Comparison of Magnetic Resonance Angiography and Digital Subtraction Angiography for the Assessment of Infrapopliteal Arterial Occlusive Lesions, Based on the TASC II Classification Criteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Overall Study Design
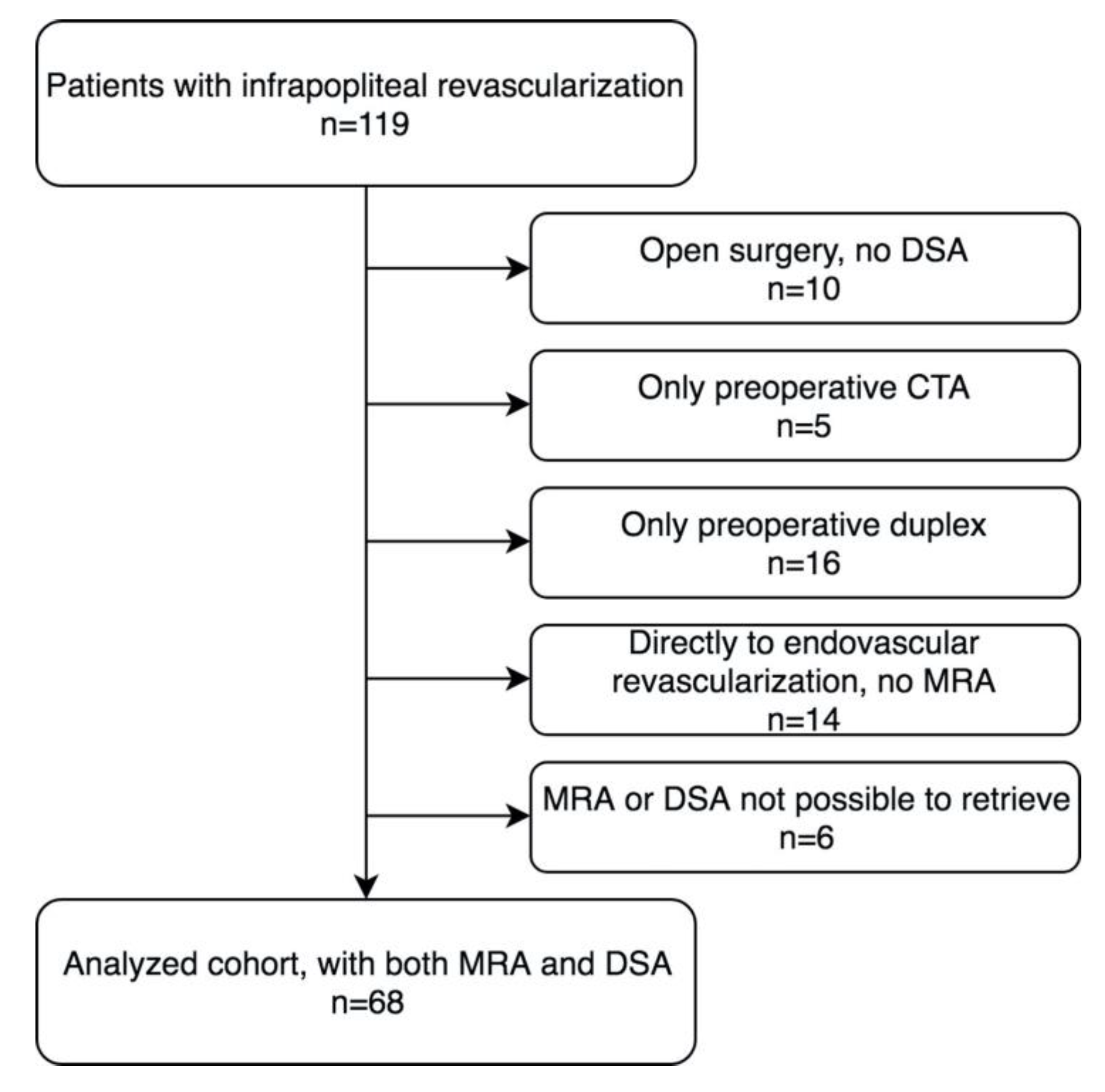
2.2. Patients and Images
2.3. Image Evaluation
2.4. Statistics
2.5. Ethics
3. Results
3.1. Modality Comparison
3.2. Assessment of TransAtlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC) II
3.3. Selection of Target Vessel
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sampson, U.K.; Fowkes, F.G.; McDermott, M.M.; Criqui, M.H.; Aboyans, V.; Norman, P.E.; Forouzanfar, M.H.; Naghavi, M.; Song, Y.; Harrell, F.E., Jr.; et al. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob. Heart 2014, 9, 145–158.e121. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet (London England) 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Baubeta Fridh, E.; Andersson, M.; Thuresson, M.; Sigvant, B.; Kragsterman, B.; Johansson, S.; Hasvold, P.; Falkenberg, M.; Nordanstig, J. Amputation rates, mortality, and pre-operative comorbidities in patients revascularised for intermittent claudication or critical limb ischaemia: A population based study. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 480–486. [Google Scholar] [CrossRef]
- Farber, A. Chronic limb-threatening ischemia. N. Engl. J. Med. 2018, 379, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Gibelli, D.; Martinenghi, C.; Oliva, G.; Floridi, C. Ct angiography of lower extremities from anatomy to traumatic and nontraumatic lesions: A pictorial review. Emerg. Radiol. 2020, 27, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Diehm, N.; Kickuth, R.; Baumgartner, I.; Srivastav, S.K.; Gretener, S.; Husmann, M.J.; Jaccard, Y.; Do do, D.; Triller, J.; Bonel, H.M. Magnetic resonance angiography in infrapopliteal arterial disease: Prospective comparison of 1.5 and 3 tesla magnetic resonance imaging. Investig. Radiol. 2007, 42, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Bonel, H.M.; Saar, B.; Hoppe, H.; Keo, H.H.; Husmann, M.; Nikolaou, K.; Ludwig, K.; Szucs-Farkas, Z.; Srivastav, S.; Kickuth, R. Mr angiography of infrapopliteal arteries in patients with peripheral arterial occlusive disease by using gadofosveset at 3.0 t: Diagnostic accuracy compared with selective dsa. Radiology 2009, 253, 879–890. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; Group, T.I.W. Inter-society consensus for the management of peripheral arterial disease (tasc ii). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef]
- Cina, A.; Di Stasi, C.; Semeraro, V.; Marano, R.; Savino, G.; Iezzi, R.; Bonomo, L. Comparison of ct and mr angiography in evaluation of peripheral arterial disease before endovascular intervention. Acta Radiol. 2016, 57, 547–556. [Google Scholar] [CrossRef]
- Wurz, C.; Davari, A.; Ackermann, H.; Vogl, T.J. Diagnostic performance of ce-mra in grading stenosis and therapy planning with tasc ii classification. Vascular 2015, 23, 403–410. [Google Scholar] [CrossRef]
- Jaff, M.R.; White, C.J.; Hiatt, W.R.; Fowkes, G.R.; Dormandy, J.; Razavi, M.; Reekers, J.; Norgren, L. An update on methods for revascularization and expansion of the tasc lesion classification to include below-the-knee arteries: A supplement to the inter-society consensus for the management of peripheral arterial disease (tasc ii): The tasc steering comittee(.). Ann. Vasc. Dis. 2015, 8, 343–357. [Google Scholar]
- Troeng, T.; Malmstedt, J.; Bjorck, M. External validation of the swedvasc registry: A first-time individual cross-matching with the unique personal identity number. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 705–712. [Google Scholar] [CrossRef]
- Swedvasc. The Swedish National Registry for Vascular Surgery. Available online: http://www.ucr.uu.se/swedvasc (accessed on 11 January 2020).
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef]
- Fontaine, R.; Kim, M.; Kieny, R. Surgical treatment of peripheral circulation disorders. Helv. Chir. Acta 1954, 21, 499–533. [Google Scholar] [PubMed]
- Borjesson, S.; Hakansson, M.; Bath, M.; Kheddache, S.; Svensson, S.; Tingberg, A.; Grahn, A.; Ruschin, M.; Hemdal, B.; Mattsson, S.; et al. A software tool for increased efficiency in observer performance studies in radiology. Radiat. Prot. Dosim. 2005, 114, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, M.; Svensson, S.; Zachrisson, S.; Svalkvist, A.; Bath, M.; Mansson, L.G. Viewdex: An efficient and easy-to-use software for observer performance studies. Radiat. Prot. Dosim. 2010, 139, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Svalkvist, A.; Svensson, S.; Hakansson, M.; Bath, M.; Mansson, L.G. Viewdex: A status report. Radiat. Prot. Dosim. 2016, 169, 38–45. [Google Scholar] [CrossRef]
- Bath, M.; Mansson, L. Visual grading characteristics (vgc) analysis: A non-parametric rank-invariant statistical method for image quality evaluation. Br. J. Radiol. 2007, 80, 169–176. [Google Scholar] [CrossRef]
- Bath, M.; Hansson, J. Vgc analyzer: A software for statistical analysis of fully crossed multiple-reader multiple-case visual grading characteristics studies. Radiat. Prot. Dosim. 2016, 169, 46–53. [Google Scholar] [CrossRef]
- Hayes, A.F.; Krippendorff, K. Answering the call for a standard reliability measure for coding data. Commun. Methods Meas. 2007, 1, 77–89. [Google Scholar] [CrossRef]
- Biagioni, R.B.; Biagioni, L.C.; Nasser, F.; Burihan, M.C.; Ingrund, J.C.; Neser, A.; Miranda, F., Jr. Infrapopliteal angioplasty of one or more than one artery for critical limb ischaemia: A randomised clinical trial. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.e140. [Google Scholar] [CrossRef] [PubMed]
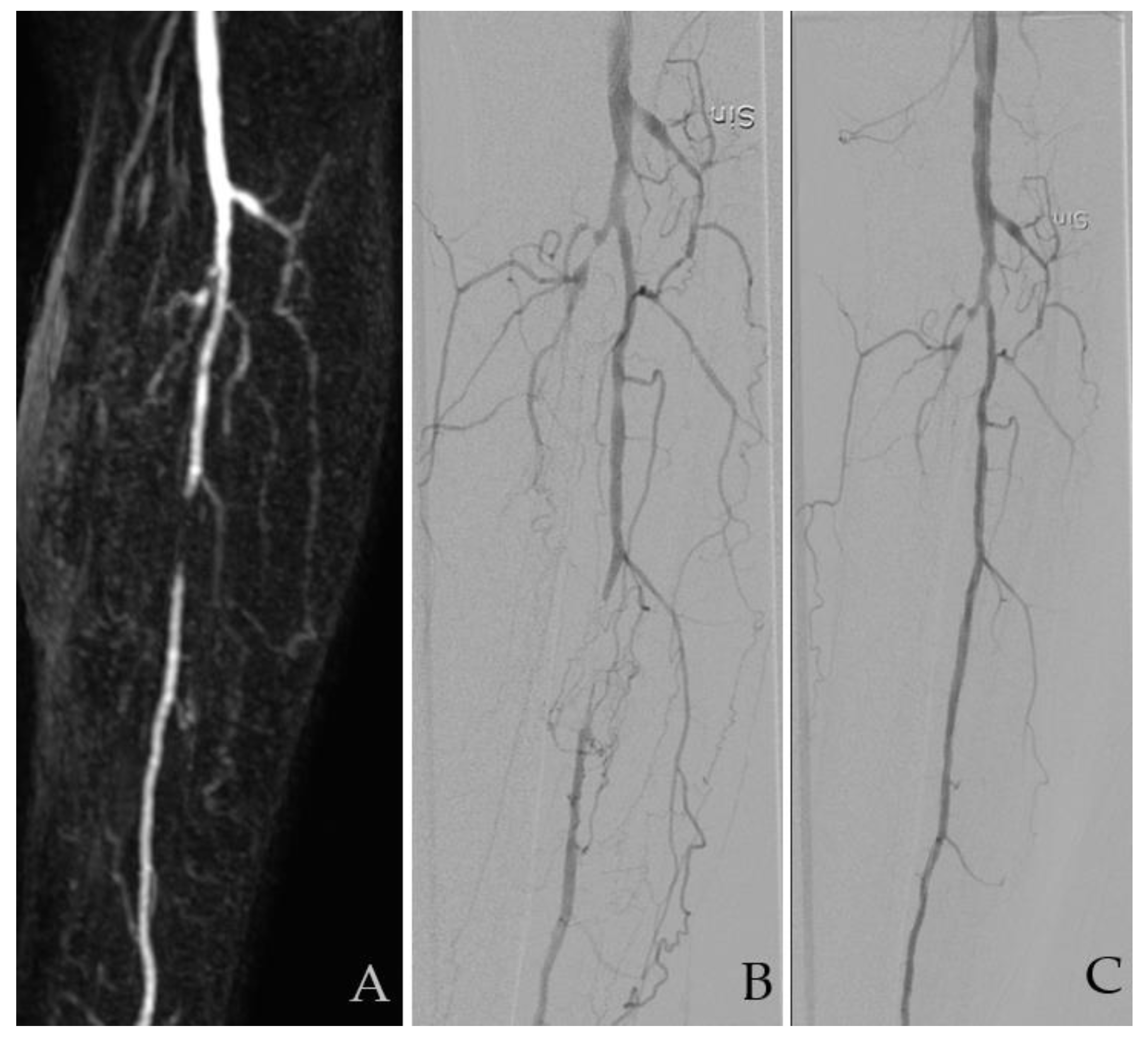
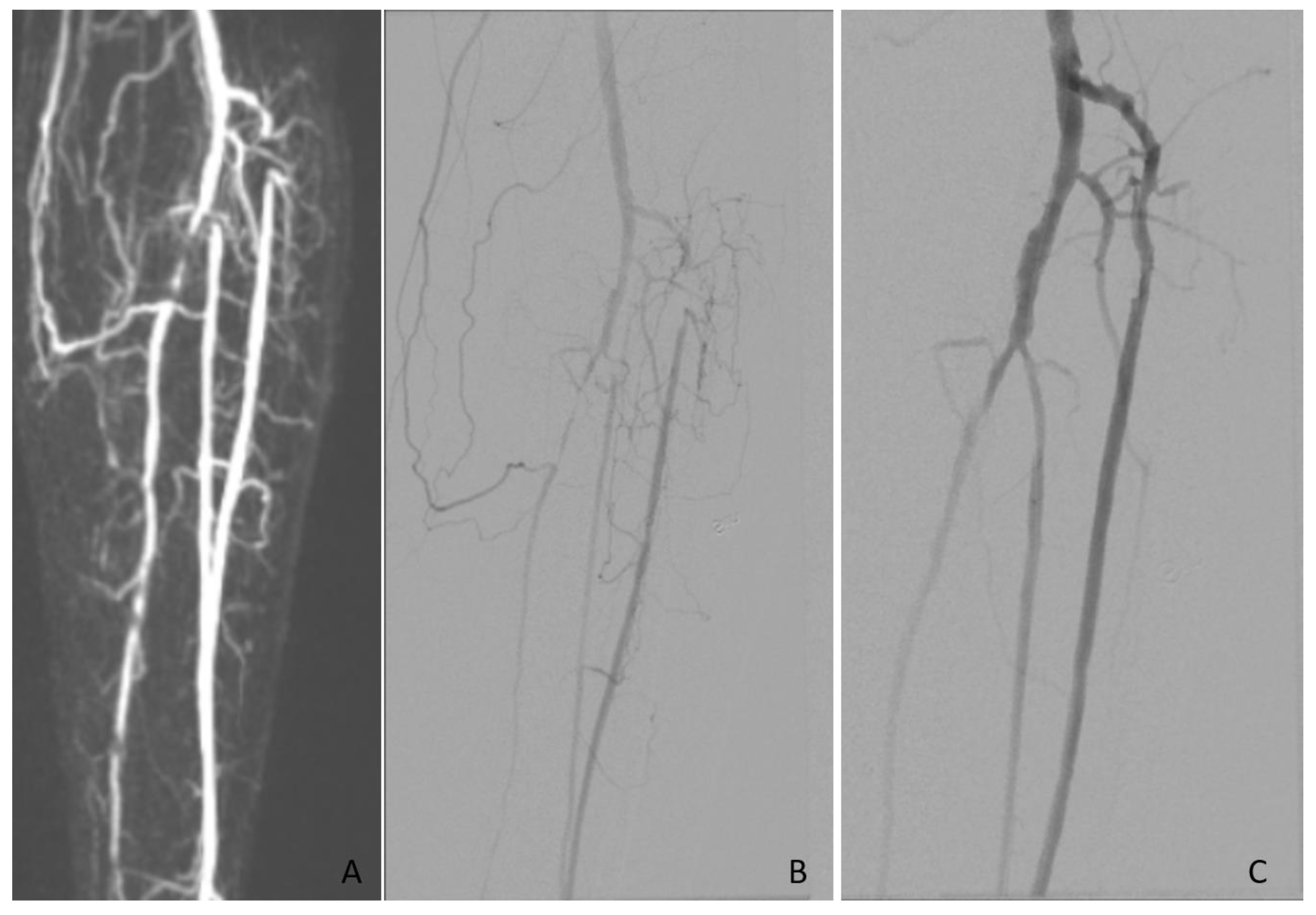
| Modality | Examinations Assessed | Good Diagnostic Quality (%) | Assessed TASC II Class, n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Observer 1 | MRA | 68 | 62 (91.2) | 0 (0.0) | 5 (8.1) | 11 (17.7) | 36 (58.1) | 10 (16.1) |
| Observer 2 | MRA | 68 | 52 (76.5) | 1 (1.9) | 16 (30.8) | 25 (48.1) | 10 (19.2) | 0 (0.0) |
| Observer 3 | MRA | 68 | 64 (94.1) | 0 (0.0) | 15 (23.4) | 25 (39.1) | 23 (35.9) | 1 (1.6) |
| Observer 1 | DSA | 68 | 62 (91.2) | 0 (0.0) | 11 (17.7) | 14 (22.6) | 30 (48.4) | 7 (11.3) |
| Observer 2 | DSA | 68 | 62 (91.2) | 0 (0.0) | 14 (22.6) | 28 (45.2) | 16 (25.8) | 4 (6.4) |
| Observer 4 | DSA | 68 | 65 (95.6) | 1 (1.5) | 18 (27.7) | 32 (49.2) | 13 (20.0) | 1 (1.5) |
| Infrapopliteal TASC | n | α (95% CI) |
|---|---|---|
| MRA Observer 1, Observer 2 and Observer 3 | 51 | 0.13 (−0.07–0.31) |
| MRA Observer 1 and Observer 2 | 51 | −0.16 (−0.56–0.2) |
| MRA Observer 1 and Observer 3 | 62 | 0.08 (−0.26–0.40) |
| MRA Observer 2 and Observer 3 | 51 | 0.25 (−0.04–0.52) |
| DSA Observer 1, Observer 2 and Observer 4 | 61 | 0.39 (0.23–0.53) |
| DSA Observer 1 and Observer 2 | 61 | 0.43 (0.18–0.64) |
| DSA Observer 1 and Observer 4 | 62 | 0.25 (−0.02–0.49) |
| DSA Observer 2 and Observer 4 | 62 | 0.45 (0.11–0.72) |
| Target Vessel | n | α (95% CI) |
|---|---|---|
| MRA Observer 1, Observer 2 and Observer 3 | 51 | 0.19 (0.01–0.36) |
| MRA Observer 1 and Observer 2 | 51 | 0.12 (−0.13–0.35) |
| MRA Observer 1 and Observer 3 | 62 | 0.26 (−0.00–0.50) |
| MRA Observer 2 and Observer 3 | 51 | 0.13 (−0.22–0.46) |
| MRA Observer 1—revascularized vessel DSA | 57 | −0.02 (−0.33–0.26) |
| MRA Observer 2—revascularized vessel DSA | 49 | 0.39 (0.06–0.67) |
| MRA Observer 3—revascularized vessel DSA | 59 | 0.14 (−0.20–0.44) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baubeta Fridh, E.; Ludwigs, K.; Svalkvist, A.; Andersson, M.; Nordanstig, J.; Falkenberg, M.; A. Johnsson, Å. Comparison of Magnetic Resonance Angiography and Digital Subtraction Angiography for the Assessment of Infrapopliteal Arterial Occlusive Lesions, Based on the TASC II Classification Criteria. Diagnostics 2020, 10, 892. https://doi.org/10.3390/diagnostics10110892
Baubeta Fridh E, Ludwigs K, Svalkvist A, Andersson M, Nordanstig J, Falkenberg M, A. Johnsson Å. Comparison of Magnetic Resonance Angiography and Digital Subtraction Angiography for the Assessment of Infrapopliteal Arterial Occlusive Lesions, Based on the TASC II Classification Criteria. Diagnostics. 2020; 10(11):892. https://doi.org/10.3390/diagnostics10110892
Chicago/Turabian StyleBaubeta Fridh, Erik, Karin Ludwigs, Angelica Svalkvist, Manne Andersson, Joakim Nordanstig, Mårten Falkenberg, and Åse A. Johnsson. 2020. "Comparison of Magnetic Resonance Angiography and Digital Subtraction Angiography for the Assessment of Infrapopliteal Arterial Occlusive Lesions, Based on the TASC II Classification Criteria" Diagnostics 10, no. 11: 892. https://doi.org/10.3390/diagnostics10110892
APA StyleBaubeta Fridh, E., Ludwigs, K., Svalkvist, A., Andersson, M., Nordanstig, J., Falkenberg, M., & A. Johnsson, Å. (2020). Comparison of Magnetic Resonance Angiography and Digital Subtraction Angiography for the Assessment of Infrapopliteal Arterial Occlusive Lesions, Based on the TASC II Classification Criteria. Diagnostics, 10(11), 892. https://doi.org/10.3390/diagnostics10110892





