Role of Endoscopic Ultrasonography-Guided Fine Needle Aspiration/Biopsy in the Diagnosis of Autoimmune Pancreatitis
Abstract
1. Introduction
2. Materials and Methods
3. Results
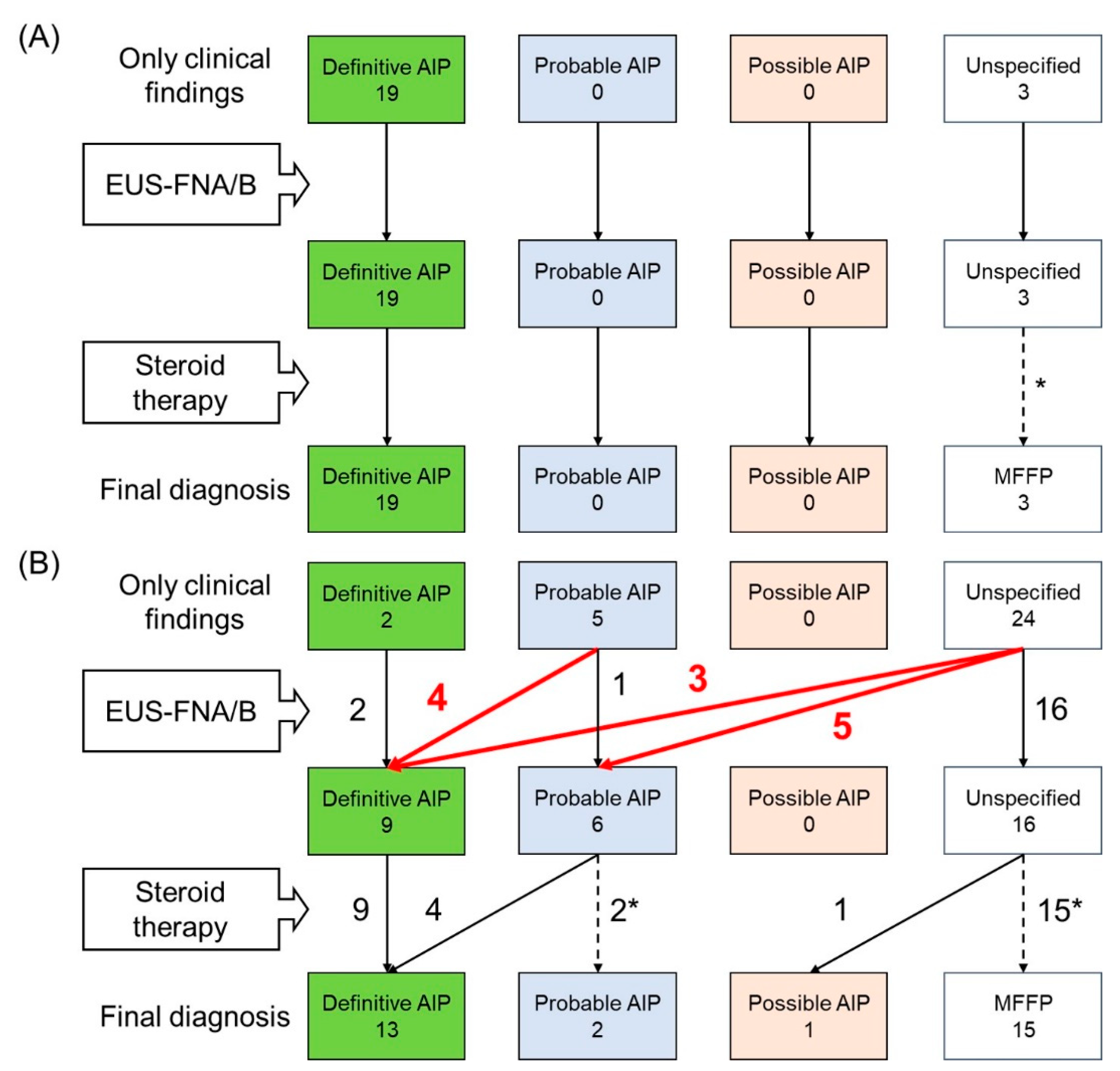
3.1. Patients Characteristics and Final Diagnosis
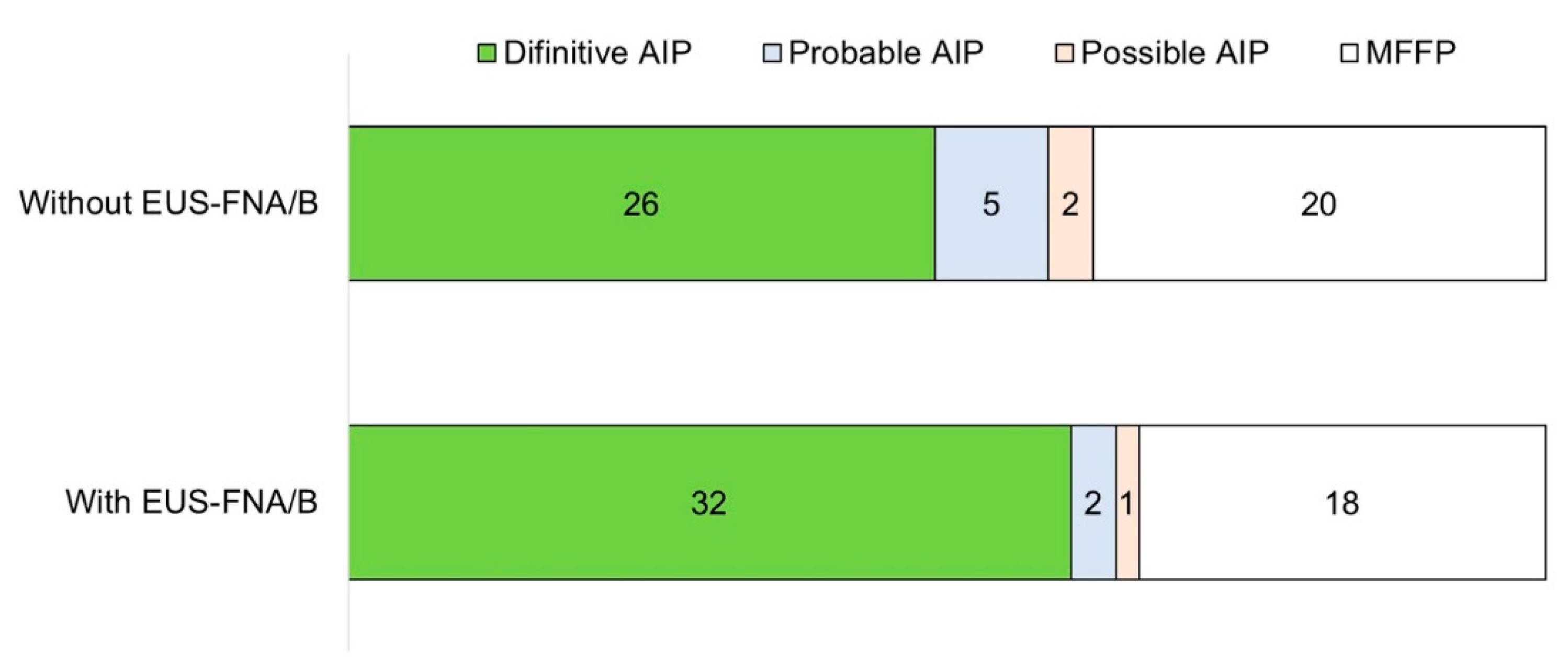
3.2. Contribution of EUS-FNA/B to Diagnosis of AIP
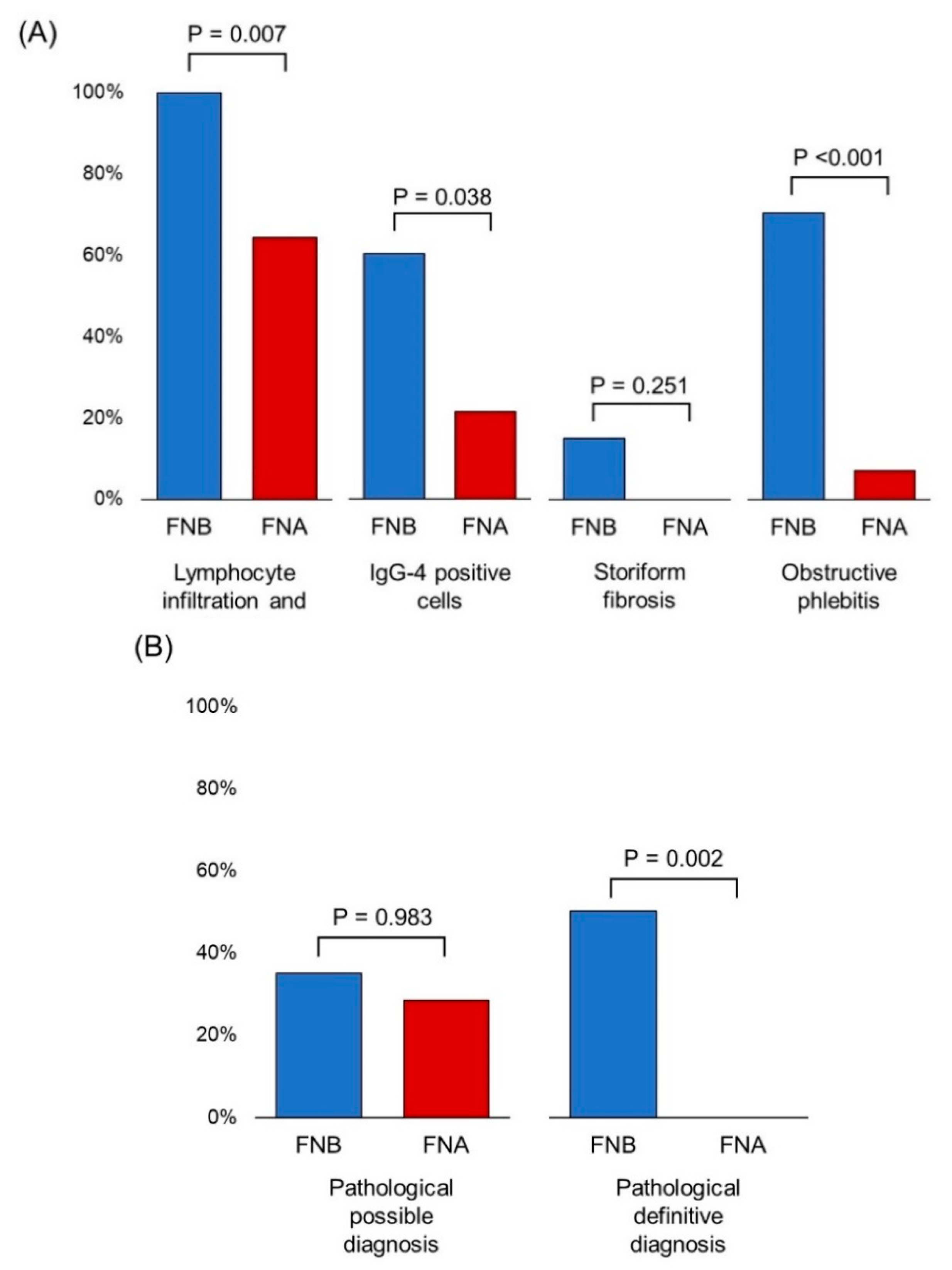
3.3. Comparison of the Diagnostic Yield of EUS-FNA/B
3.4. Adverse Events of EUS-FNA/B
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kamisawa, T.; Okazaki, K.; Kawa, S.; Ito, T.; Inui, K.; Irie, H.; Nishino, T.; Notohara, K.; Nishimori, I.; Tanaka, S.; et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 III. Treatment and prognosis of autoimmune pancreatitis. J. Gastroenterol. 2014, 49, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Kawa, S.; Okazaki, K.; Kamisawa, T.; Kubo, K.; Ohara, H.; Hasebe, O.; Fujinaga, Y.; Irisawa, A.; Notohara, K.; Ito, T.; et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 II. Extrapancreatic lesions, differential diagnosis. J. Gastroenterol. 2014, 49, 765–784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okazaki, K.; Kawa, S.; Kamisawa, T.; Ito, T.; Inui, K.; Irie, H.; Nishino, T.; Notohara, K.; Nishimori, I.; Tanaka, S.; et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 I. Concept and diagnosis of autoimmune pancreatitis. J. Gastroenterol. 2014, 49, 567–588. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Toki, F.; Takeuchi, T.; Watanabe, S.; Shiratori, K.; Hayashi, N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig. Dis. Sci. 1995, 40, 1561–1568. [Google Scholar] [CrossRef]
- Shimosegawa, T.; Chari, S.T.; Frulloni, L.; Kamisawa, T.; Kawa, S.; Mino-Kenudson, M.; Kim, M.H.; Kloppel, G.; Lerch, M.M.; Lohr, M.; et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas 2011, 40, 352–358. [Google Scholar] [CrossRef]
- Hirano, K.; Tada, M.; Isayama, H.; Sasahira, N.; Umefune, G.; Akiyama, D.; Watanabe, T.; Saito, T.; Takagi, K.; Takahara, N.; et al. Outcome of Long-term Maintenance Steroid Therapy Cessation in Patients With Autoimmune Pancreatitis: A Prospective Study. J. Clin. Gastroenterol. 2016, 50, 331–337. [Google Scholar] [CrossRef]
- Hirano, K.; Tada, M.; Isayama, H.; Yagioka, H.; Sasaki, T.; Kogure, H.; Nakai, Y.; Sasahira, N.; Tsujino, T.; Yoshida, H.; et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut 2007, 56, 1719–1724. [Google Scholar] [CrossRef]
- Kamisawa, T.; Okazaki, K.; Kawa, S.; Shimosegawa, T.; Tanaka, M.; Society, R.C.f.I.P.D.a.J.P. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J. Gastroenterol. 2010, 45, 471–477. [Google Scholar] [CrossRef]
- Hirano, K.; Tada, M.; Sasahira, N.; Tsujino, T.; Isayama, H.; Kawabe, T.; Omata, M. Are all pancreatic lesions responsive to steroid therapy [corrected] autoimmune pancreatitis? Gut 2009, 58, 1031–1032; author reply 1032. [Google Scholar]
- Lohr, J.M.; Beuers, U.; Vujasinovic, M.; Alvaro, D.; Frokjaer, J.B.; Buttgereit, F.; Capurso, G.; Culver, E.L.; de-Madaria, E.; Della-Torre, E.; et al. European Guideline on IgG4-related digestive disease—UEG and SGF evidence-based recommendations. United Eur. Gastroenterol. J. 2020, 8, 637–666. [Google Scholar] [CrossRef]
- Hewitt, M.J.; McPhail, M.J.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest. Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Volmar, K.E.; Vollmer, R.T.; Jowell, P.S.; Nelson, R.C.; Xie, H.B. Pancreatic FNA in 1000 cases: A comparison of imaging modalities. Gastrointest. Endosc. 2005, 61, 854–861. [Google Scholar] [CrossRef]
- Williams, D.B.; Sahai, A.V.; Aabakken, L.; Penman, I.D.; van Velse, A.; Webb, J.; Wilson, M.; Hoffman, B.J.; Hawes, R.H. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experience. Gut 1999, 44, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.L.; Kalade, A.; Prasad, S.; Cade, R.; Thomson, B.; Banting, S.; Mackay, S.; Desmond, P.V.; Chen, R.Y. Diagnosis of solid pancreatic masses by endoscopic ultrasound-guided fine-needle aspiration. Intern. Med. J. 2009, 39, 32–37. [Google Scholar] [CrossRef]
- Abdelfatah, M.M.; Grimm, I.S.; Gangarosa, L.M.; Baron, T.H. Cohort study comparing the diagnostic yields of 2 different EUS fine-needle biopsy needles. Gastrointest. Endosc. 2018, 87, 495–500. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hawes, R.; Varadarajulu, S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy 2016, 48, 339–349. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hebert-Magee, S.; Hasan, M.K.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig. Endosc. 2017, 29, 338–346. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hebert-Magee, S.; Navaneethan, U.; Hasan, M.K.; Hawes, R.; Varadarajulu, S. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest. Endosc. 2018, 87, 1432–1438. [Google Scholar] [CrossRef]
- Ishigaki, K.; Nakai, Y.; Oyama, H.; Kanai, S.; Suzuki, T.; Nakamura, T.; Sato, T.; Hakuta, R.; Saito, K.; Saito, T.; et al. Endoscopic Ultrasound-Guided Tissue Acquisition by 22-Gauge Franseen and Standard Needles for Solid Pancreatic Lesions. Gut Liver 2020. [Google Scholar] [CrossRef]
- Iwashita, T.; Nakai, Y.; Mukai, T.; Togawa, O.; Matsubara, S.; Hatano, Y.; Hara, A.; Tanaka, M.; Shibahara, J.; Fukayama, M.; et al. A 19-Gauge Histology Needle Versus a 19-Gauge Standard Needle in Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Solid Lesions: A Multicenter Randomized Comparison Study (GREATER Study). Dig. Dis. Sci. 2018, 63, 1043–1051. [Google Scholar] [CrossRef]
- Kandel, P.; Tranesh, G.; Nassar, A.; Bingham, R.; Raimondo, M.; Woodward, T.A.; Gomez, V.; Wallace, M.B. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: A case-control study. Gastrointest. Endosc. 2016, 84, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Nayar, M.K.; Paranandi, B.; Dawwas, M.F.; Leeds, J.S.; Darne, A.; Haugk, B.; Majumdar, D.; Ahmed, M.M.; Oppong, K.W. Comparison of the diagnostic performance of 2 core biopsy needles for EUS-guided tissue acquisition from solid pancreatic lesions. Gastrointest. Endosc. 2017, 85, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, N.; Ogura, T.; Kurisu, Y.; Imanishi, M.; Onda, S.; Takagi, W.; Sano, T.; Okuda, A.; Miyano, A.; Amano, M.; et al. Prospective histological evaluation of a 20G core trap with a forward-cutting bevel needle for EUS-FNA of pancreatic lesions. Surg. Endosc. 2018, 32, 4125–4131. [Google Scholar] [CrossRef] [PubMed]
- van Riet, P.A.; Larghi, A.; Attili, F.; Rindi, G.; Nguyen, N.Q.; Ruszkiewicz, A.; Kitano, M.; Chikugo, T.; Aslanian, H.; Farrell, J.; et al. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest. Endosc. 2019, 89, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Cruise, M.; Chahal, P. Endoscopic ultrasound guided 22 gauge core needle biopsy for the diagnosis of Autoimmune pancreatitis. Pancreatology 2018, 18, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, Y.; Wang, J.; Guo, Q.; Chen, Q.; Wu, X.; Tang, S.J.; Cheng, B. The role of EUS-guided fine needle aspiration in autoimmune pancreatitis: A single center prospective study. Scand. J. Gastroenterol. 2018, 53, 1604–1610. [Google Scholar] [CrossRef]
- Imai, K.; Matsubayashi, H.; Fukutomi, A.; Uesaka, K.; Sasaki, K.; Ono, H. Endoscopic ultrasonography-guided fine needle aspiration biopsy using 22-gauge needle in diagnosis of autoimmune pancreatitis. Dig. Liver Dis. 2011, 43, 869–874. [Google Scholar] [CrossRef]
- Ishikawa, T.; Itoh, A.; Kawashima, H.; Ohno, E.; Matsubara, H.; Itoh, Y.; Nakamura, Y.; Hiramatsu, T.; Nakamura, M.; Miyahara, R.; et al. Endoscopic ultrasound-guided fine needle aspiration in the differentiation of type 1 and type 2 autoimmune pancreatitis. World J. Gastroenterol. 2012, 18, 3883–3888. [Google Scholar] [CrossRef]
- Iwashita, T.; Yasuda, I.; Doi, S.; Ando, N.; Nakashima, M.; Adachi, S.; Hirose, Y.; Mukai, T.; Iwata, K.; Tomita, E.; et al. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin. Gastroenterol. Hepatol. 2012, 10, 316–322. [Google Scholar] [CrossRef]
- Kanno, A.; Ishida, K.; Hamada, S.; Fujishima, F.; Unno, J.; Kume, K.; Kikuta, K.; Hirota, M.; Masamune, A.; Satoh, K.; et al. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest. Endosc. 2012, 76, 594–602. [Google Scholar] [CrossRef]
- Kanno, A.; Masamune, A.; Fujishima, F.; Iwashita, T.; Kodama, Y.; Katanuma, A.; Ohara, H.; Kitano, M.; Inoue, H.; Itoi, T.; et al. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: A prospective multicenter study. Gastrointest. Endosc. 2016, 84, 797–804.e791. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Shimosegawa, T. Endoscopic approaches for the diagnosis of autoimmune pancreatitis. Dig. Endosc. 2015, 27, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Bhatia, V.; Hosoda, W.; Sawaki, A.; Hoki, N.; Hara, K.; Takagi, T.; Ko, S.B.; Yatabe, Y.; Goto, H.; et al. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: A comparison study with EUS-FNA. J. Gastroenterol. 2009, 44, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Morishima, T.; Kawashima, H.; Ohno, E.; Yamamura, T.; Funasaka, K.; Nakamura, M.; Miyahara, R.; Watanabe, O.; Ishigami, M.; Shimoyama, Y.; et al. Prospective multicenter study on the usefulness of EUS-guided FNA biopsy for the diagnosis of autoimmune pancreatitis. Gastrointest. Endosc. 2016, 84, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Takagi, T.; Suzuki, R.; Konno, N.; Asama, H.; Watanabe, K.; Nakamura, J.; Kikuchi, H.; Waragai, Y.; Takasumi, M.; et al. Endoscopic Ultrasonography-Guided Fine Needle Aspiration Can Be Used to Rule Out Malignancy in Autoimmune Pancreatitis Patients. J. Ultrasound. Med. 2017, 36, 2237–2244. [Google Scholar] [CrossRef]
- Kurita, A.; Yasukawa, S.; Zen, Y.; Yoshimura, K.; Ogura, T.; Ozawa, E.; Okabe, Y.; Asada, M.; Nebiki, H.; Shigekawa, M.; et al. Comparison of a 22-gauge Franseen-tip needle with a 20-gauge forward-bevel needle for the diagnosis of type 1 autoimmune pancreatitis: A prospective, randomized, controlled, multicenter study (COMPAS study). Gastrointest. Endosc. 2020, 91, 373–381.e372. [Google Scholar] [CrossRef] [PubMed]
- Kawa, S.; Kamisawa, T.; Notohara, K.; Fujinaga, Y.; Inoue, D.; Koyama, T.; Okazaki, K. Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2018: Revision of Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2011. Pancreas 2020, 49, e13–e14. [Google Scholar] [CrossRef]
- Notohara, K.; Kamisawa, T.; Fukushima, N.; Furukawa, T.; Tajiri, T.; Yamaguchi, H.; Aishima, S.; Fukumura, Y.; Hirabayashi, K.; Iwasaki, E.; et al. Guidance for diagnosing autoimmune pancreatitis with biopsy tissues. Pathol. Int. 2020, 70, 699–711. [Google Scholar] [CrossRef]
- Barresi, L.; Tacelli, M.; Crino, S.F.; Attili, F.; Petrone, M.C.; De Nucci, G.; Carrara, S.; Manfredi, G.; Capurso, G.; De Angelis, C.G.; et al. Multicentric Italian survey on daily practice for autoimmune pancreatitis: Clinical data, diagnosis, treatment, and evolution toward pancreatic insufficiency. United Eur. Gastroenterol. J. 2020, 8, 705–715. [Google Scholar] [CrossRef]
- Notohara, K.; Kamisawa, T.; Kanno, A.; Naitoh, I.; Iwasaki, E.; Shimizu, K.; Kuraishi, Y.; Motoya, M.; Kodama, Y.; Kasashima, S.; et al. Efficacy and limitations of the histological diagnosis of type 1 autoimmune pancreatitis with endoscopic ultrasound-guided fine needle biopsy with large tissue amounts. Pancreatology 2020, 20, 834–843. [Google Scholar] [CrossRef]
- Laquiere, A.; Lefort, C.; Maire, F.; Aubert, A.; Gincul, R.; Prat, F.; Grandval, P.; Croizet, O.; Boulant, J.; Vanbiervliet, G.; et al. 19 G nitinol needle versus 22 G needle for transduodenal endoscopic ultrasound-guided sampling of pancreatic solid masses: A randomized study. Endoscopy 2019, 51, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Polkowski, M.; Jenssen, C.; Kaye, P.; Carrara, S.; Deprez, P.; Gines, A.; Fernandez-Esparrach, G.; Eisendrath, P.; Aithal, G.P.; Arcidiacono, P.; et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline—March 2017. Endoscopy 2017, 49, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Crino, S.F.; Le Grazie, M.; Manfrin, E.; Conti Bellocchi, M.C.; Bernardoni, L.; Granato, A.; Locatelli, F.; Parisi, A.; Di Stefano, S.; Frulloni, L.; et al. Randomized trial comparing fork-tip and side-fenestrated needles for EUS-guided fine-needle biopsy of solid pancreatic lesions. Gastrointest. Endosc. 2020, 92, 648–658.e2. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.N.; Moon, J.H.; Kim, H.K.; Choi, H.J.; Choi, M.H.; Kim, D.C.; Lee, T.H.; Cha, S.W.; Cho, Y.D.; Park, S.H. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: A randomized parallel-group study. Endoscopy 2014, 46, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
| n = 53 | |
|---|---|
| Sex: male | 30 (56.6%) |
| Age * (year) | 68 (17–83) |
| Enlargement of the pancreas: diffuse/focal | 22/31 (41.5%/58.5%) |
| Elevated levels of serum IgG4 (≥ 135 mg/dl) | 33 (62.3%) |
| Other organ involvement | 21 (40.0%) |
| Locus of the target of EUS-FNA/B | |
| Head | 14 (26.4%) |
| Body | 25 (47.2%) |
| Tail | 14 (26.4%) |
| Diameter of the lesion * (mm) | 25 (20–36) |
| Needles: FNA/FNB | 21/32 (39.6%/60.4%) |
| Final diagnosis | |
| Definitive AIP | 32 (60.4%) |
| Probable AIP | 2 (3.8%) |
| Possible AIP | 1 (1.9%) |
| Mass-forming focal pancreatitis | 18 (34.0%) |
| Enlargement of the Pancreas | Needle | Adverse Event | Diagnosis without EUS-FNA/B | Diagnosis with EUS-FNA/B | |
|---|---|---|---|---|---|
| 1 | Focal | FNB 22-gauge Franseen | Pancreatic fistula | Unspecified diagnosis | MFFP |
| 2 | Focal | FNB 22-gauge Franseen | Pancreatic fistula | Unspecified diagnosis | Probable AIP |
| 3 | Focal | FNB 20-gauge forward-bevel | Mild pancreatitis | Unspecified diagnosis | MFFP |
| 4 | Focal | FNB 20-gauge forward-bevel | Mild pancreatitis | Possible AIP | Definitive AIP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, K.; Nakai, Y.; Mizuno, S.; Hirano, K.; Kanai, S.; Suzuki, Y.; Inokuma, A.; Sato, T.; Hakuta, R.; Ishigaki, K.; et al. Role of Endoscopic Ultrasonography-Guided Fine Needle Aspiration/Biopsy in the Diagnosis of Autoimmune Pancreatitis. Diagnostics 2020, 10, 954. https://doi.org/10.3390/diagnostics10110954
Noguchi K, Nakai Y, Mizuno S, Hirano K, Kanai S, Suzuki Y, Inokuma A, Sato T, Hakuta R, Ishigaki K, et al. Role of Endoscopic Ultrasonography-Guided Fine Needle Aspiration/Biopsy in the Diagnosis of Autoimmune Pancreatitis. Diagnostics. 2020; 10(11):954. https://doi.org/10.3390/diagnostics10110954
Chicago/Turabian StyleNoguchi, Kensaku, Yousuke Nakai, Suguru Mizuno, Kenji Hirano, Sachiko Kanai, Yukari Suzuki, Akiyuki Inokuma, Tatsuya Sato, Ryunosuke Hakuta, Kazunaga Ishigaki, and et al. 2020. "Role of Endoscopic Ultrasonography-Guided Fine Needle Aspiration/Biopsy in the Diagnosis of Autoimmune Pancreatitis" Diagnostics 10, no. 11: 954. https://doi.org/10.3390/diagnostics10110954
APA StyleNoguchi, K., Nakai, Y., Mizuno, S., Hirano, K., Kanai, S., Suzuki, Y., Inokuma, A., Sato, T., Hakuta, R., Ishigaki, K., Saito, K., Saito, T., Hamada, T., Takahara, N., Kogure, H., Isayama, H., & Koike, K. (2020). Role of Endoscopic Ultrasonography-Guided Fine Needle Aspiration/Biopsy in the Diagnosis of Autoimmune Pancreatitis. Diagnostics, 10(11), 954. https://doi.org/10.3390/diagnostics10110954






