Dynamic Enhancement Pattern on CT for Predicting Pancreatic Neuroendocrine Neoplasms with Low PAX6 Expression: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
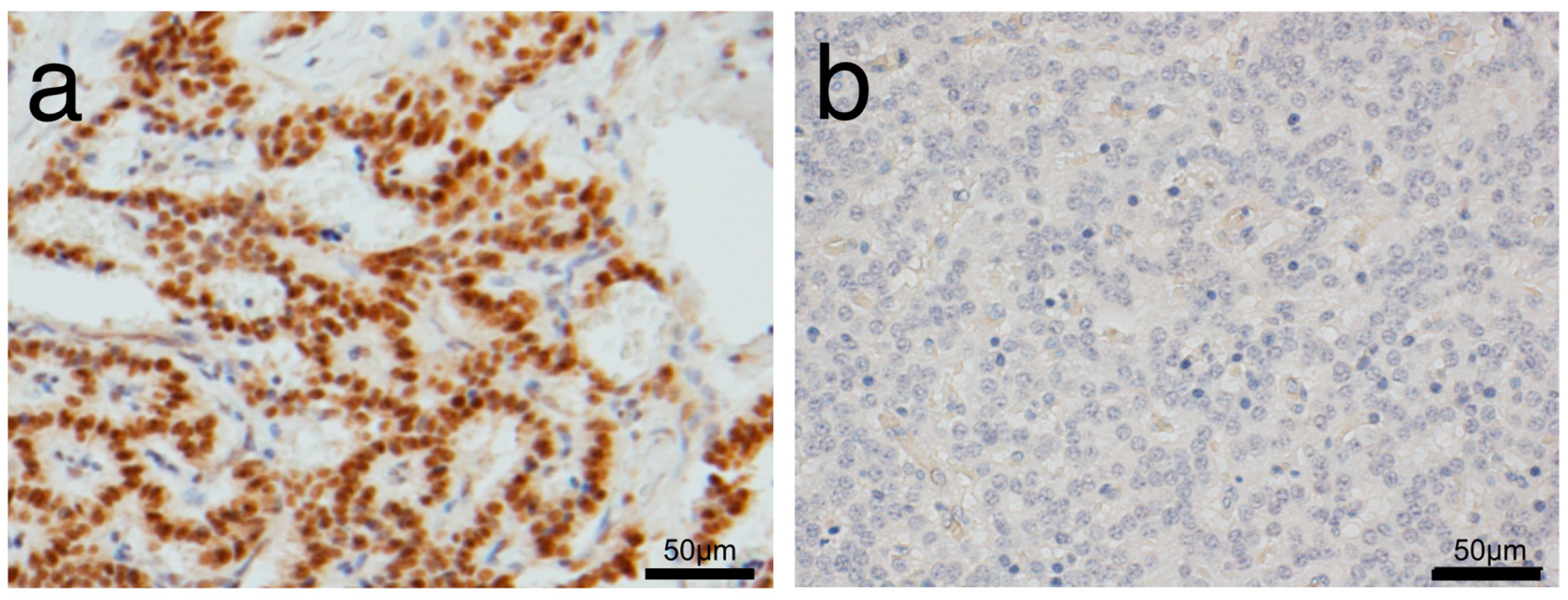
2.2. Pathological Analysis
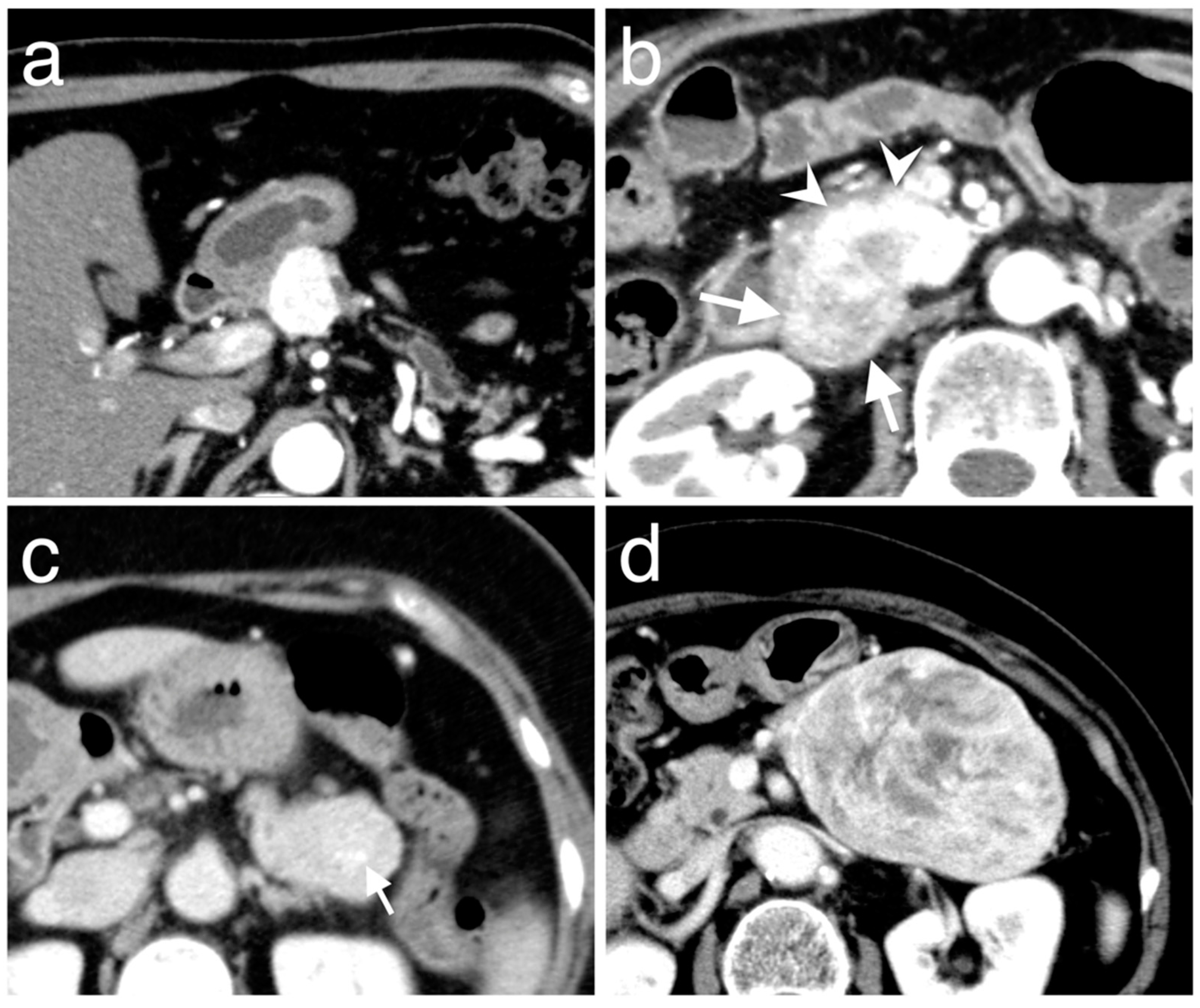
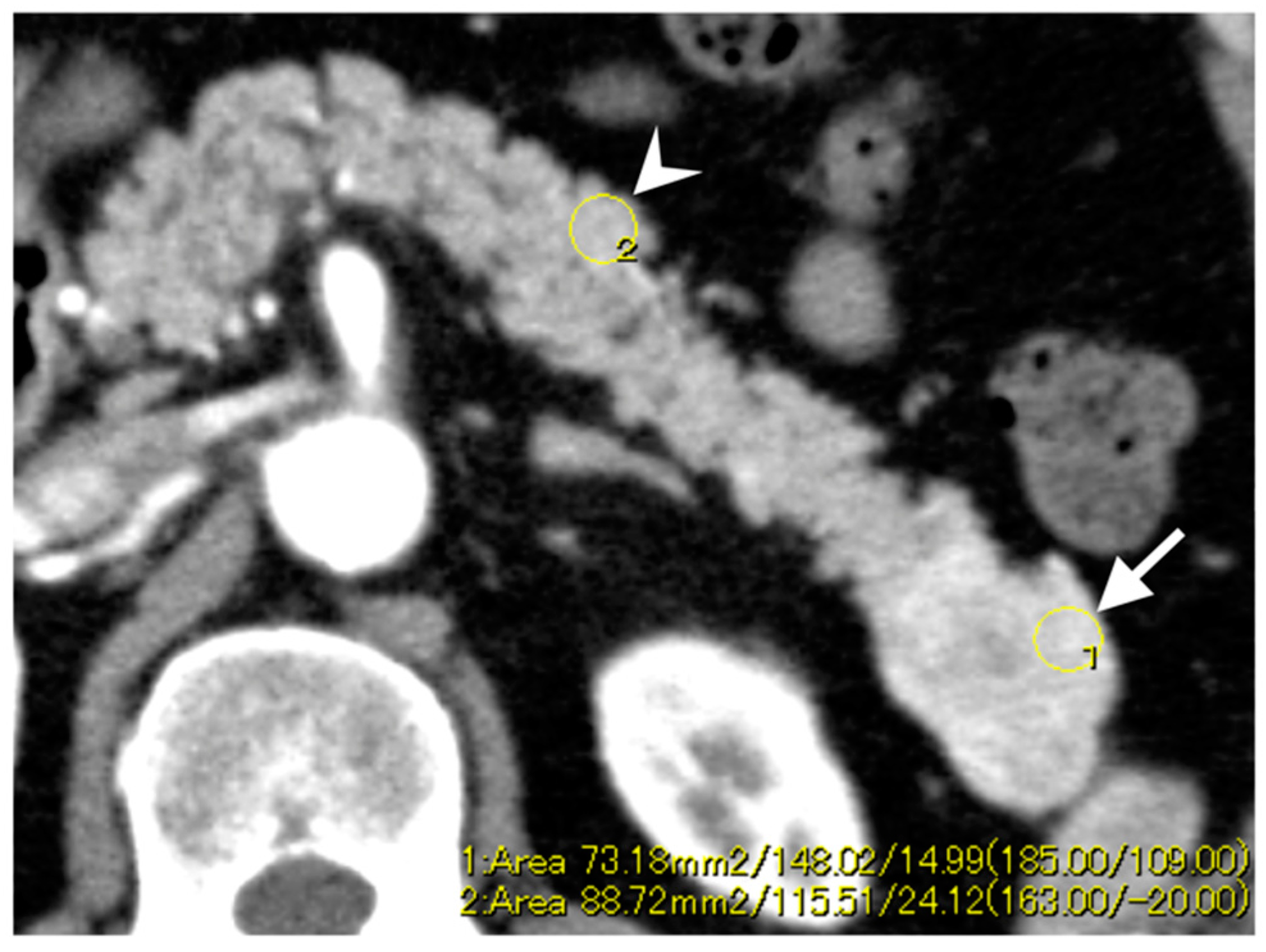
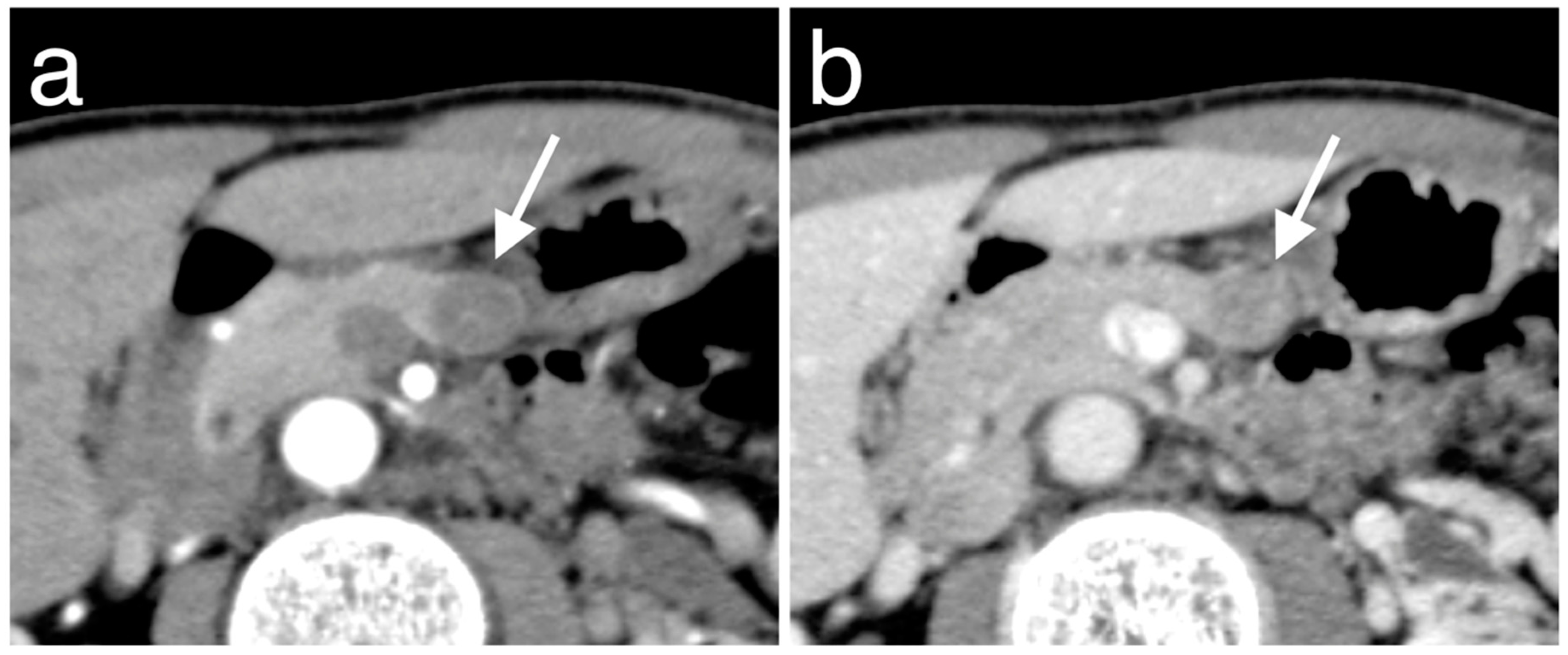
2.3. CT Imaging Techniques and Analysis
2.4. Statistical Analysis
3. Results
3.1. Comparison of Characteristics between Low and High PAX6 Expression Groups
3.2. Morphological Features of Pan-NENs Associated with Low or High PAX6 Expression
3.3. Enhancement Patterns of Pan-NENs Related to Low or High PAX6 Expression
3.4. Morphological Features and Enhancement Patterns of Pan-NENs Associated with Ki-67 Proliferative Index and Mitotic Count
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
A.1. Injection protocol of CT for our institution
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Rabe, K.G.; Rubin, J.; Petersen, G.M. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann. Oncol. 2008, 19, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Or, K.G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Pape, U.F.; Böhmig, M.; Berndt, U.; Tiling, N.; Wiedenmann, B.; Plöckinger, U. Survival and clinical outcome of patients with neuroendocrine tumors of the gastroenteropancreatic tract in a German referral center. Ann. N. Y. Acad. Sci. 2004, 1014, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.B.; van Heerden, J.A.; Grant, C.S.; Carney, J.A.; Ilstrup, D.M. Islet cell carcinomas of the pancreas: A twenty-year experience. Surgery 1988, 104, 1011–1017. [Google Scholar]
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.Y.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef]
- Strachan, T.; Read, A.P. PAX genes. Curr. Opin. Genet. Dev. 1994, 4, 427–438. [Google Scholar] [CrossRef]
- Simpson, T.I.; Price, D.J. Pax6; a pleiotropic player in development. BioEssays 2002, 24, 1041–1051. [Google Scholar] [CrossRef]
- Cohen-Tayar, Y.; Cohen, H.; Mitiagin, Y.; Abravanel, Z.; Levy, C.; Idelson, M.; Reubinoff, B.; Itzkovitz, S.; Raviv, S.; Kaestner, K.H.; et al. Pax6 regulation of Sox9 in the mouse retinal pigmented epithelium controls its timely differentiation and choroid vasculature development. Development 2018, 145, dev163691. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, A.; Long, J.E.; Crandall, J.E.; Rubenstein, J.L.; Bhide, P.G. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat. Neurosci. 2008, 11, 429–439. [Google Scholar] [CrossRef]
- St-Onge, L.; Sosa-Pineda, B.; Chowdhury, K.; Mansouri, A.; Gruss, P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature 1997, 387, 406–409. [Google Scholar] [CrossRef]
- Sander, M.; Neubüser, A.; Kalamaras, J.; Ee, H.C.; Martin, G.R.; German, M.S. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997, 11, 1662–1673. [Google Scholar] [CrossRef]
- Hart, A.W.; Mella, S.; Mendrychowski, J.; van Heyningen, V.; Kleinjan, D.A. The developmental regulator Pax6 is essential for maintenance of islet cell function in the adult mouse pancreas. PLoS ONE 2013, 8, e54173. [Google Scholar] [CrossRef]
- Ahmad, Z.; Rafeeq, M.; Collombat, P.; Mansouri, A. Pax6 inactivation in the adult pancreas reveals ghrelin as endocrine cell maturation marker. PLoS ONE 2015, 10, e0144597. [Google Scholar] [CrossRef]
- Ashery-Padan, R.; Zhou, X.; Marquardt, T.; Herrera, P.; Toube, L.; Berry, A.; Gruss, P. Conditional inactivation of Pax6 in the pancreas causes early onset of diabetes. Dev. Biol. 2004, 269, 479–488. [Google Scholar] [CrossRef]
- Muratovska, A.; Zhou, C.; He, S.; Goodyer, P.; Eccles, M.R. Paired-Box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene 2003, 22, 7989–7997. [Google Scholar] [CrossRef]
- Robson, E.J.D.; He, S.J.; Eccles, M.R. A PANorama of PAX genes in cancer and development. Nat. Rev. Cancer 2006, 6, 52–62. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Wu, X.; Tan, F.; Shi, Y.X.; Glass, T.; Liu, T.J.; Wathen, K.; Hess, K.R.; Gumin, J.; Lang, F.; et al. PAX6 suppresses growth of human glioblastoma cells. J. Neurooncol. 2005, 71, 223–229. [Google Scholar] [CrossRef]
- Mayes, D.A.; Hu, Y.; Teng, Y.; Siegel, E.; Wu, X.; Panda, K.; Tan, F.; Yung, W.K.; Zhou, Y.H. PAX6 suppresses the invasiveness of glioblastoma cells and the expression of the matrix metalloproteinase-2 gene. Cancer Res. 2006, 66, 9809–9817. [Google Scholar] [CrossRef]
- Shyr, C.R.; Tsai, M.Y.; Yeh, S.; Kang, H.Y.; Chang, Y.C.; Wong, P.L.; Huang, C.C.; Huang, K.E.; Chang, C. Tumor suppressor PAX6 functions as androgen receptor co-repressor to inhibit prostate cancer growth. Prostate 2010, 70, 190–199. [Google Scholar] [CrossRef]
- Kudo, A.; Akahoshi, K.; Ito, S.; Akashi, T.; Shimada, S.; Toshiro, O.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Ban, D.; et al. Downregulated pancreatic beta cell genes indicate poor prognosis in patients with pancreatic neuroendocrine neoplasms. Ann. Surg. 2020, 271, 732–739. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Wang, J.; Liang, T.; Gu, Y.; Yang, D. Down-regulation of PAX6 by promoter methylation is associated with poor prognosis in non small cell lung cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11452–11457. [Google Scholar]
- Kiselev, Y.; Andersen, S.; Johannessen, C.; Fjukstad, B.; Standahl Olsen, K.; Stenvold, H.; Al-Saad, S.; Donnem, T.; Richardsen, E.; Bremnes, R.M.; et al. Transcription factor PAX6 as a novel prognostic factor and putative tumour suppressor in non-small cell lung cancer [sci. rep., 5059]. Sci. Rep. 2018, 8, 5059. [Google Scholar]
- Zhou, Y.H.; Tan, F.; Hess, K.R.; Yung, W.K.A. The expression of PAX6, PTEN, vascular endothelial growth factor, and epidermal growth factor receptor in gliomas: Relationship to tumor grade and survival. Clin. Cancer Res. 2003, 9, 3369–3375. [Google Scholar]
- Lai, J.P.; Mertens, R.B.; Mirocha, J.; Koo, J.; Venturina, M.; Chung, F.; Mendez, A.B.; Kahn, M.; Dhall, D. Comparison of PAX6 and PAX8 as immunohistochemical markers for pancreatic neuroendocrine tumors. Endocr. Pathol. 2015, 26, 54–62. [Google Scholar]
- Zhang, L.; Smyrk, T.C.; Oliveira, A.M.; Lohse, C.M.; Zhang, S.; Johnson, M.R.; Lloyd, R.V. KIT is an independent prognostic marker for pancreatic endocrine tumors: A finding derived from analysis of islet cell differentiation markers. Am. J. Surg. Pathol. 2009, 33, 1562–1569. [Google Scholar]
- Kartalis, N.; Mucelli, R.M.P.; Sundin, A. Recent developments in imaging of pancreatic neuroendocrine tumors. Ann. Gastroenterol. 2015, 28, 193–202. [Google Scholar]
- Lewis, R.B.; Lattin, G.E., Jr.; Paal, E. Pancreatic endocrine tumors: Radiologic-clinicopathologic correlation. RadioGraphics 2010, 30, 1445–1464. [Google Scholar]
- Humphrey, P.E.; Alessandrino, F.; Bellizzi, A.M.; Mortele, K.J. Non-hyperfunctioning pancreatic endocrine tumors: Multimodality imaging features with histopathological correlation. Abdom. Imaging 2015, 40, 2398–2410. [Google Scholar] [PubMed]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Lee, E.S.; Park, H.J.; Jang, J.Y.; Ryu, J.K.; Lee, K.B.; Han, J.K. Nonhypervascular pancreatic neuroendocrine tumors: Differential diagnosis from pancreatic ductal adenocarcinomas at MR imaging-retrospective cross-sectional study. Radiology 2017, 284, 77–87. [Google Scholar] [PubMed]
- Worhunsky, D.J.; Krampitz, G.W.; Poullos, P.D.; Visser, B.C.; Kunz, P.L.; Fisher, G.A.; Norton, J.A.; Poultsides, G.A. Pancreatic neuroendocrine tumours: Hypoenhancement on arterial phase computed tomography predicts biological aggressiveness. HPB 2014, 16, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, R.; Suzuki, K.; Ogawa, H.; Komada, T.; Naganawa, S. Pancreatic neuroendocrine tumors containing areas of iso- or hypoattenuation in dynamic contrast-enhanced computed tomography: Spectrum of imaging findings and pathological grading. Eur. J. Radiol. 2015, 84, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Belousova, E.; Karmazanovsky, G.; Kriger, A.; Kalinin, D.; Mannelli, L.; Glotov, A.; Karelskaya, N.; Paklina, O.; Kaldarov, A. Contrast-enhanced MDCT in patients with pancreatic neuroendocrine tumours: Correlation with histological findings and diagnostic performance in differentiation between tumour grades. Clin. Radiol. 2017, 72, 150–158. [Google Scholar] [CrossRef]
- Rodallec, M.; Vilgrain, V.; Couvelard, A.; Rufat, P.; O’Toole, D.; Barrau, V.; Sauvanet, A.; Ruszniewski, P.; Menu, Y. Endocrine pancreatic tumours and helical CT: Contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatology 2006, 6, 77–85. [Google Scholar] [CrossRef]
- Vicentini, C.; Fassan, M.; D’Angelo, E.; Corbo, V.; Silvestris, N.; Nuovo, G.J.; Scarpa, A. Clinical application of microRNA testing in neuroendocrine tumors of the gastrointestinal tract. Molecules 2014, 19, 2458–2468. [Google Scholar] [CrossRef]
- Lo Gullo, R.; Daimiel, I.; Morris, E.A.; Pinker, K. Combining molecular and imaging metrics in cancer: Radiogenomics. Insights Imaging 2020, 11, 1. [Google Scholar] [CrossRef]
- d’Assignies, G.; Couvelard, A.; Bahrami, S.; Vullierme, M.P.; Hammel, P.; Hentic, O.; Sauvanet, A.; Bedossa, P.; Ruszniewski, P.; Vilgrain, V. Pancreatic endocrine tumors: Tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology 2009, 250, 407–416. [Google Scholar] [CrossRef]
- Kim, J.H.; Eun, H.W.; Kim, Y.J.; Lee, J.M.; Han, J.K.; Choi, B.I. Pancreatic neuroendocrine tumour (PNET): Staging accuracy of MDCT and its diagnostic performance for the differentiation of PNET with uncommon CT findings from pancreatic adenocarcinoma. Eur. Radiol. 2016, 26, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, H.J.; Kim, K.W.; Byun, J.H.; Song, K.B.; Kim, J.H.; Hong, S.M. Neuroendocrine neoplasms of the pancreas at dynamic enhanced CT: Comparison between grade 3 neuroendocrine carcinoma and grade 1/2 neuroendocrine tumour. Eur. Radiol. 2015, 25, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Okamura, Y.; Uemura, S.; Sugiura, T.; Ito, T.; Ashida, R.; Kato, Y.; Ohgi, K.; Yamada, M.; Sasaki, K.; et al. Vascularity and tumor size are significant predictors for recurrence after resection of a pancreatic neuroendocrine tumor. Ann. Surg. Oncol. 2017, 24, 2363–2370. [Google Scholar] [CrossRef]
- Takumi, K.; Fukukura, Y.; Higashi, M.; Ideue, J.; Umanodan, T.; Hakamada, H.; Kanetsuki, I.; Yoshiura, T. Pancreatic neuroendocrine tumors: Correlation between the contrast-enhanced computed tomography features and the pathological tumor grade. Eur. J. Radiol. 2015, 84, 1436–1443. [Google Scholar] [CrossRef]
- Gallotti, A.; Johnston, R.P.; Bonaffini, P.A.; Ingkakul, T.; Deshpande, V.; Fernández-del Castillo, C.; Sahani, D.V. Incidental neuroendocrine tumors of the pancreas: MDCT findings and features of malignancy. Am. J. Roentgenol. 2013, 200, 355–362. [Google Scholar] [CrossRef]
- Karmazanovsky, G.; Belousova, E.; Schima, W.; Glotov, A.; Kalinin, D.; Kriger, A. Nonhypervascular pancreatic neuroendocrine tumors: Spectrum of MDCT imaging findings and differentiation from pancreatic ductal adenocarcinoma. Eur. J. Radiol. 2019, 110, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.E.; Sherman, S.K.; Howe, J.R. Translational diagnostics and therapeutics in pancreatic neuroendocrine tumors. Clin. Cancer Res. 2016, 22, 5022–5029. [Google Scholar] [CrossRef]
- Tang, C.T.; Zhang, Q.W.; Wu, S.; Tang, M.Y.; Liang, Q.; Lin, X.L.; Gao, Y.; Ge, Z. Thalidomide Targets EGFL6 to Inhibit EGFL6/PAX6 Axis-Driven Angiogenesis in Small Bowel Vascular Malformation. Cell. Mol. Life Sci. 2020. [Tang, C.; Zhang, Q.; Wu, S.; Tang, M.; Liang, Q.; Lin, X.; Gao, Y.; Ge, Z. Online. Cell. Mol. Life Sci. Ahead of print]. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Hu, Y.; Mayes, D.; Siegel, E.; Kim, J.G.; Mathews, M.S.; Hsu, N.; Eskander, D.; Yu, O.; Tromberg, B.J.; et al. PAX6 suppression of glioma angiogenesis and the expression of vascular endothelial growth factor A. J. Neurooncol. 2010, 96, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Corrias, G.; Monti, S.; Horvat, N.; Tang, L.; Basturk, O.; Saba, L.; Mannelli, L. Imaging features of malignant abdominal neuroendocrine tumors with rare presentation. Clin. Imaging 2018, 51, 59–64. [Google Scholar] [CrossRef]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von, G.E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef]
- Ambrosini, V.; Campana, D.; Tomassetti, P.; Fanti, S. 68Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur. J. Nucl. Med. Mol. Imaging 2012, 39 (Suppl 1), S52–S60. [Google Scholar] [CrossRef]
- Kayani, I.; Conry, B.G.; Groves, A.M.; Win, T.; Dickson, J.; Caplin, M.; Bomanji, J.B. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. J. Nucl. Med. 2009, 50, 1927–1932. [Google Scholar]
- Hofman, M.S.; Lau, W.F.; Hicks, R.J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation. RadioGraphics 2015, 35, 500–516. [Google Scholar]
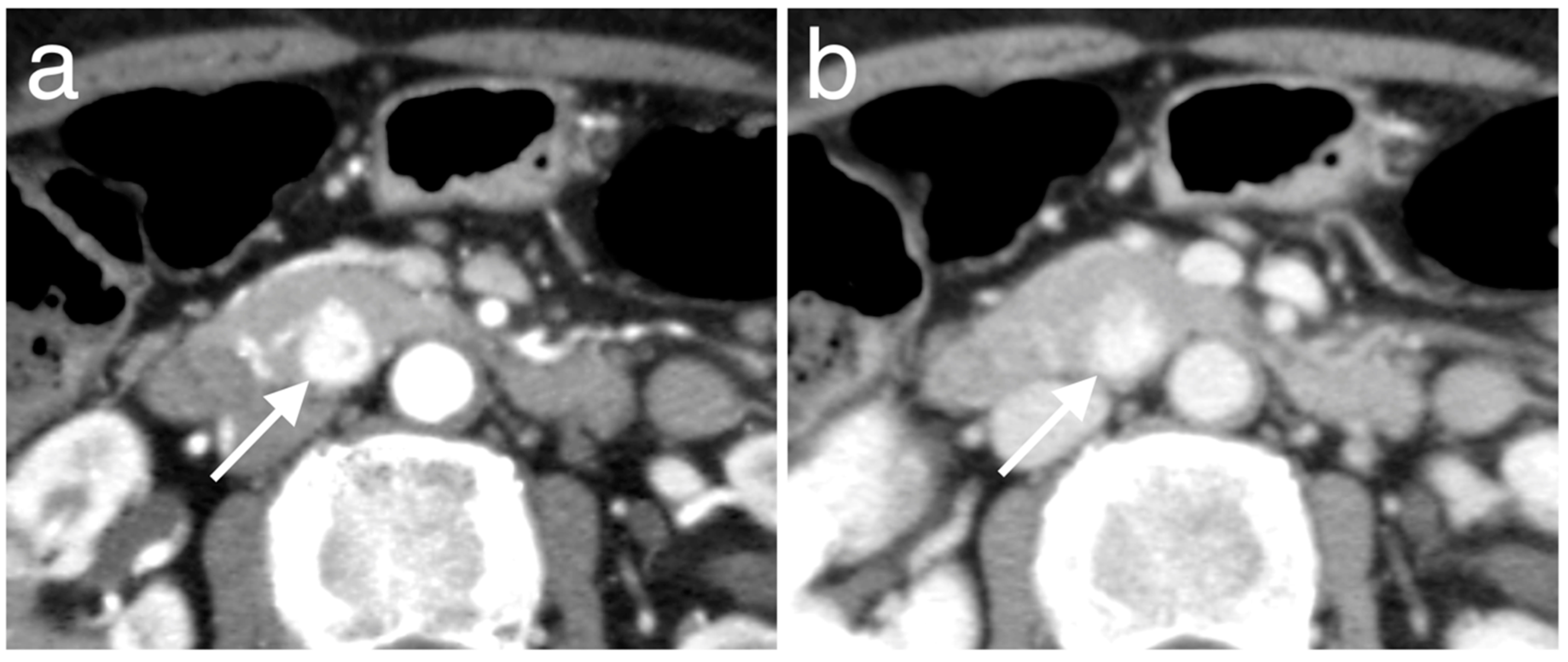

| Characteristic | Low PAX6 Expression Group (n = 11) | High PAX6 Expression Group (n = 40) | p Value a |
|---|---|---|---|
| Age (y) b | 53 ± 15 | 61 ± 11 | 0.14 |
| Male sex | 6 | 16 | 0.50 |
| Genetic disorder | 1 | 1 | 0.39 |
| Time from CT to surgery (d) c | 24 [6.5, 43] | 34 [8.75, 76.5] | 0.43 |
| 2010 WHO classification | |||
| NET G1 | 6 | 33 | 0.049 |
| NET G2 | 4 | 7 | |
| NEC G3 | 1 | 0 | |
| Ki-67 index (%) b | 6.25 ± 8.85 | 1.81 ± 1.72 | 0.021 |
| Mitotic count (n/10 HPF) b | 1.45 ± 1.86 | 0.80 ± 0.94 | 0.11 |
| Symptomatic tumor | 2 | 13 | 0.47 |
| Metachronous hepatic metastasis | 2 | 1 | 0.11 |
| Death | 2 | 0 | 0.043 |
| Features | Low PAX6 Expression Group (n = 11) | High PAX6 Expression Group (n = 40) | k | p Value a |
|---|---|---|---|---|
| Size (mm) b | 18.6 [13.5, 44.0] | 15.5 [10.1, 21.8] | 0.24 | |
| Location | ||||
| Head | 5 | 16 | 0.76 | 0.28 |
| Body | 0 | 9 | ||
| Tail | 6 | 15 | ||
| Shape | ||||
| Round | 2 | 15 | 0.50 | 0.44 |
| Oval | 7 | 21 | ||
| Lobulated | 2 | 4 | ||
| Margin (well-defined) | 9 | 35 | 0.45 | 0.63 |
| Cystic change | 1 | 5 | 0.46 | 1.00 |
| Calcification | 4 | 4 | 0.92 | 0.055 |
| Visible intratumoral vessel | 1 | 5 | 0.73 | 1.00 |
| Invasion into adjacent vascular | 2 | 3 | 0.63 | 0.29 |
| Main pancreatic duct dilatation | 1 | 2 | 0.84 | 0.52 |
| Upstream pancreatic atrophy | 2 | 1 | 0.64 | 0.11 |
| Low PAX6 Expression Group (n = 11) | High PAX6 Expression Group (n = 40) | k | p Value a | |
|---|---|---|---|---|
| Tumor homogeneity | ||||
| Arterial phase | ||||
| Homogeneous | 8 | 26 | 0.69 | 0.73 |
| Heterogeneous | 3 | 14 | ||
| Portal phase | ||||
| Homogeneous | 8 | 29 | 0.77 | 1.00 |
| Heterogeneous | 3 | 11 | ||
| Main enhancement types | ||||
| Arterial phase | ||||
| Hyperenhancement | 4 | 32 | 0.64 | 0.009 |
| Iso- or hypoenhancement b | 7 (3, 4) | 8 (6, 2) | ||
| Portal phase | ||||
| Hyperenhancement | 3 | 33 | 0.76 | 0.001 |
| Iso- or hypoenhancement b | 8 (6, 2) | 7 (6, 1) | ||
| Enhancement ratio c | ||||
| Arterial phase | 1.19 ± 0.44 | 1.38 ± 0.36 | 0.28 | |
| Portal phase | 1.12 ± 0.28 | 1.30 ± 0.22 | 0.044 |
| Parameter | Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive LR | Negative LR |
|---|---|---|---|---|---|
| Iso- or hypoenhancement types on the arterial phase | 63.6 (30.8, 89.1) [7/11] | 80.0 (64.4, 90.9) [32/40] | 76.5 (62.5, 87.2) [39/51] | 3.18 (1.48, 6.83) | 0.46 (0.21, 1.01) |
| Iso- or hypoenhancement types on the portal phase | 72.7 (39.0, 94.0) [8/11] | 82.5 (67.2, 92.7) [33/40] | 80.4 (66.9, 90.2) [41/51] | 4.16 (1.94, 8.92) | 0.33 (0.13, 0.88) |
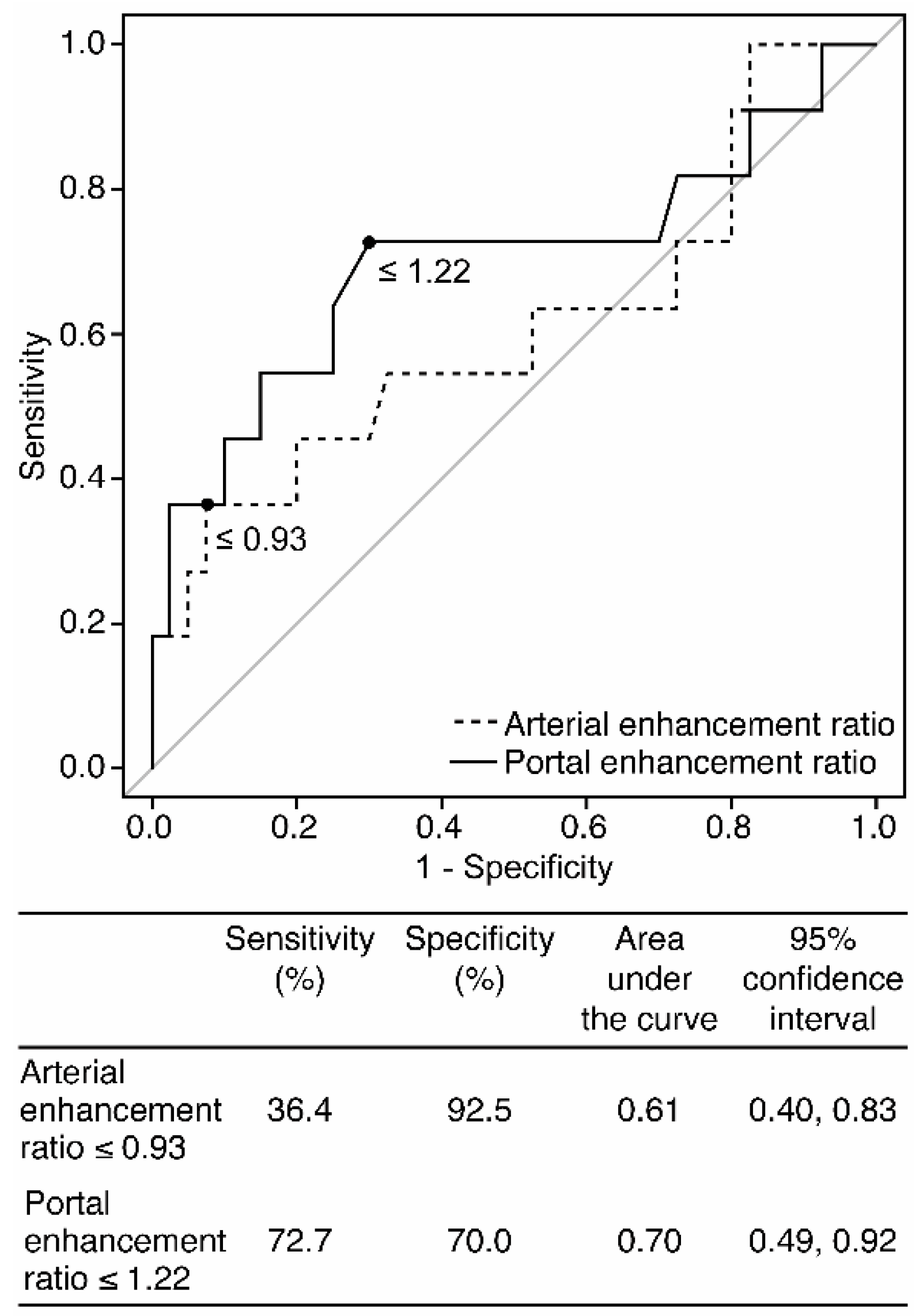
| Enhancement ratio on the portal phase ≤ 1.22 | 72.7 (39.0, 94.0) [8/11] | 70.0 (53.5, 83.4) [28/40] | 70.6 (56.2, 82.5) [36/51] | 2.42 (1.34, 4.40) | 0.39 (0.15, 1.04) |
| At least one parameter | 81.8 (48.2, 97.7) [9/11] | 60.0 (43.3, 75.1) [24/40] | 64.7 (50.1, 77.6) [33/51] | 2.05 (1.28, 3.26) | 0.30 (0.08, 1.09) |
| Any two parameters | 72.7 (39.0, 94.0) [8/11] | 80.0 (64.4, 90.9) [32/40] | 78.4 (64.7, 88.7) [40/51] | 3.64 (1.77, 7.45) | 0.34 (0.13, 0.91) |
| All three parameters | 54.5 (23.4, 83.3) [6/11] | 92.5 (79.6, 98.4) [37/40] | 84.3 (71.4, 93.0) [43/51] | 7.27 (2.16, 24.5) | 0.49 (0.26, 0.94) |
| Ki-67 Proliferative Index | Mitotic Count | |||||
|---|---|---|---|---|---|---|
| Features | ≤2%: Low Group (n = 35) | >2%: High Group (n = 16) | p Value a | <2 n/10 HPF: Low Group (n = 47) | ≥2 n/10 HPF: High Group (n = 4) | p Value a |
| Size (mm) b | 15.0 [10.6, 17.8] | 28.3 [14.5, 54.3] | 0.012 | 15.3 [10.0, 20.1] | 57.9 [42.2, 76.7] | 0.004 |
| Location | ||||||
| Head | 14 | 7 | 0.85 | 19 | 2 | 1.00 |
| Body | 7 | 2 | 9 | 0 | ||
| Tail | 14 | 7 | 19 | 2 | ||
| Shape | ||||||
| Round | 13 | 4 | 0.15 | 17 | 0 | 0.24 |
| Oval | 20 | 8 | 25 | 3 | ||
| Lobulated | 2 | 4 | 5 | 1 | ||
| Margin (well-defined) | 33 | 11 | 0.025 | 41 | 3 | 0.46 |
| Cystic change | 3 | 3 | 0.36 | 4 | 2 | 0.063 |
| Calcification | 2 | 6 | 0.008 | 5 | 3 | 0.009 |
| Visible intratumoral vessel | 2 | 4 | 0.069 | 5 | 1 | 0.40 |
| Invasion into adjacent vascular | 2 | 3 | 0.309 | 4 | 1 | 0.35 |
| Main pancreatic duct dilatation | 1 | 2 | 0.23 | 2 | 1 | 0.22 |
| Upstream pancreatic atrophy | 1 | 2 | 0.23 | 2 | 1 | 0.22 |
| Ki-67 Proliferative Index | Mitotic Count | |||||
|---|---|---|---|---|---|---|
| Features | ≤2%: Low Group (n = 35) | >2%: High Group (n = 16) | p Value a | <2 n/10 HPF: Low Group (n = 47) | ≥2 n/10 HPF: High Group (n = 4) | p Value a |
| Tumor homogeneity | ||||||
| Arterial phase | ||||||
| Homogeneous | 24 | 10 | 0.75 | 33 | 1 | 0.10 |
| Heterogeneous | 11 | 6 | 14 | 3 | ||
| Portal phase | ||||||
| Homogeneous | 27 | 10 | 0.32 | 36 | 1 | 0.057 |
| Heterogeneous | 8 | 6 | 11 | 3 | ||
| Main enhancement types | ||||||
| Arterial phase | ||||||
| Hyperenhancement | 28 | 8 | 0.046 | 34 | 2 | 0.57 |
| Iso- or hypoenhancement b | 7 (3, 4) | 8 (6, 2) | 13 (8, 5) | 2 (1, 1) | ||
| Portal phase | ||||||
| Hyperenhancement | 25 | 11 | 1.00 | 33 | 3 | 1.00 |
| Iso- or hypoenhancement b | 10 (8, 2) | 5 (4, 1) | 14 (12, 2) | 1 (0, 1) | ||
| Enhancement ratio c | ||||||
| Arterial phase | 1.38 ± 0.41 | 1.24 ± 0.32 | 0.096 | 1.36 ± 0.39 | 1.13 ± 0.20 | 0.19 |
| Portal phase | 1.27 ± 0.23 | 1.27 ± 0.27 | 0.96 | 1.26 ± 0.23 | 1.24 ± 0.39 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, K.; Tsuchiya, J.; Kitazume, Y.; Kishino, M.; Akahoshi, K.; Kudo, A.; Tanaka, S.; Tanabe, M.; Tateishi, U. Dynamic Enhancement Pattern on CT for Predicting Pancreatic Neuroendocrine Neoplasms with Low PAX6 Expression: A Retrospective Observational Study. Diagnostics 2020, 10, 919. https://doi.org/10.3390/diagnostics10110919
Kimura K, Tsuchiya J, Kitazume Y, Kishino M, Akahoshi K, Kudo A, Tanaka S, Tanabe M, Tateishi U. Dynamic Enhancement Pattern on CT for Predicting Pancreatic Neuroendocrine Neoplasms with Low PAX6 Expression: A Retrospective Observational Study. Diagnostics. 2020; 10(11):919. https://doi.org/10.3390/diagnostics10110919
Chicago/Turabian StyleKimura, Koichiro, Junichi Tsuchiya, Yoshio Kitazume, Mitsuhiro Kishino, Keiichi Akahoshi, Atsushi Kudo, Shinji Tanaka, Minoru Tanabe, and Ukihide Tateishi. 2020. "Dynamic Enhancement Pattern on CT for Predicting Pancreatic Neuroendocrine Neoplasms with Low PAX6 Expression: A Retrospective Observational Study" Diagnostics 10, no. 11: 919. https://doi.org/10.3390/diagnostics10110919
APA StyleKimura, K., Tsuchiya, J., Kitazume, Y., Kishino, M., Akahoshi, K., Kudo, A., Tanaka, S., Tanabe, M., & Tateishi, U. (2020). Dynamic Enhancement Pattern on CT for Predicting Pancreatic Neuroendocrine Neoplasms with Low PAX6 Expression: A Retrospective Observational Study. Diagnostics, 10(11), 919. https://doi.org/10.3390/diagnostics10110919






