Routine Fluorescence Imaging to Detect Wound Bacteria Reduces Antibiotic Use and Antimicrobial Dressing Expenditure While Improving Healing Rates: Retrospective Analysis of 229 Foot Ulcers
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard of Care
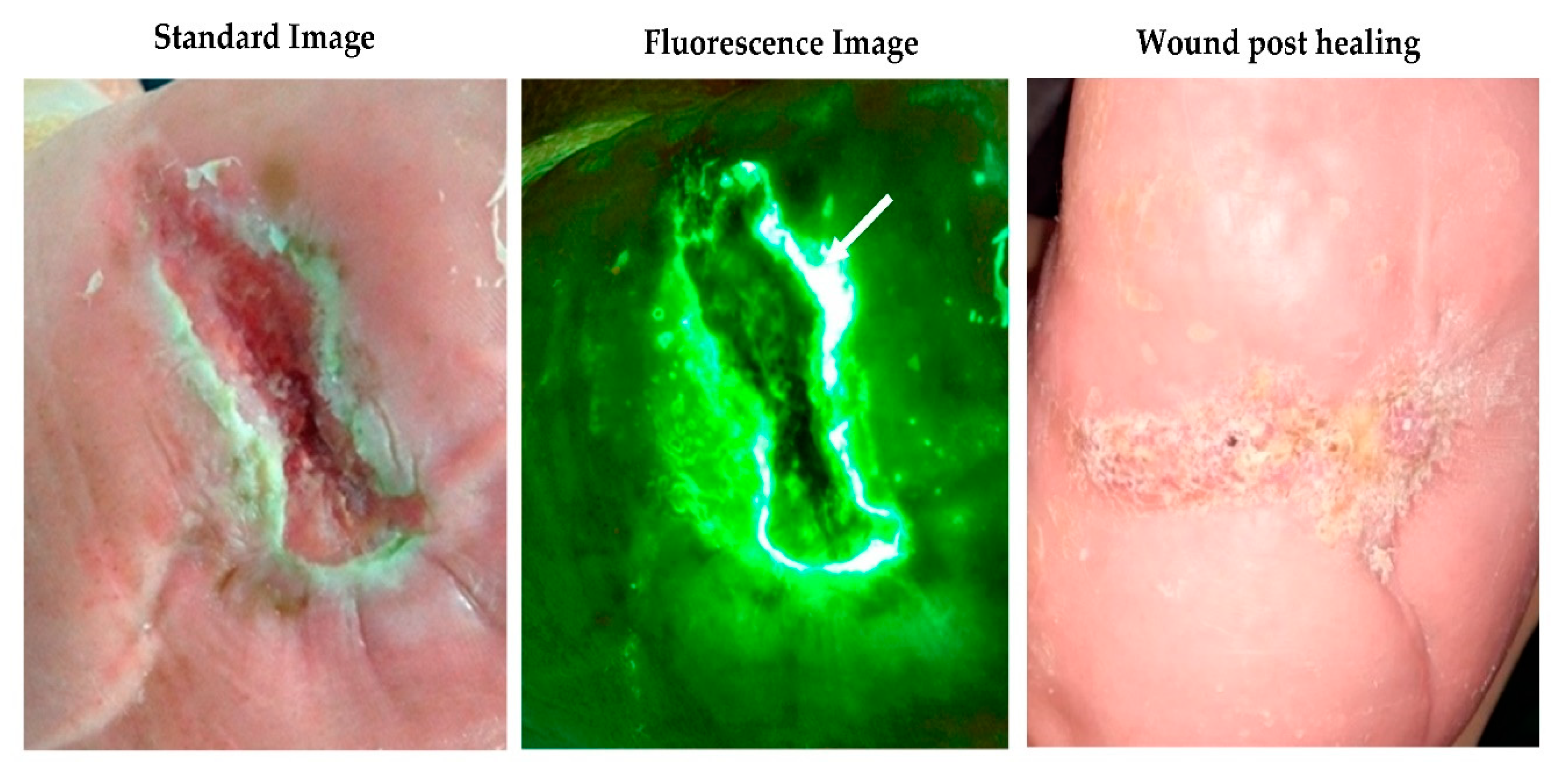
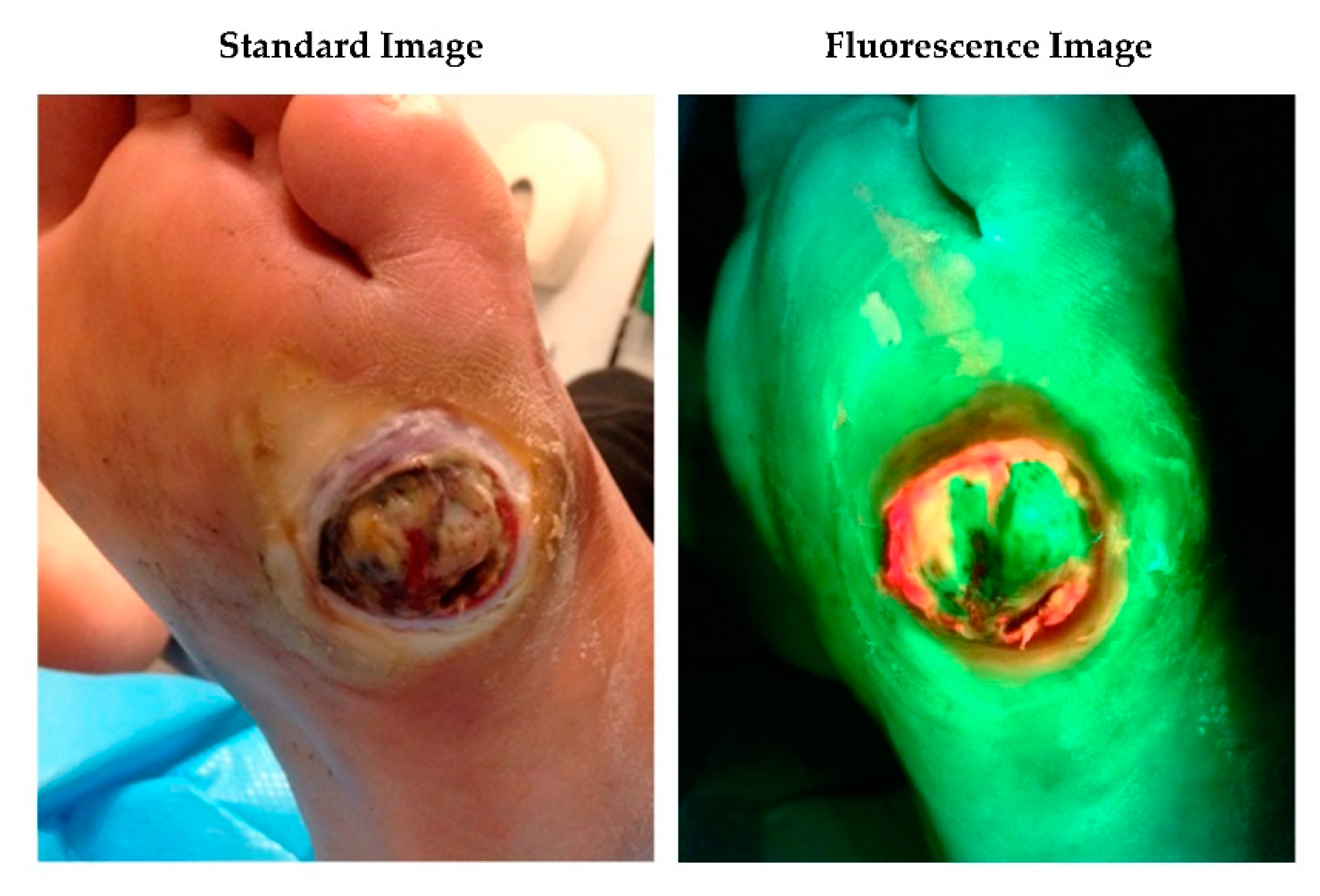
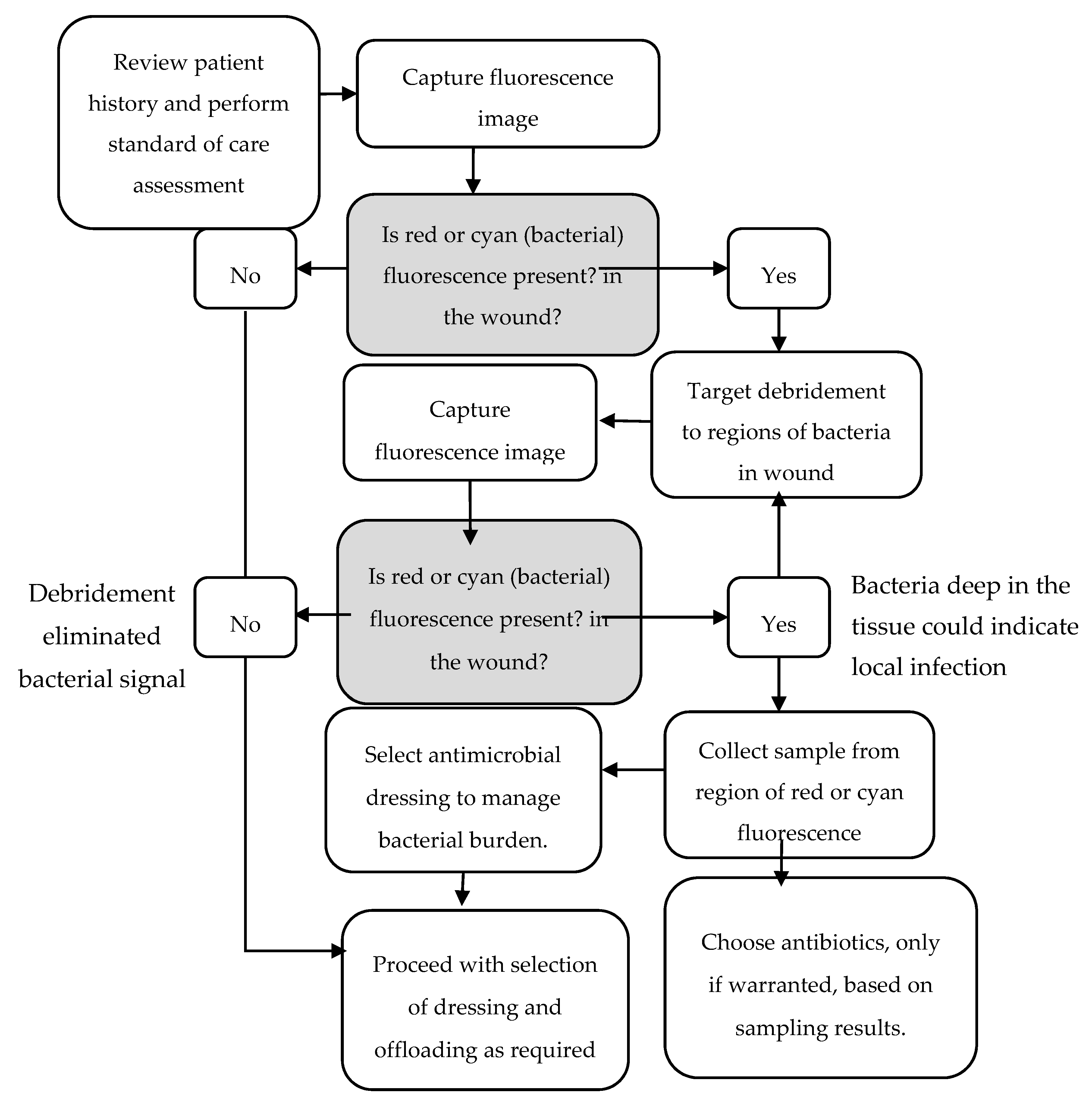
2.2. Fluorescence Imaging Procedure
2.3. Extraction of Data on Antimicrobial Dressing Use and Cost Analysis
3. Results
3.1. Patient Demographics and Throughput
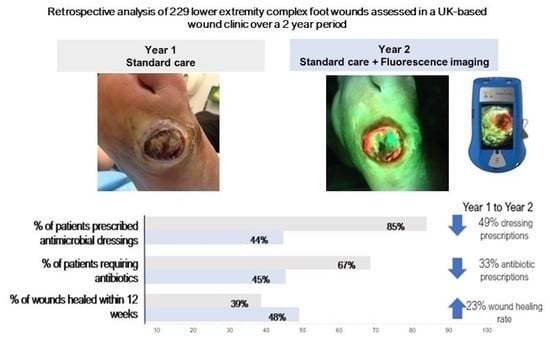
3.2. Antimicrobial and Antibiotic Usage and 12-Week Healing Rates
3.3. Antimicrobial Expenditure
3.4. Estimating Impact of Healing Rates on Total Wound Care Expenditure
4. Discussion
5. Strengths and Weaknesses
6. Conclusions
Funding
Conflicts of Interest
References
- Lavery, L.A.; Armstrong, D.G.; Wunderlich, R.P.; Mohler, M.J.; Wendel, C.S.; Lipsky, B.A. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006, 29, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, P.; Kelly, L.; Hurley, L.; McIntosh, C.; Dinneen, S. The effect of foot ulcers on costs of care for people with diabetes in Ireland. Diabet. Foot J. 2015, 3, 107–112. [Google Scholar]
- Kumar, S.; Ashe, H.; Parnell, L.; Fernando, D.; Tsigos, C.; Young, R.; Ward, J.; Boulton, A. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: A population-based study. Diabet. Med. 1994, 11, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Jupiter, D.C.; Thorud, J.C.; Buckley, C.J.; Shibuya, N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int. Wound J. 2016, 13, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.; Barron, E.; Chadwick, P.; Evans, T.; Kong, W.M.; Rayman, G.; Sutton-Smith, M.; Todd, G.; Young, B.; Jeffcoate, W.J. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet. Med. 2019, 36, 995–1002. [Google Scholar] [CrossRef]
- Kerr, M. Cost of Diabetic Foot Disease in England. Foot Diabet. 2020, 17–29. [Google Scholar]
- Guest, J.F.; Fuller, G.W.; Vowden, P. Diabetic foot ulcer management in clinical practice in the UK: Costs and outcomes. Int. Wound J. 2018, 15, 43–52. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Chronic Wounds: Advanced Wound Dressings and Antimicrobial Dressings; National Institute for Health and Care Excellence: London, UK, 2016. [Google Scholar]
- Lipsky, B.A.; Senneville, E.; Abbas, Z.G.; Aragon-Sanchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; van Asten, S.A.; et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020, 36 (Suppl. 1), e3280. [Google Scholar] [CrossRef]
- Le, L.; Baer, M.; Briggs, P.; Bullock, N.; Cole, W.; DiMarco, D.; Hamil, R.; Harrell, K.; Kasper, M.; Li, W.; et al. Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Adv. Wound Care 2020. [Google Scholar] [CrossRef]
- Jones, R.E.; Foster, D.S.; Longaker, M.T. Management of Chronic Wounds—2018. JAMA 2018, 320, 1481–1482. [Google Scholar] [CrossRef]
- Pagnamenta, F. Evidence generation for wound care dressing selection: Reviewing the issues. J. Wound Care 2017, 26, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Best Practice Statement: Improving Holistic Assessment of Chronic Wounds; Wounds UK: London, UK, 2018.
- Rennie, M.Y.; Lindvere-Teene, L.; Tapang, K.; Linden, R. Point-of-care fluorescence imaging predicts the presence of pathogenic bacteria in wounds: A clinical study. J. Wound Care 2017, 26, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, E.L.; Jeffery, S.L.A. Efficacy of an imaging device at identifying the presence of bacteria in wounds at a plastic surgery outpatients clinic. J. Wound Care 2018, 27, 20–26. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Healthcare and Clinical Excellence, NICE. Diabetic Foot Problems: Prevention and Management (NG19); NICE: London, UK, 2019. [Google Scholar]
- Jones, L.M.; Dunham, D.; Rennie, M.Y.; Kirman, J.; Lopez, A.J.; Keim, K.C.; Little, W.; Gomez, A.; Bourke, J.; Ng, H.; et al. In vitro detection of porphyrin-producing wound bacteria with real-time fluorescence imaging. Future Microbiol. 2020, 15, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.Y.; Dunham, D.; Lindvere-Teene, L.; Raizman, R.; Hill, R.; Linden, R. Understanding Real-Time Fluorescence Signals from Bacteria and Wound Tissues Observed with the MolecuLight i:X(TM). Diagnostics 2019, 9, 22. [Google Scholar] [CrossRef]
- Serena, T.E.; Harrell, K.; Serena, L.; Yaakov, R.A. Real-time bacterial fluorescence imaging accurately identifies wounds with moderate-to-heavy bacterial burden. J. Wound Care 2019, 28, 346–357. [Google Scholar] [CrossRef]
- Hussey, L.; Stocks, S.J.; Wilson, P.; Dumville, J.C.; Cullum, N. Use of antimicrobial dressings in England and the association with published clinical guidance: Interrupted time series analysis. BMJ Open 2019, 9, e028727. [Google Scholar] [CrossRef]
- Caldwell, M.D. Bacteria and Antibiotics in Wound Healing. Surg. Clin. N. Am. 2020, 100, 757–776. [Google Scholar] [CrossRef]
- Xu, L.; McLennan, S.V.; Lo, L.; Natfaji, A.; Bolton, T.; Liu, Y.; Twigg, S.M.; Yue, D.K. Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care 2007, 30, 378–380. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Dryden, M.; Gottrup, F.; Nathwani, D.; Seaton, R.A.; Stryja, J. Antimicrobial stewardship in wound care: A Position Paper from the British Society for Antimicrobial Chemotherapy and European Wound Management Association. J. Antimicrob. Chemother 2016, 71, 3026–3035. [Google Scholar] [CrossRef]
- Wilcox, J.R.; Carter, M.J.; Covington, S. Frequency of Debridements and Time to Heal: A Retrospective Cohort Study of 312 744 Wounds. JAMA Dermatol. 2013, 149, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Hurlow, J.; Blanz, E.; Gaddy, J.A. Clinical investigation of biofilm in non-healing wounds by high resolution microscopy techniques. J. Wound Care 2016, 25 (Suppl. 9), S11–S22. [Google Scholar] [CrossRef]
- Attinger, C.; Wolcott, R. Clinically Addressing Biofilm in Chronic Wounds. Adv. Wound Care (New Rochelle) 2012, 1, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.; Coe, S. Use of a bacterial fluorescence imaging system to target wound debridement and accelerate healing: A pilot study. J. Wound Care 2020, 29, S44–S52. [Google Scholar] [CrossRef] [PubMed]
- IWII. Wound Infection in Clinical Practice; International Wound Infection Institute, Wounds International: Londin, UK, 2016. [Google Scholar]
- Wu, S.C.; Driver, V.R.; Wrobel, J.S.; Armstrong, D.G. Foot ulcers in the diabetic patient, prevention and treatment. Vasc. Health Risk Manag. 2007, 3, 65–76. [Google Scholar] [PubMed]
- Gardner, S.E.; Hillis, S.L.; Frantz, R.A. Clinical signs of infection in diabetic foot ulcers with high microbial load. Biol. Res. Nurs. 2009, 11, 119–128. [Google Scholar] [CrossRef]
- Morley, G.L.; Wacogne, I.D. UK recommendations for combating antimicrobial resistance: A review of ‘antimicrobial stewardship: Systems and processes for effective antimicrobial medicine use’ (NICE guideline NG15, 2015) and related guidance. Arch. Dis. Child. Educ. Pract. Ed. 2018, 103, 46–49. [Google Scholar] [CrossRef]
- Fishman, N. Policy Statement on Antimicrobial Stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect. Control. Hosp. Epidemiol. 2012, 33, 322–327. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Blumenthal, E.; Jeffery, S.L.A. The Use of the MolecuLight i:X in Managing Burns: A Pilot Study. J. Burn Care Res. 2018, 39, 154–161. [Google Scholar]
- Farhan, N.; Jeffery, S. Utility of MolecuLight i:X for managing bacterial burden in paediatric burns. J. Burn Care Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Healthcare and Clinical Excellence, NICE. MolecuLight i:X for Wound Imaging; NICE: London, UK, 2020. [Google Scholar]
|
| Year 1 12 Months Prior to Fluorescence Imaging of Bacteria (2018/2019) | Year 2 12 Months Post-Fluorescence Imaging of Bacteria (2019/2020) | % Change | |
|---|---|---|---|
| Patient demographics | |||
| Average patient age | 58.4 | 60.1 | |
| Male/Female | 51/21 | 50/27 | |
| % of patients with diabetes | 92% | 83% | |
| # of wounds seen in clinic | 101 | 128 | +27% |
| # of patients | 72 | 77 | +7% |
| Outcomes and expenditures | |||
| Antimicrobial dressing expenditure | £2,291 | £1531 | −33% |
| Antimicrobial spending normalised to # of wounds | £22.68 | £11.96 | −47% |
| % of wounds healed within 12 weeks | 39% | 48% | +23% |
| % of patients who required antibiotics for their wound | 67% | 45% | −33% |
| % of patients prescribed antimicrobial dressings | 85% | 44% | −49% |
| % of patients requiring an amputation | 18% | 16% | −11% |
| Healed Foot Ulcer | Amputated Foot Ulcer | Unhealed Foot Ulcer | Total Cost of Wound Care (Annual per Patient) | |
|---|---|---|---|---|
| Estimated cost [7] | £2138 | £16,941 | £8786 | |
| Year 1 standard of care | 39% | 18% | 43% | £7662 |
| Year 2 standard of care + fluorescence imaging | 48% | 16% | 36% | £6900 |
| Estimated cost savings per patient | £762 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Price, N. Routine Fluorescence Imaging to Detect Wound Bacteria Reduces Antibiotic Use and Antimicrobial Dressing Expenditure While Improving Healing Rates: Retrospective Analysis of 229 Foot Ulcers. Diagnostics 2020, 10, 927. https://doi.org/10.3390/diagnostics10110927
Price N. Routine Fluorescence Imaging to Detect Wound Bacteria Reduces Antibiotic Use and Antimicrobial Dressing Expenditure While Improving Healing Rates: Retrospective Analysis of 229 Foot Ulcers. Diagnostics. 2020; 10(11):927. https://doi.org/10.3390/diagnostics10110927
Chicago/Turabian StylePrice, Nadine. 2020. "Routine Fluorescence Imaging to Detect Wound Bacteria Reduces Antibiotic Use and Antimicrobial Dressing Expenditure While Improving Healing Rates: Retrospective Analysis of 229 Foot Ulcers" Diagnostics 10, no. 11: 927. https://doi.org/10.3390/diagnostics10110927
APA StylePrice, N. (2020). Routine Fluorescence Imaging to Detect Wound Bacteria Reduces Antibiotic Use and Antimicrobial Dressing Expenditure While Improving Healing Rates: Retrospective Analysis of 229 Foot Ulcers. Diagnostics, 10(11), 927. https://doi.org/10.3390/diagnostics10110927