Abstract
Abnormal uterine bleeding (AUB) is a common symptom in the female population, with an estimated prevalence of 10 to 30% in fertile age and up to 90% in perimenopausal women. In most cases, AUB is due to a benign cause. However, it can also be a symptom of atypical endometrial hyperplasia or endometrial cancer, a more common disease during menopause which can also affect women in their reproductive age. Considering the high prevalence of this symptom an appropriate diagnostic algorithm is needed. Concerns about the risks, pain, and stress associated with an endometrial biopsy and its impact on the healthcare system make the choice of AUB diagnostic strategy extremely relevant. Even if the scientific community agrees on the definition of AUB, International Guidelines show some differences in the management of women of reproductive age with AUB, especially regarding the age cut-off as an independent indication for endometrial biopsy. This study compared different diagnostic strategies to identify a diagnostic pathway with high sensitivity and specificity but low impact on the health system’s resources. The analysis was based on three diagnostic algorithms defined as part of the guidelines of leading scientific societies. Women of reproductive age with AUB (n = 625) and without risk of endometrial cancer were included in the study. Results showed that the best criterion to investigate AUB in women at low risk of endometrial cancer is not age cut-off but the presence or absence of focal endometrial pathology at the ultrasound and the response to the progestin therapy. This approach makes it possible to perform fewer outpatient hysteroscopic biopsies without excluding positive cases from the examination.
1. Introduction
Abnormal uterine bleeding (AUB), also known as dysfunctional uterine bleeding, is a common symptom in the female population, with an estimated prevalence of 10 to 30% in fertile age and up to 90% in perimenopausal women [1,2,3]. According to the 2011 criteria by the International Federation of Gynecology and Obstetrics (FIGO), AUB is defined as abnormal bleeding from the uterine cavity by regularity, volume, and timing present for most of the past six months [4]. The FIGO systems have also given an acronym for common etiologies, which apply to chronic AUB. PALM-COEIN is the acronym provided by the FIGO to classify the underlying etiologies of chronic AUB [5]. The first portion, PALM, describes structural issues (P, Polyp; A, Adenomyosis; L, Leiomyoma; M, Malignancy, and hyperplasia). The second portion, COEI, reports non-structural issues (C, Coagulopathy; O, Ovulatory dysfunction; E, Endometrial disorders; I, Iatrogenic). The N stands for “not otherwise classified” [5].
The prevalence of AUB increases mostly in adolescents [6] and perimenopause women [7], when the anovulatory cycles are frequent [8,9]. About 20% of the affected people belong to the adolescent age group, and 50% of the affected individuals are between 40 and 50. It is estimated that about 90% of women present at least one episode of AUB in the pre-menopausal transition period, and 78% of them have a recurrence of at least three episodes [2]. In most cases, AUB is due to a benign cause. However, it can also be a symptom of atypical endometrial hyperplasia or endometrial cancer, a more common disease during menopause which can also affect women in their reproductive age, with an incidence that the National Cancer Institute estimated in 16.7% in the 45–54 age group, and 34.5% in the 55–64 age group [10]. Nevertheless, 5% of women of reproductive age suffer from a malignant pathology [11].
The selection of patients for the endometrial biopsy has the foremost aim of detect endometrial cancer and atypical hyperplasia, for which the early diagnosis is of utmost importance for proper treatment of the disease [12]. It is important to consider that atypical hyperplasia is associated with a concomitant carcinoma in up to 50% of cases [13] and has a risk of neoplastic progression of 12.5% in the following ten years [14]. On the contrary, the histological diagnosis of hyperplasia without atypia is associated with a low risk of neoplastic progression (less than 5% in 20 years) [11,13], and it generally regresses spontaneously during follow-up or responds well to local or systemic progestogen therapy [11,15].
Even if the scientific community agrees on the definition of AUB, International Guidelines show some differences in the management of women of reproductive age with AUB. All guidelines consider the presence of risk factors, such as unopposed estrogen use, tamoxifen treatment, obesity, polycystic ovary syndrome (PCOS), and genetic factors, e.g., families with hereditary non-polypoid cancer of the colon for endometrial cancer, as an independent indication for endometrial biopsy [12,16,17]. But some guidelines consider patient’s age as an independent indication for endometrial biopsy [12,16,17].
Hysteroscopy associated with targeted biopsy is considered the ‘gold standard’ approach for the most accurate evaluation of the endometrium: endometrial hysteroscopic achieves higher sensitivity (78.4–98.0%) and specificity (92.0–95.8%) if compared to blind biopsy [14]. In a previous study, we evaluated the appropriateness of the indications for biopsy to detect endometrial cancer. In women of reproductive-aged, we found that 57% of hysteroscopic biopsies were inappropriate [18]. A consequence of this over-investigation is that many individuals are subjected to the potential harms and costs of treatment without almost none benefits [19].
Since the most frequent indication for endometrial evaluation in reproductive and perimenopausal age is the AUB, it is advisable to establish the optimal management of this symptom in order to decrease the number of inappropriate examinations and to therefore reduce the incidence of complications related to the procedure, such as infection, perforations, visceral injuries vasovagal attack, adverse reaction to anesthetics, and excessive pain [20,21,22,23]. Other than that, inappropriate biopsies are associated with a higher rate of dysfunctional endometrium rather than malignancy. Reducing these procedures allows for an increase in the sensitivity of hysteroscopy for pre-neoplastic and neoplastic lesions [24,25].
The present study aims to compare different diagnostic strategies recommended by the leading scientific societies to manage AUB in patients of reproductive age without risk of endometrial cancer [26,27].
2. Materials and Methods
2.1. Study Design and Patient Selection
We designed a retrospective cohort study including patients who underwent office hysteroscopy for AUB from January 2012 to December 2014 in the third level hysteroscopy service of the Gynecology Department of Institute for Maternal and Child Health—IRCCS Burlo Garofolo. This study represents a single-center experience.
Women of reproductive age with AUB, defined according to FIGO criteria 2011, were included in the study [4]. Exclusions criteria were: insufficient clinical data, menopause (defined as at least 12 months of amenorrhea), referral due to infertility, cervical disease, isthmocele, pyometra, ongoing endometrial infections, an incomplete procedure for excessive pain, or vagal reaction or risk factors of endometrial cancer: PCOS, ovarian tumors, obesity (body mass index > 30), tamoxifen therapy, family history of endometrial, ovarian, or intestinal cancer, unopposed estrogen therapy, and diabetes. Considering the possible association between endometrial cancer and hypertension described by some authors, we decided to exclude also patients with hypertension [28]. Considering that the sample also includes young women we did not take into account parity, and we considered only infertility as a risk factor. All women underwent hysteroscopically guided biopsy for the histological diagnosis. The study was approved by the Institutional review board (Scientific committee), reference number RC 17-2008, and all participants gave the consent for use their clinical details in scientific researches and scientific publications.
2.2. Diagnostic Algorithms Design
All patients were evaluated based on three algorithms set up according to the criteria of three different guidelines of scientific societies, which are: (A) the “EMAS clinical guide: assessment of the endometrium in peri e postmenopausal women” of the European Menopause and Andropause Society (EMAS); (B) the “Diagnosis of AUB in reproductive-aged women” of the American Congress of Obstetricians and Gynecologists (ACOG); and (C) the “AUB in premenopausal women” of the Society of Obstetricians and Gynecologists of Canada (SOGC) [12,16,17]. The EMAS algorithm considers hysteroscopy as appropriate if there is a focal growing lesion or in the case of lack of response to the progestin therapy. ACOG and SOGC guidelines add the patient’s age as an independent risk factor: ACOG considers hysteroscopy appropriate for every patient with AUB and ≥ 45 years old, whereas SOGC puts the cut off at ≥ 40 years of age [12,16,17]. Differences between the three algorithms used to define the need for endometrial biopsy are highlighted in Figure 1.
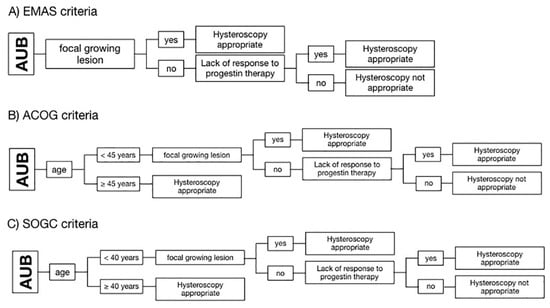
Figure 1.
Schematization of the algorithms used to define hysteroscopic biopsy appropriateness according to the criteria of three different guidelines of scientific societies. (A) European Menopause and Andropause Society (EMAS); (B) American Congress of Obstetricians and Gynecologists (ACOG); and (C) Society of Obstetricians and Gynecologists of Canada (SOGC). Diagrams show that ACOG and SOGC consider the age of 45 and 40 years, respectively, as the first criterion of appropriateness.
According to the PALM-COEIN classification, we considered as focal growing lesion ultrasound imagines suggestive for polyps or myomas [4]. Regarding the endometrial thickness there is no consensus in the current literature about a cut-off in premenopausal population associated with endometrial cancer, so we did not take into account this value in our analysis [1,29]. The diagnosis were all histological according to WHO 2014 classification. This separates endometrial hyperplasia into two groups based upon the presence of cytological atypia: hyperplasia without atypia and atypical hyperplasia [30]. All the positive exams have been reviewed by two pathologist experts in this field, and there was no discrepancy in the reports.
2.3. Statistical Analysis
Descriptive analyses were carried out following the characteristics of the studied variables. We calculated frequencies and percentages, means, and standard deviations or median and interquartile ranges, as appropriate. We calculated sensitivities, specificities, positive predictive values (PPV), negative predictive value (NPV), positive likehood ratio (LR+), and negative likehood ratio (LR-) in detecting endometrial carcinoma, atypical hyperplasia, and atypical polyp. All analyses were conducted with Stata/IC 14.2 (StataCorp LLC, College Station, TX, USA).
3. Results
3.1. Characteristics of the Study Population
From January 2012 to December 2014, 3036, patients underwent office hysteroscopy for AUB in our center. Following inclusion and exclusion criteria, a study population of 625 women was considered for the analysis. The population characteristics are reported in Table 1.

Table 1.
Population’s demographic characteristics.
In 365 (58.4%) patients, a focal growing lesion was detected by ultrasound, whereas, in 260 (41.6%) patients, the imaging evaluation was negative. Histological diagnosis are reported in Table 2.

Table 2.
Distribution of histological reports.
Of all biopsies, 7 women had a positive histological report of a malignant or premalignant lesion (1.1%) (Table 3).

Table 3.
Clinical characteristics of positive histological reports of malignant or premalignant lesions in the study population.
3.2. Application of the Diagnostic Algorithms on the Study Population
Using the EMAS algorithm, 456 procedures resulted appropriate (73%) and 169 inappropriate (27%). According to the ACOG guidelines, 545 procedures have been classified as appropriate (87%) and only 80 as inappropriate (13%). With the SOGC algorithm, 588 hysteroscopies resulted appropriate (94%) and 37 inappropriate (6%) (Figure 2). Remarkably, no pathological cases were ignored by any of the algorithms, meaning that the sensitivity was 100% for all. What appeared to be different was specificity. Algorithm A had a specificity of 27.3% (95% CI 23.9–31.0%), a PPV of 1.5%, an NPV of 100%, a LR+ of 1.38, and LR- of 0. Algorithm B had a specificity of 12.9% (95% CI 10.4–15.9%), a PPV of 1.28%, an NPV of 100%, a LR+ of 1.15, and a LR of 0. Specificity of algorithm C was 6.0% (95% CI 4.2–8.2%), PPV 1.19%, and NPV 100%, LR+ 1.06, and a LR of 0. By looking at the different guidelines, we could observe that the specificity of algorithm A, assessing the non-response to progesterone, without taking the age as risk factor, was more than double than algorithm B based on the 45 years cut-off and more than four times the algorithm C based on the 40 years cut-off. The confidence intervals of the three results do not overlap. We performed a post-test probability analysis, and we found that the incidence of positive cases was 1.5% with EMAS criteria, while it was 1.3% with ACOG guideline and 1.2% with SOGC criteria.
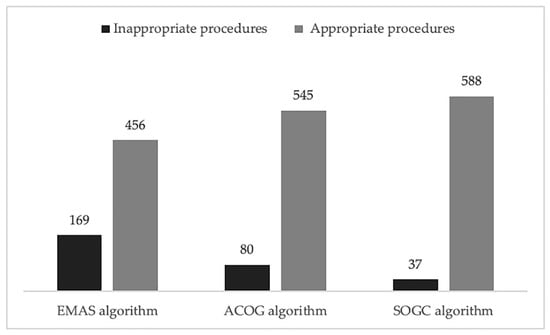
Figure 2.
Comparison between different algorithms. The bar chart shows the different number of appropriate and inappropriate hysteroscopies according the three different algorithms considered.
4. Discussion
There is no agreement among scientific societies on the criteria and indications that recommend endometrial evaluation in cases with AUB. The choice of the most suitable path must balance the prevalence of AUB in the population, the risk of cancer in symptomatic patients, and the feasibility and risk associated with diagnostic methods [20,21].
About 90% of women present at least one episode of AUB in the pre-menopausal transition period, and 78% of them have a recurrence of at least three episodes [2]. Our results suggest that up to 50% of patients have dysfunctional endometrium or hyperplasia without atypia probably due to transient anovulatory cycles, as already described in literature [13]. The risk of endometrial hyperplasia without atypia progressing to endometrial cancer is less than 5% over 20 years, and, in premenopausal women, the majority will regress spontaneously during follow-up [13]. Treatment with progesterone has a high disease regression rate.
Endometrial cancer is a typical postmenopausal disease, only 4.5–14% affects premenopausal women. In these women, 5-year survival at stage I is 92–100% [31,32].
In our clinical practice, we choose to perform hysteroscopy associated with targeted biopsy, considered the procedure high sensitivity and specificity [16,24,25]. It is possible to obtain an endometrial sample also with curettage, but this technique loses 11% of malignancy and 60% of atypical hyperplasia, and it carries all the collateral effects related to general anesthesia and complications due to the procedure itself. Endometrial sampling in an outpatient setting presents fewer risks, the complications’ rate is estimated between 1.2% and 3.8% (vasovagal reactions, perforation, and infections) but, even if it is well accepted in the majority of the patients, it is not free from pain [33]. This procedure presents some limitations: in 17% of cases, it is not possible to complete the procedure, and, in 7% of patients, the sample is inadequate for examination [16]. In these cases, could be necessary to perform an operative hysteroscopy in theatre, in order to reduce patient’s discomfort and allow an accurate diagnosis and treatment when possible. The prevalence of complications in operative hysteroscopy is estimated between 0.1 and 1.6% for uterine perforations, 0.02% for visceral injuries (urinary), and 1.9% for endometritis [20,21,33,34]. With a suspect of malignancy, the gold standard is to perform an endometrial sampling after hysteroscopy. A positive hysteroscopy result increases the probability of malignancy from <5% to 71.8%, whereas a negative result reduces the probability to 0.6%. Hysteroscopy in fact is more accurate in detecting endometrial disease rather than excluding it, and the accuracy is higher for endometrial cancer than for endometrial hyperplasia [11,35]. However, we found in a previous study that 57% of requests for hysteroscopy in reproductive-aged women were inappropriate according to the indications of the scientific societies [18].
Considering the high prevalence of AUB in premenopausal women, we have to find the diagnostic strategy that exposes our patients to less unnecessary procedures without losing positive cancer cases [36,37,38].
Based on the prevalence and the natural history of endometrial cancer in fertile age, it may be questionable whether the risks of diagnostic hysteroscopy do not outweigh the benefits [18].
The guidelines of the scientific societies (ACOG, SOGC, and EMA) agree that endometrial biopsy should be proposed to women with AUB who have risk factors for endometrial cancer [12,16,17]. There is also agreement that women who present ultrasound focal growing lesion suggestive of endometrial polyp or submucosal myoma must have direct access to hysteroscopy to avail themselves of the diagnostic and therapeutic treatment and that endometrial thickness in reproductive and premenopausal age, being very variable, cannot be used as a diagnostic criterion [1,12,16,17,29].
Instead, they differ from the criteria for low-risk women without focal lesion. The main difference is the age criterion, considered as an independent risk factor for malignancy and, therefore, an indication for immediate histological evaluation according to ACOG and SOGC algorithm [12,17]. The European guidelines, considering the therapeutic action of progesterone on hyperplasia without atypia, suggest treating all low-risk patients with progesterone and reserving the biopsy only for those who do not respond to treatment, continuing to experience bleeding [16]. In our cohort of patients, adding the age parameter in the diagnostic flow chart seems to increase the numbers of unnecessary procedures, considering the low cancer prevalence in this population [29]. This result is coherent with the recent literature [29,39]. Pennant et al. demonstrates in their review that it is not possible to identify a specific age cut-off in pre-menopausal population above which the risk of malignancy becomes meaningful [40].
Overall, the present study’s findings help to underline and clarify the importance of a progestin therapy trial and the crucial role of ultrasound uterus imaging. In particular, we strengthen the notion that, according to all the examined guidelines, without a recognized intrauterine lesion at transvaginal ultrasound, a trial of progestin therapy is useful to exclude hormonal dysfunctions, avoiding unnecessary invasive intervention. On the other hand, as we have shown in an Italian multicenter study, the presence of intrauterine polyps does not increase the risk of cancer if these are less than 22 mm, but progestin treatment does not resolve AUB in the presence of polyps [41].
Depending on the algorithm used for comparison, between 14% and 21% of biopsies could have been avoided when choosing as an exclusion criterion the response to medical therapy, with no apparent missed diagnosis.
By looking at the different guidelines, we could observe that the specificity of algorithm A, assessing the non-response to progesterone, without taking the age as risk factor, was more than double than algorithm B based on the 45 years cut-off (27.3% vs. 12.9%), and more than four times the algorithm C based on the 40 years cut-off (27.3% vs. 6%). This consequently determines a different incidence of malignancy: 1.5% with EMAS criteria, 1.3% with ACOG guidelines, and 1.2% with SOGC criteria.
Other than that, these findings agree with a previous observation that associated inappropriate hysteroscopic biopsies with a high rate of dysfunctional endometrium (up to 15% of cases), pointing out the importance of being able to rule out any underlying dysfunctional disturbance before the biopsy [11]. Delaying the biopsy of three months to allow a trial of progesterone therapy should not modify the prognosis of patients with endometrial cancer when a focal growing lesion is not detectable [42,43]. Indeed, AUB represents an early symptom of endometrial cancer characterized by a generally slow course that allows making the diagnosis at the initial stages in 70–90% of cases [44,45]. Furthermore, the prognosis of endometrial cancer in young women would appear to be even better than the already good prognosis of women in menopause [31,45,46].
To summarize, in case of AUB, an accurate anamnesis is the first necessary step that allows the clinician to exclude risk factors. The recognized risk factors for premenopausal endometrial cancer are PCOS, infertility, ovarian tumors, obesity (body mass index > 30), tamoxifen therapy, family history of endometrial, ovarian or intestinal cancer, unopposed estrogen therapy, diabetes, and hypertension [47]. In women with these characteristics, abnormal uterine bleeding indicates endometrial biopsy according to all the guidelines examined in the study [12,16,17,48]. Laboratory evaluation could be helpful to rule out menopause in some cases. We know the risk of malignancy to be higher in this group therefore a different management is needed. Whereas sex hormones’ level has not been correlated with endometrial cancer, so to know their hematic concentration is not helpful in the management of women with AUB considering also the high variability of sex hormones’ level at this age [49,50]. Thyroid’s disfunction, such as elevated TSH with low fT3 and fT4, and hyperprolactinemia could be associated with AUB, so, if there is clinical suspicious of endocrine disease, laboratory investigation could be useful [51]. After an accurate anamnesis and, if necessary, a clinical-laboratory evaluation, the ultimate decision on whether the biopsy of a patient can be delayed, to investigate the presence of a dysfunctional disturbance, depends on the ultrasound imaging analysis, which, therefore, plays a fundamental role, allowing to exclude the presence of focal growing lesions (i.e., polyps or myomas) that require surgical treatment, as well as ovarian anomalies. As we mentioned before, there is no consensus regarding the endometrial cut-off in premenopausal women associated with endometrial cancer because increase thickness is associated with dysfunctional endometrium, as well [1,29]. In case of negative ultrasound, we suggest three month of progestin therapy, according with all the three guidelines considered. If the patient still has AUB, endometrial biopsy is appropriate. With the suggested algorithm, we still have a high number of false positive cases, but this is acceptable if it does not miss any positive case. The diagnostic algorithm is synthetized in Figure 3.
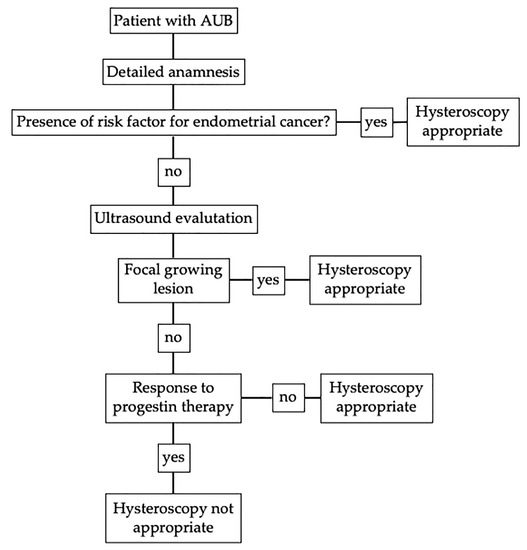
Figure 3.
Diagnostic algorithm without age criteria for patient with abnormal uterine bleeding (AUB) in fertile and pre-menopausal age.
Therefore, considering the study limitations related to the small sample size, we should concede that we selected a population at low risk of malignancy to compare the three algorithms, with a lower incidence of positive biopsies (1.1%) compared to postmenopausal women, but this data is consistent with the prevalence reported in literature [29,40].
In conclusion, the study shows that in our population, the best strategy to investigate AUB in women at low risk of endometrial cancer does not take into account an age cut-off, but the presence or absence of focal endometrial pathology at the ultrasound and the response to progestin therapy. This approach makes it possible to perform fewer hysteroscopic biopsies without excluding positive cases from the examination. As we suggested in a previous study [18], scientific societies should point out the risks of over-investigation and related overtreatment in their guidelines.
Author Contributions
Conceptualization, F.S. and G.R.; methodology, L.M.; software, S.B.; validation, F.S., F.R., and G.R.; formal analysis, L.M.; investigation, E.F.G.; resources, G.R.; data curation, L.M.; writing—original draft preparation, C.B.; writing—review and editing, F.S., C.B., S.B., and A.M.; visualization, F.S., C.B., and A.M.; supervision, F.R.; project administration, F.S.; funding acquisition, G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health (RC 08/2020-Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste, Italy).
Acknowledgments
The authors thank Martina Bradaschia for the English revision of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, M.J.; Kim, J.J.; Kim, S.M. Endometrial evaluation with transvaginal ultrasonography for the screening of endometrial hyperplasia or cancer in premenopausal and perimenopausal women. Obstet. Gynecol. Sci. 2016, 59, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, P.; Harlow, S.D.; Greendale, G.A.; Gold, E.B.; Crawford, S.L.; Elliott, M.R.; Lisabeth, L.D.; Randolph, J.F., Jr. Bleeding patterns during the menopausal transition in the multi-ethnic Study of Women’s Health Across the Nation (SWAN): A prospective cohort study. BJOG 2014, 121, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Astrup, K.; Olivarius Nde, F.; Moller, S.; Gottschau, A.; Karlslund, W. Menstrual bleeding patterns in pre- and perimenopausal women: A population-based prospective diary study. Acta Obstet. Gynecol. Scand. 2004, 83, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Critchley, H.O.; Broder, M.S.; Fraser, I.S.; Disorders, F.W.G.o.M. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int. J. Gynaecol. Obstet. 2011, 113, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Critchley, H.O.D.; Fraser, I.S.; Committee, F.M.D. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int. J. Gynaecol. Obstet. 2018, 143, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.R.; Gray, S.H. What to do when she’s bleeding through: The recognition, evaluation, and management of abnormal uterine bleeding in adolescents. Curr. Opin. Pediatr. 2014, 26, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Rezk, M.; Masood, A.; Dawood, R. Perimenopausal bleeding: Patterns, pathology, response to progestins and clinical outcome. J. Obstet. Gynaecol. 2015, 35, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Committee on Practice Bulletins-Gynecology. Practice bulletin no. 136: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet. Gynecol. 2013, 122, 176–185. [Google Scholar] [CrossRef]
- Wise, M.R.; Gill, P.; Lensen, S.; Thompson, J.M.; Farquhar, C.M. Body mass index trumps age in decision for endometrial biopsy: Cohort study of symptomatic premenopausal women. Am. J. Obstet. Gynecol. 2016, 215, 598 e591–598 e598. [Google Scholar] [CrossRef]
- (NIH), N.C.I. Cancer Stat Facts: Endometrial Cancer. Available online: http://seer.cancer.gov/statfacts/html/corp.html (accessed on 27 November 2017).
- RCOG/BSGE. Management of Endometrial Hyperplasia (Green-top Guideline No. 67). Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_67_endometrial_hyperplasia.pdf (accessed on 1 February 2017).
- Committee on Practice Bulletins-Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet. Gynecol. 2012, 120, 197–206. [Google Scholar] [CrossRef]
- Lacey, J.V., Jr.; Chia, V.M. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas 2009, 63, 39–44. [Google Scholar] [CrossRef]
- Lacey, J.V., Jr.; Sherman, M.E.; Rush, B.B.; Ronnett, B.M.; Ioffe, O.B.; Duggan, M.A.; Glass, A.G.; Richesson, D.A.; Chatterjee, N.; Langholz, B. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J. Clin. Oncol. 2010, 28, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Abu Hashim, H.; Ghayaty, E.; El Rakhawy, M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: A systematic review and metaanalysis of randomized trials. Am. J. Obstet. Gynecol. 2015, 213, 469–478. [Google Scholar] [CrossRef]
- Dreisler, E.; Poulsen, L.G.; Antonsen, S.L.; Ceausu, I.; Depypere, H.; Erel, C.T.; Lambrinoudaki, I.; Perez-Lopez, F.R.; Simoncini, T.; Tremollieres, F.; et al. EMAS clinical guide: Assessment of the endometrium in peri and postmenopausal women. Maturitas 2013, 75, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Best, C.; Dunn, S.; Leyland, N.; Wolfman, W.L.; Clinical Practice, G.C. Abnormal uterine bleeding in pre-menopausal women. J. Obstet. Gynaecol. Can. 2013, 35, 473–475. [Google Scholar] [CrossRef]
- Scrimin, F.; Wiesenfeld, U.; Galati, E.F.; Monasta, L.; Ricci, G. Hysteroscopic chasing for endometrial cancer in a low-risk population: Risks of overinvestigation. Arch. Gynecol. Obstet. 2016, 293, 851–856. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Cooper, R.J. Overdiagnosis of disease: A modern epidemic. Arch. Intern. Med. 2012, 172, 1123–1124. [Google Scholar] [CrossRef]
- Moynihan, R.; Doust, J.; Henry, D. Preventing overdiagnosis: How to stop harming the healthy. BMJ 2012, 344, e3502. [Google Scholar] [CrossRef]
- Glasziou, P.; Moynihan, R.; Richards, T.; Godlee, F. Too much medicine; too little care. BMJ 2013, 347, f4247. [Google Scholar] [CrossRef] [PubMed]
- van Kerkvoorde, T.C.; Veersema, S.; Timmermans, A. Long-term complications of office hysteroscopy: Analysis of 1028 cases. J. Minim. Invasive Gynecol. 2012, 19, 494–497. [Google Scholar] [CrossRef] [PubMed]
- McGurgan, P.M.; McIlwaine, P. Complications of hysteroscopy and how to avoid them. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Garuti, G.; Cellani, F.; Colonnelli, M.; Garzia, D.; Gonfiantini, C.; Luerti, M. Hysteroscopically targeted biopsies compared with blind samplings in endometrial assessment of menopausal women taking tamoxifen for breast cancer. J. Am. Assoc. Gynecol. Laparosc. 2004, 11, 62–67. [Google Scholar] [CrossRef]
- Cooper, J.M.; Erickson, M.L. Endometrial sampling techniques in the diagnosis of abnormal uterine bleeding. Obstet. Gynecol. Clin. N. Am. 2000, 27, 235–244. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Yang, H.P.; Pike, M.C.; McCann, S.E.; Yu, H.; Xiang, Y.B.; Wolk, A.; Wentzensen, N.; Weiss, N.S.; Webb, P.M.; et al. Type I and II endometrial cancers: Have they different risk factors? J. Clin. Oncol. 2013, 31, 2607–2618. [Google Scholar] [CrossRef]
- Smith, R.A.; von Eschenbach, A.C.; Wender, R.; Levin, B.; Byers, T.; Rothenberger, D.; Brooks, D.; Creasman, W.; Cohen, C.; Runowicz, C.; et al. American Cancer Society guidelines for the early detection of cancer: Update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: Update 2001—Testing for early lung cancer detection. CA Cancer J. Clin. 2001, 51, 38–75, quiz 77–80. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Vatten, L.J. Hypertension and the risk of endometrial cancer: A systematic review and meta-analysis of case-control and cohort studies. Sci. Rep. 2017, 7, 44808. [Google Scholar] [CrossRef]
- Giannella, L.; Cerami, L.B.; Setti, T.; Bergamini, E.; Boselli, F. Prediction of Endometrial Hyperplasia and Cancer among Premenopausal Women with Abnormal Uterine Bleeding. Biomed. Res. Int. 2019, 2019, 8598152. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Young, R.H.; Herrington, C.S. WHO Classification of Tumours of Female Reproductive Organs; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Pellerin, G.P.; Finan, M.A. Endometrial cancer in women 45 years of age or younger: A clinicopathological analysis. Am. J. Obstet. Gynecol. 2005, 193, 1640–1644. [Google Scholar] [CrossRef]
- Leo, L.; Arduino, S.; Febo, G.; Bellino, R.; Tessarolo, M.; Wierdis, T.; Lanza, A. Endometrial carcinoma in women 45 years of age or younger. Eur. J. Gynaecol. Oncol. 1996, 17, 403–405. [Google Scholar]
- Deffieux, X.; Gauthier, T.; Menager, N.; Legendre, G.; Agostini, A.; Pierre, F. Hysteroscopy: Guidelines for clinical practice from the French College of Gynaecologists and Obstetricians. Eur. J. Obstet. Gynecol. Reprod. Biol 2014, 178, 114–122. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: Management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet. Gynecol. 2013, 121, 891–896. [Google Scholar] [CrossRef]
- Clark, T.J.; Voit, D.; Gupta, J.K.; Hyde, C.; Song, F.; Khan, K.S. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: A systematic quantitative review. JAMA 2002, 288, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Marsh, F.A.; Rogerson, L.J.; Duffy, S.R. A randomised controlled trial comparing outpatient versus daycase endometrial polypectomy. BJOG 2006, 113, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Kremer, C.; Duffy, S.; Moroney, M. Patient satisfaction with outpatient hysteroscopy versus day case hysteroscopy: Randomised controlled trial. BMJ 2000, 320, 279–282. [Google Scholar] [CrossRef]
- van Hanegem, N.; Breijer, M.C.; Khan, K.S.; Clark, T.J.; Burger, M.P.; Mol, B.W.; Timmermans, A. Diagnostic evaluation of the endometrium in postmenopausal bleeding: An evidence-based approach. Maturitas 2011, 68, 155–164. [Google Scholar] [CrossRef]
- Corbacioglu Esmer, A.; Akbayir, O.; Goksedef, B.P.; Gunduz, N.; Kisacik, S.; Dagdeviren, H.; Guraslan, B.; Ark, C. Is there an appropriate cutoff age for sampling the endometrium in premenopausal bleeding? Gynecol. Obstet. Invest. 2014, 77, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Pennant, M.E.; Mehta, R.; Moody, P.; Hackett, G.; Prentice, A.; Sharp, S.J.; Lakshman, R. Premenopausal abnormal uterine bleeding and risk of endometrial cancer. BJOG 2017, 124, 404–411. [Google Scholar] [CrossRef]
- Garuti, G.; Luerti, M.; Leone, F.P.G.; Perrini, G.; Dealberti, D.; Vitelli, V.; Angioni, S.; Vitagliano, A.; Sardo, A.D.S.; Benassi, G.; et al. Prevalence and predictors of atypical histology in endometrial polyps removed by hysteroscopy: A secondary analysis from the SICMIG hysteroscopy trial. Facts Views Vis. Obgyn. 2019, 11, 127–134. [Google Scholar]
- Menczer, J.; Krissi, H.; Chetrit, A.; Gaynor, J.; Lerner, L.; Ben-Baruch, G.; Modan, B. The effect of diagnosis and treatment delay on prognostic factors and survival in endometrial carcinoma. Am. J. Obstet. Gynecol. 1995, 173, 774–778. [Google Scholar] [CrossRef]
- Levy, T.; Golan, A.; Menczer, J. Endometrial endometrioid carcinoma: A glimpse at the natural course. Am. J. Obstet. Gynecol. 2006, 195, 454–457. [Google Scholar] [CrossRef]
- Duska, L.R.; Garrett, A.; Rueda, B.R.; Haas, J.; Chang, Y.; Fuller, A.F. Endometrial cancer in women 40 years old or younger. Gynecol. Oncol. 2001, 83, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Cheung, M.K.; Shin, J.Y.; Husain, A.; Teng, N.N.; Berek, J.S.; Kapp, D.S.; Osann, K.; Chan, J.K. Prognostic factors for uterine cancer in reproductive-aged women. Obstet. Gynecol. 2007, 109, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Yoshida, M.; Kimura, M.; Kinoshita, K. Clinicopathologic study of uterine endometrial carcinoma in young women aged 40 years and younger. Int. J. Gynecol. Cancer 2005, 15, 657–662. [Google Scholar] [CrossRef]
- Opmeer, B.C.; van Doorn, H.C.; Heintz, A.P.; Burger, C.W.; Bossuyt, P.M.; Mol, B.W. Improving the existing diagnostic strategy by accounting for characteristics of the women in the diagnostic work up for postmenopausal bleeding. BJOG 2007, 114, 51–58. [Google Scholar] [CrossRef]
- Singh, S.; Best, C.; Dunn, S.; Leyland, N.; Wolfman, W.L. No. 292-Abnormal Uterine Bleeding in Pre-Menopausal Women. J. Obstet. Gynaecol. Can. 2018, 40, e391–e415. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Hankinson, S.E. Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids 2015, 99, 8–10. [Google Scholar] [CrossRef]
- Allen, N.E.; Key, T.J.; Dossus, L.; Rinaldi, S.; Cust, A.; Lukanova, A.; Peeters, P.H.; Onland-Moret, N.C.; Lahmann, P.H.; Berrino, F.; et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. Relat. Cancer 2008, 15, 485–497. [Google Scholar] [CrossRef]
- Abdel Hamid, A.M.; Borg, T.F.; Madkour, W.A. Prevalence of hyperprolactinemia and thyroid disorders among patients with abnormal uterine bleeding. Int. J. Gynaecol. Obstet. 2015, 131, 273–276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).