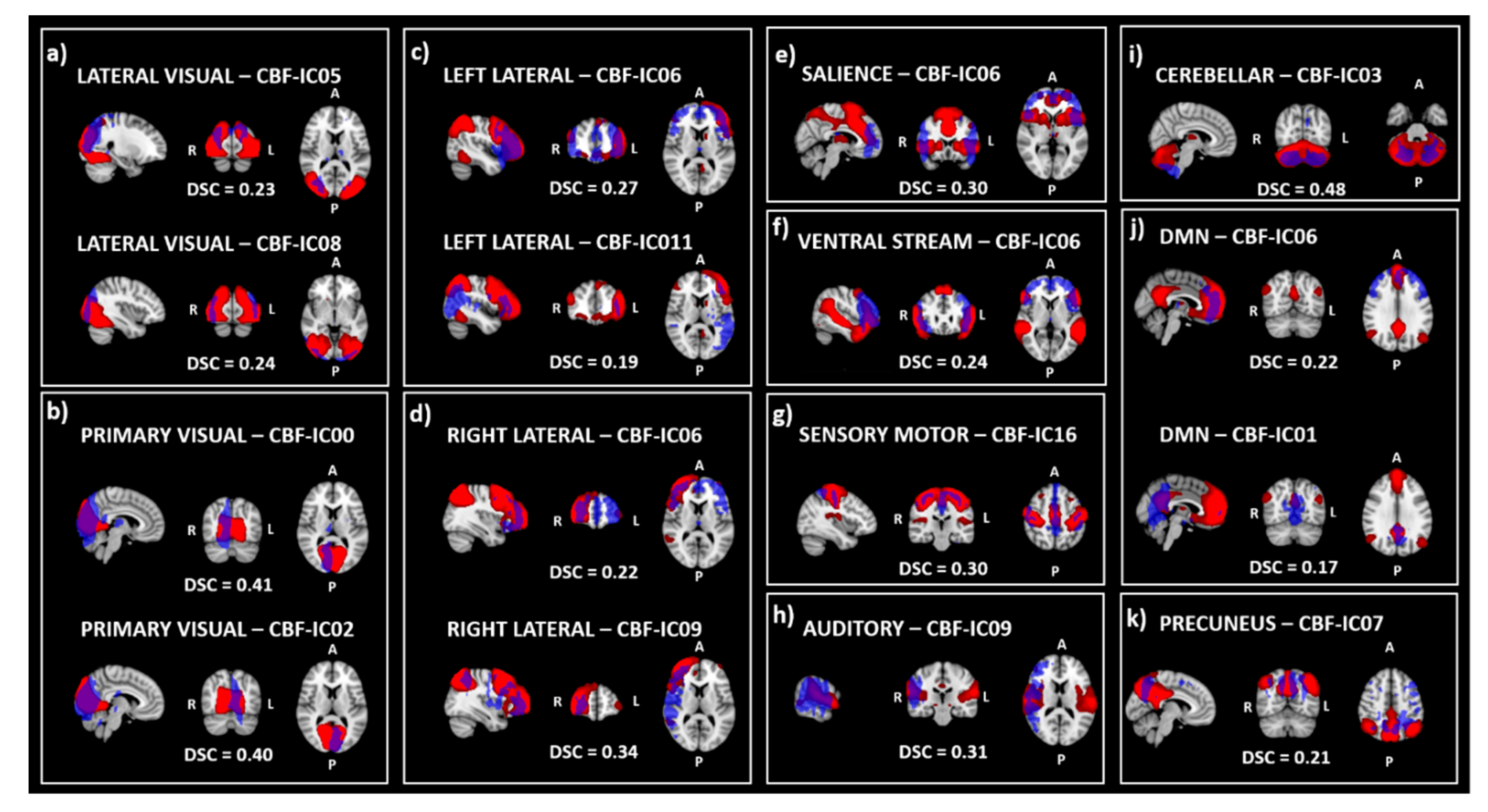
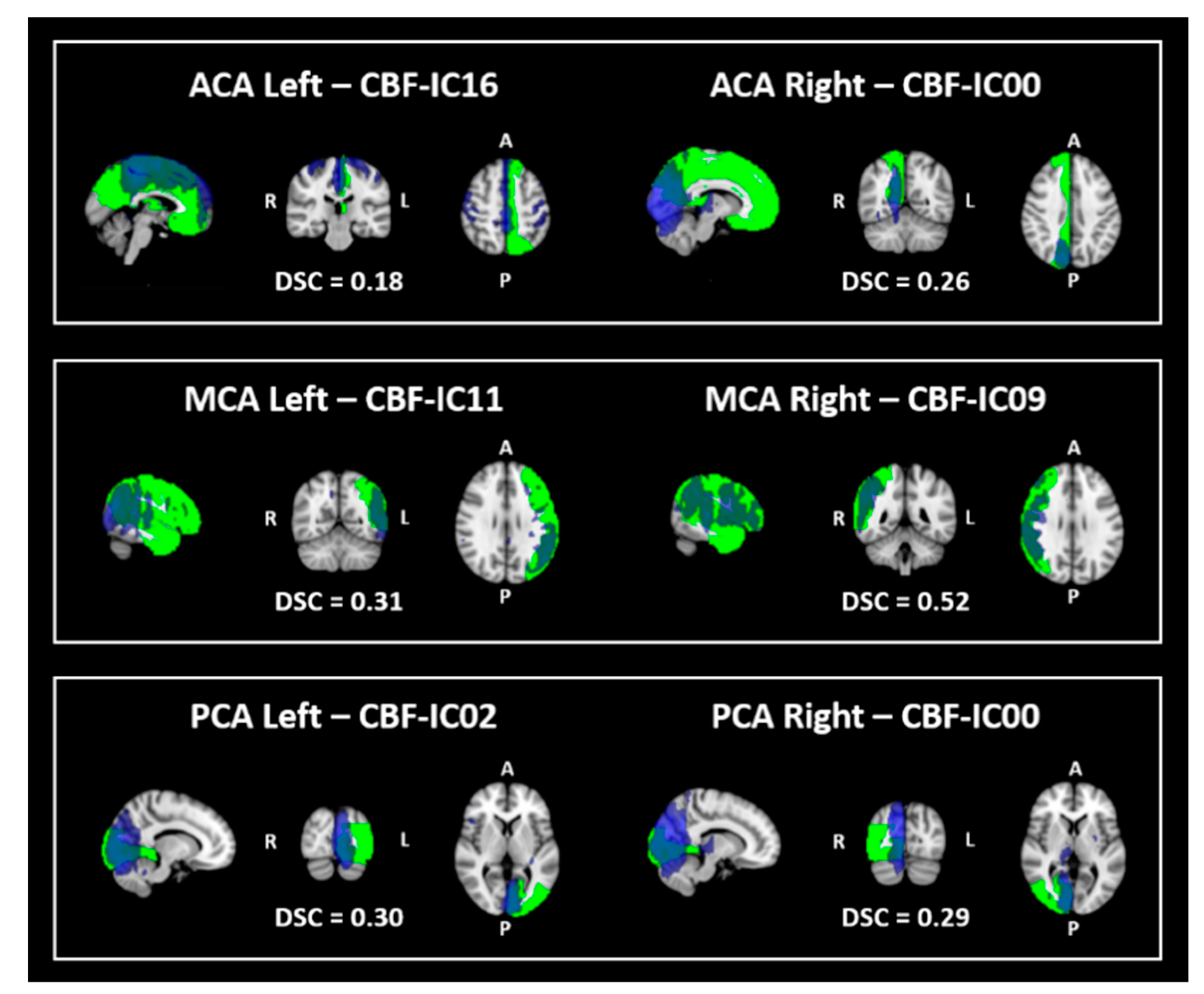
| Sub-Sample 1 | Sub-Sample 2 | p-Value | |
|---|---|---|---|
| Split 1: Random | n = 46 | n = 46 | |
| Age in yr, mean ± SD | 42.4 ± 17.6 | 43.1 ± 17.7 | 0.876 a |
| Males n (%) | 24 (52%) | 23 (50%) | 0.835 b |
| Split 2: Age | n = 46 | n = 46 | |
| Age in yr, mean ± SD | 58.3 ± 10.3 | 27.2 ± 4.8 | <0.0001 a |
| Males n (%) | 25 (54%) | 22 (48%) | 0.531 b |
| Split 3: Sex | n = 47 | n = 45 | |
| Age in yr, mean ± SD | 44.6 ± 17.9 | 40.8 ± 17.1 | 0.276 a |
| Males n (%) | 47 (100%) | 0 (0%) | <0.0001 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirastru, A.; Pelizzari, L.; Bergsland, N.; Cazzoli, M.; Cecconi, P.; Baglio, F.; Laganà, M.M. Consistent Cerebral Blood Flow Covariance Networks across Healthy Individuals and Their Similarity with Resting State Networks and Vascular Territories. Diagnostics 2020, 10, 963. https://doi.org/10.3390/diagnostics10110963
Pirastru A, Pelizzari L, Bergsland N, Cazzoli M, Cecconi P, Baglio F, Laganà MM. Consistent Cerebral Blood Flow Covariance Networks across Healthy Individuals and Their Similarity with Resting State Networks and Vascular Territories. Diagnostics. 2020; 10(11):963. https://doi.org/10.3390/diagnostics10110963
Chicago/Turabian StylePirastru, Alice, Laura Pelizzari, Niels Bergsland, Marta Cazzoli, Pietro Cecconi, Francesca Baglio, and Maria Marcella Laganà. 2020. "Consistent Cerebral Blood Flow Covariance Networks across Healthy Individuals and Their Similarity with Resting State Networks and Vascular Territories" Diagnostics 10, no. 11: 963. https://doi.org/10.3390/diagnostics10110963
APA StylePirastru, A., Pelizzari, L., Bergsland, N., Cazzoli, M., Cecconi, P., Baglio, F., & Laganà, M. M. (2020). Consistent Cerebral Blood Flow Covariance Networks across Healthy Individuals and Their Similarity with Resting State Networks and Vascular Territories. Diagnostics, 10(11), 963. https://doi.org/10.3390/diagnostics10110963






