Longitudinal Magnetic Resonance Imaging of Cerebral Microbleeds in Multiple Sclerosis Patients
Abstract
1. Introduction
2. Materials and Methods
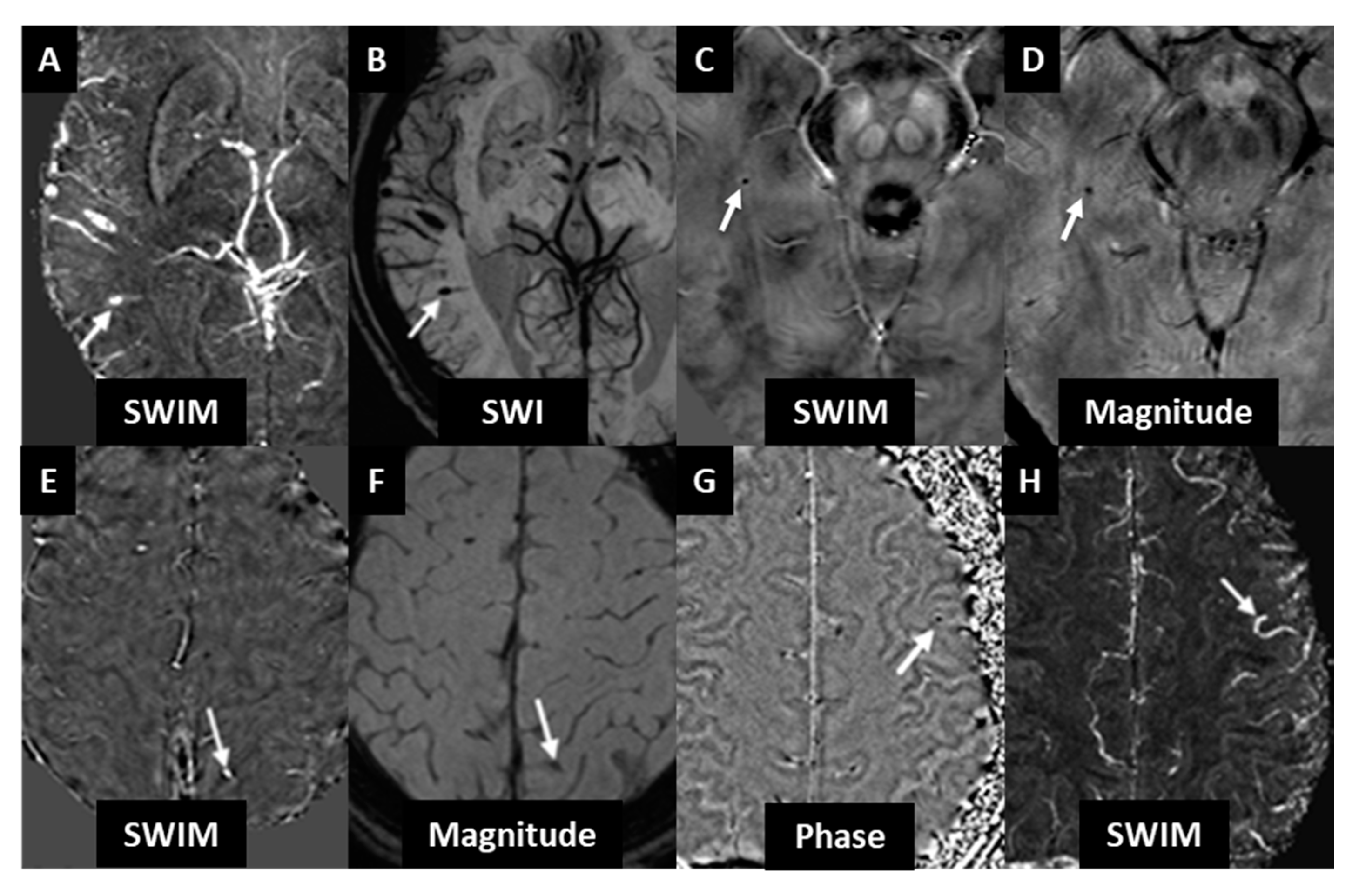
3. Results
3.1. Cohort 1
3.2. Cohort 2
3.3. Longitudinal CMB Analysis
3.4. CMB vs. Subject Demographics
4. Discussion
4.1. CMB Detection and Guidelines
4.2. CMB Prevalence in MS and Other Diseases
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Greenberg, S.M.; Vernooij, M.W.; Cordonnier, C.; Viswanathan, A.; Al-Shahi Salman, R.; Warach, S.; Launer, L.J.; Van Buchem, M.A.; Breteler, M.M. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009, 8, 165–174. [Google Scholar] [CrossRef]
- Haller, S.; Montandon, M.-L.; Lazeyras, F.; Scheffler, M.; Meckel, S.; Herrmann, F.R.; Giannakopoulos, P.; Kövari, E. Radiologic-Histopathologic Correlation of Cerebral Microbleeds Using Pre-Mortem and Post-Mortem MRI. PLoS ONE 2016, 11, e0167743. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Kwok, C.S.; Benavente, O. Cerebral microbleeds: Histopathological correlation of neuroimaging. Cerebrovasc. Dis. 2011, 32, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Roob, G.; Schmidt, R.; Kapeller, P.; Lechner, A.; Hartung, H.P.; Fazekas, F. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology 1999, 52, 991. [Google Scholar] [CrossRef] [PubMed]
- Poels, M.M.; Ikram, M.A.; van der Lugt, A.; Hofman, A.; Krestin, G.P.; Breteler, M.M.; Vernooij, M.W. Incidence of cerebral microbleeds in the general population: The Rotterdam Scan Study. Stroke 2011, 42, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, C.; van der Flier, W.M. Brain microbleeds and Alzheimer’s disease: Innocent observation or key player? Brain 2011, 134, 335–344. [Google Scholar] [CrossRef]
- Van der Flier, W.M.; Cordonnier, C. Microbleeds in vascular dementia: Clinical aspects. Exp. Gerontol. 2012, 47, 853–857. [Google Scholar] [CrossRef]
- Ayaz, M.; Boikov, A.S.; Haacke, E.M.; Kido, D.K.; Kirsch, W.M. Imaging cerebral microbleeds using susceptibility weighted imaging: One step toward detecting vascular dementia. J. Magn. Reson. Imaging 2010, 31, 142–148. [Google Scholar] [CrossRef]
- Fan, Y.H.; Zhang, L.; Lam, W.W.; Mok, V.C.; Wong, K.S. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke 2003, 34, 2459–2462. [Google Scholar] [CrossRef]
- Lee, S.H.; Ryu, W.S.; Roh, J.K. Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology 2009, 72, 171–176. [Google Scholar] [CrossRef]
- Lupo, J.M.; Chuang, C.F.; Chang, S.M.; Barani, I.J.; Jimenez, B.; Hess, C.P.; Nelson, S.J. 7-Tesla susceptibility-weighted imaging to assess the effects of radiotherapy on normal-appearing brain in patients with glioma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e493–e500. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, C.; Al-Shahi Salman, R.; Wardlaw, J. Spontaneous brain microbleeds: Systematic review, subgroup analyses and standards for study design and reporting. Brain 2007, 130, 1988–2003. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Izumiyama, M.; Izumiyama, K.; Takahashi, A.; Itoyama, Y. Silent cerebral microbleeds on T2*-weighted MRI: Correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke 2002, 33, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.R.; Gattu, R.; Sewick, B.; Kou, Z.; Zakariah, N.; Cavanaugh, J.M.; Haacke, E.M. Detection of hemorrhagic and axonal pathology in mild traumatic brain injury using advanced MRI: Implications for neurorehabilitation. NeuroRehabilitation 2012, 31, 261–279. [Google Scholar] [CrossRef]
- Gregoire, S.M. Cerebral microbleeds and long-term cognitive outcome: Longitudinal cohort study of stroke clinic patients. Cerebrovasc. Dis. 2012, 33, 430–435. [Google Scholar] [CrossRef]
- Cheng, A.L.; Batool, S.; McCreary, C.R.; Lauzon, M.L.; Frayne, R.; Goyal, M.; Smith, E.E. Susceptibility-weighted imaging is more reliable than T2*-weighted gradient-recalled echo MRI for detecting microbleeds. Stroke 2013, 44, 2782–2786. [Google Scholar] [CrossRef]
- Nandigam, R.N.K.; Viswanathan, A.; Delgado, P.; Skehan, M.E.; Smith, E.E.; Rosand, J.; Greenberg, S.M.; Dickerson, B.C. MR imaging detection of cerebral microbleeds: Effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am. J. Neuroradiol. 2009, 30, 338–343. [Google Scholar] [CrossRef]
- Tong, K.A.; Ashwal, S.; Holshouser, B.A.; Nickerson, J.P.; Wall, C.J.; Shutter, L.A.; Osterdock, R.J.; Haacke, E.M.; Kido, D. Diffuse axonal injury in children: Clinical correlation with hemorrhagic lesions. Ann. Neurol. 2004, 56, 36–50. [Google Scholar] [CrossRef]
- Haacke, E.M.; Tang, J.; Neelavalli, J.; Cheng, Y.C. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. J. Magn. Reson. Imaging 2010, 32, 663–676. [Google Scholar] [CrossRef]
- Tang, J.; Liu, S.; Neelavalli, J.; Cheng, Y.C.; Buch, S.; Haacke, E.M. Improving susceptibility mapping using a threshold-based K-space/image domain iterative reconstruction approach. Magn. Reson. Med. 2013, 69, 1396–1407. [Google Scholar] [CrossRef]
- Haacke, E.M.; Liu, S.; Buch, S.; Zheng, W.; Wu, D.; Ye, Y. Quantitative susceptibility mapping: Current status and future directions. Magn. Reson. Imaging 2015, 33, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Eisele, P.; Alonso, A.; Griebe, M.; Szabo, K.; Hennerici, M.G.; Gass, A. Investigation of cerebral microbleeds in multiple sclerosis as a potential marker of blood-brain barrier dysfunction. Mult. Scler. Relat. Disord. 2016, 7, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Rudick, R.; Horwitz, R.; Cutter, G.; Tyry, T.; Campagnolo, D.; Vollmer, T. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010, 74, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Zivadinov, R.; Ramasamy, D.P.; Benedict, R.R.; Polak, P.; Hagemeier, J.; Magnano, C.; Dwyer, M.G.; Bergsland, N.; Bertolino, N.; Weinstock-Guttman, B.; et al. Cerebral Microbleeds in Multiple Sclerosis Evaluated on Susceptibility-weighted Images and Quantitative Susceptibility Maps: A Case-Control Study. Radiology 2016, 281, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Pacurar, E.E.; Sethi, S.K.; Habib, C.; Laze, M.O.; Martis-Laze, R.; Haacke, E.M. Database integration of protocol-specific neurological imaging datasets. Neuroimage 2016, 124, 1220–1224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, S.; Utriainen, D.; Chai, C.; Chen, Y.; Wang, L.; Sethi, S.K.; Xia, S.; Haacke, E.M. Cerebral microbleed detection using Susceptibility Weighted Imaging and deep learning. Neuroimage 2019, 198, 271–282. [Google Scholar] [CrossRef]
- Gregoire, S.M.; Chaudhary, U.J.; Brown, M.M.; Yousry, T.A.; Kallis, C.; Jäger, H.R.; Werring, D.J. The Microbleed Anatomical Rating Scale (MARS). Neurology 2009, 73, 1759. [Google Scholar] [CrossRef]
- Haacke, E.M.; Makki, M.; Ge, Y.; Maheshwari, M.; Sehgal, V.; Hu, J.; Selvan, M.; Wu, Z.; Latif, Z.; Xuan, Y.; et al. Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J. Magn. Reson. Imaging 2009, 29, 537–544. [Google Scholar] [CrossRef]
- Williams, R.; Buchheit, C.L.; Berman, N.E.J.; LeVine, S.M. Pathogenic implications of iron accumulation in multiple sclerosis. J. Neurochem. 2012, 120, 7–25. [Google Scholar] [CrossRef]
- Jeon, S.B.; Parikh, G.; Choi, H.A.; Badjatia, N.; Lee, K.; Schmidt, J.M.; Lantigua, H.; Connolly, E.S.; Mayer, S.A.; Claassen, J. Cerebral microbleeds in patients with acute subarachnoid hemorrhage. Neurosurgery 2014, 74, 176–181, discussion 181. [Google Scholar] [CrossRef]
- Tsushima, Y.; Tanizaki, Y.; Aoki, J.; Endo, K. MR detection of microhemorrhages in neurologically healthy adults. Neuroradiology 2002, 44, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Jeerakathil, T.; Wolf, P.A.; Beiser, A.; Hald, J.K.; Au, R.; Kase, C.S.; Massaro, J.M.; DeCarli, C. Cerebral microbleeds: Prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke 2004, 35, 1831–1835. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Haacke, E.M.; DelProposto, Z.S.; Chaturvedi, S.; Sehgal, V.; Tenzer, M.; Neelavalli, J.; Kido, D. Imaging Cerebral Amyloid Angiopathy with Susceptibility-Weighted Imaging. Am. J. Neuroradiol. 2007, 28, 316. [Google Scholar] [PubMed]
- Buch, S.; Cheng, Y.-C.N.; Hu, J.; Liu, S.; Beaver, J.; Rajagovindan, R.; Haacke, E.M. Determination of detection sensitivity for cerebral microbleeds using susceptibility-weighted imaging. NMR Biomed. 2017, 30. [Google Scholar] [CrossRef]
- Goos, J.D.; Henneman, W.J.; Sluimer, J.D.; Vrenken, H.; Sluimer, I.C.; Barkhof, F.; Blankenstein, M.A.; Scheltens, P.H.; van der Flier, W.M. Incidence of cerebral microbleeds: A longitudinal study in a memory clinic population. Neurology 2010, 74, 1954–1960. [Google Scholar] [CrossRef]
- Gregoire Simone, M.; Brown Martin, M.; Kallis, C.; Jäger, H.R.; Yousry Tarek, A.; Werring David, J. MRI Detection of New Microbleeds in Patients With Ischemic Stroke. Stroke 2010, 41, 184–186. [Google Scholar] [CrossRef]
- Cordonnier, C.; van der Flier, W.M.; Sluimer, J.D.; Leys, D.; Barkhof, F.; Scheltens, P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology 2006, 66, 1356. [Google Scholar] [CrossRef]
- Ham, J.H.; Yi, H.; Sunwoo, M.K.; Hong, J.Y.; Sohn, Y.H.; Lee, P.H. Cerebral microbleeds in patients with Parkinson’s disease. J. Neurol. 2014, 261, 1628–1635. [Google Scholar] [CrossRef]
- Vernooij, M.W.; van der Lugt, A.; Ikram, M.A.; Wielopolski, P.A.; Niessen, W.J.; Hofman, A.; Krestin, G.P.; Breteler, M.M. Prevalence and risk factors of cerebral microbleeds: The Rotterdam Scan Study. Neurology 2008, 70, 1208–1214. [Google Scholar] [CrossRef]
- Trifan, G.; Gattu, R.; Haacke, E.M.; Kou, Z.; Benson, R.R. MR imaging findings in mild traumatic brain injury with persistent neurological impairment. Magn. Reson. Imaging 2017, 37, 243–251. [Google Scholar] [CrossRef] [PubMed]

| Sequence | T2WI | T2 FLAIR | 3D T1WI | 2D T1WI | SWI | SWI |
|---|---|---|---|---|---|---|
| Orientation | Axial | Sagittal | Axial | Sagittal | Axial | Axial |
| TR (ms) | 7080 | 6000 | 1750 | 306 | 30 | 30 |
| TE (ms) | 77 | 396 | 2.93 | 9.4 | 7/21 | 6/21 |
| FA (degrees) | 120 | 120 | 9 | 75 | 15 | 15 |
| FOV (mm2) | 256 × 192 | 256 × 256 | 256 × 256 | 229 × 229 | 224 × 168 | 256 × 192 |
| Matrix (Nx × Ny) | 512 × 192 | 258 × 256 | 512 × 256 | 384 × 326 | 448 × 336 | 512 × 384 |
| Resn (mm3) | 0.5 × 0.5 × 2 | 0.5 × 0.5 × 1 | 0.5 × 0.5 × 1 | 0.6 × 0.6 × 5 | 0.5 × 0.5 × 1 | 0.5 × 0.5 × 1.5 |
| Nz | 100 | 160 | 192 | 20 | 128 | 120 |
| Sequence | T2WI | T2 FLAIR | 3D T1WI | SWI |
|---|---|---|---|---|
| Orientation | Axial | Axial | Axial | Axial |
| TR (ms) | 5300 | 8500 | 5.9 | 40 |
| TE (ms) | 98 | 120 | 2.8 | 22 |
| FA (degrees) | 90 | 90 | 10 | 12 |
| FOV (mm2) | 256 × 192 | 256 × 192 | 256 × 192 | 256 × 192 |
| Matrix (Nx × Ny) | 256 × 192 | 256 × 192 | 256 × 192 | 512 × 192 |
| Resn (mm3) | 1 × 1 × 3 | 1 × 1 × 3 | 1 × 1 × 1 | 0.5 × 1 × 2 |
| Nz | 64 | 64 | 184 | 64 |
| Brain Regions | Number of CMBs in MS Subjects |
|---|---|
| Brainstem | 2 |
| Cerebellum | 3 |
| Frontal | 7 |
| Occipital | 3 |
| Parietal | 6 |
| Temporal | 4 |
| Basal ganglia | 1 |
| Deep periventricular white matter | 2 |
| Insula | 0 |
| Thalamus | 1 |
| Internal capsule | 0 |
| External capsule | 0 |
| Parameter | Total Patients with MS | Patients > 50 years | Patients < 50 years | |||
|---|---|---|---|---|---|---|
| Scan 1 | Scan 2 | Scan 1 | Scan 2 | Scan 1 | Scan 2 | |
| Patients with CMB | 16 | 20 | 9 | 13 | 7 | 7 |
| Demographics: | ||||||
| Mean age (years) | 49.3 | 52.3 | 56.6 | 57.7 | 44.9 | 42.9 |
| Disease duration (years) | 14.8 | 17.8 | 18.2 | 19.9 | 12.9 | 15 |
| Mean EDSS | 3.7 | 3.7 | 3.7 | 4.3 | 3.1 | 2.9 |
| CMB frequency: | ||||||
| 0 CMB | 5 | 1 * | 1 | 0 | 4 | 1 * |
| 1 CMB | 13 | 15 | 7 | 9 | 6 | 6 |
| 2 CMB | 2 | 4 | 1 | 3 | 1 | 1 |
| 3 CMB | 1 | 1 | 1 | 1 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanian, K.; Utriainen, D.; Ramasamy, D.P.; Sethi, S.K.; Schweser, F.; Beaver, J.; Hagemeier, J.; Weinstock-Guttman, B.; Rajagovindan, R.; Zivadinov, R.; et al. Longitudinal Magnetic Resonance Imaging of Cerebral Microbleeds in Multiple Sclerosis Patients. Diagnostics 2020, 10, 942. https://doi.org/10.3390/diagnostics10110942
Subramanian K, Utriainen D, Ramasamy DP, Sethi SK, Schweser F, Beaver J, Hagemeier J, Weinstock-Guttman B, Rajagovindan R, Zivadinov R, et al. Longitudinal Magnetic Resonance Imaging of Cerebral Microbleeds in Multiple Sclerosis Patients. Diagnostics. 2020; 10(11):942. https://doi.org/10.3390/diagnostics10110942
Chicago/Turabian StyleSubramanian, Karthikeyan, David Utriainen, Deepa P. Ramasamy, Sean K. Sethi, Ferdinand Schweser, John Beaver, Jesper Hagemeier, Bianca Weinstock-Guttman, Rajasimhan Rajagovindan, Robert Zivadinov, and et al. 2020. "Longitudinal Magnetic Resonance Imaging of Cerebral Microbleeds in Multiple Sclerosis Patients" Diagnostics 10, no. 11: 942. https://doi.org/10.3390/diagnostics10110942
APA StyleSubramanian, K., Utriainen, D., Ramasamy, D. P., Sethi, S. K., Schweser, F., Beaver, J., Hagemeier, J., Weinstock-Guttman, B., Rajagovindan, R., Zivadinov, R., & Haacke, E. M. (2020). Longitudinal Magnetic Resonance Imaging of Cerebral Microbleeds in Multiple Sclerosis Patients. Diagnostics, 10(11), 942. https://doi.org/10.3390/diagnostics10110942





