Nucleic Acid and Immunological Diagnostics for SARS-CoV-2: Processes, Platforms and Pitfalls
Abstract
1. Introduction
2. Nucleic Acid Amplification Techniques for SARS-CoV-2 Diagnosis
2.1. Specimen Types and Sampling Procedure
2.1.1. Specimen Types for Testing
2.1.2. Respiratory Sampling
2.1.3. Self-Collected Samples
2.1.4. Variation in the Viral Load
2.1.5. Sample Collection, Storage and Transport
2.2. RNA Isolation
RNA Isolation Quality Control
2.3. cDNA Synthesis
2.3.1. Reverse Transcription Systems
2.3.2. Primers for Reverse Transcription
2.3.3. RNase P as Control
2.3.4. Positive and Negative Controls
2.4. RT-qPCR for SARS-CoV-2
2.4.1. Primers for SARS-CoV-2 Detection
2.4.2. Comparative Sensitivity of the Primer Probe Sets Used for SARS-CoV-2
2.4.3. Amplifications Conditions
2.4.4. Data Analysis
2.5. Other Nucleic Acid Detection Technologies
2.6. Reverse Transcriptional Loop-Mediated Isothermal Amplification (RT-LAMP) for SARS-CoV-2 Detection
2.7. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas (CRISPR-Associated) Based Molecular Diagnostics
2.7.1. SHERLOCK
2.7.2. DETECTR
2.7.3. FELUDA
2.7.4. CARMEN-Cas13 System
2.8. Truenat–Affordable Chip-Based Portable PCR
2.9. Pooled Testing for Population Screening by RT-qPCR
2.10. Environmental Sampling for SARS-CoV-2
3. Immunological/Serological Tests for SARS-CoV-2
3.1. Enzyme Immunoassays
3.2. Classical Indirect ELISA
3.3. Solid-Phase Antibody Capture ELISA
3.4. Double Antigen ELISA
3.5. ELISA Employing Novel Solid Surfaces—(Bead-Based Automated ELISA)
3.6. Different Detection Methods
3.6.1. ELISA Using Chemiluminescence Detection Methods
3.6.2. Fluorescence Based ELISA
3.6.3. AlphaLISA
3.7. Antigens Used In Antibody Detection ELISA
4. Lateral Flow Tests (LFT)
4.1. Antigen Detection LFA/Immunochromatographic Tests
4.2. Precautionary Measures to Avoid Erroneous Results with Antigen Detection LFA Tests
4.2.1. Sampling
4.2.2. Sample Storage and Transportation
4.2.3. Sample Application
4.2.4. Factors Influencing Antigen Detection LFAs
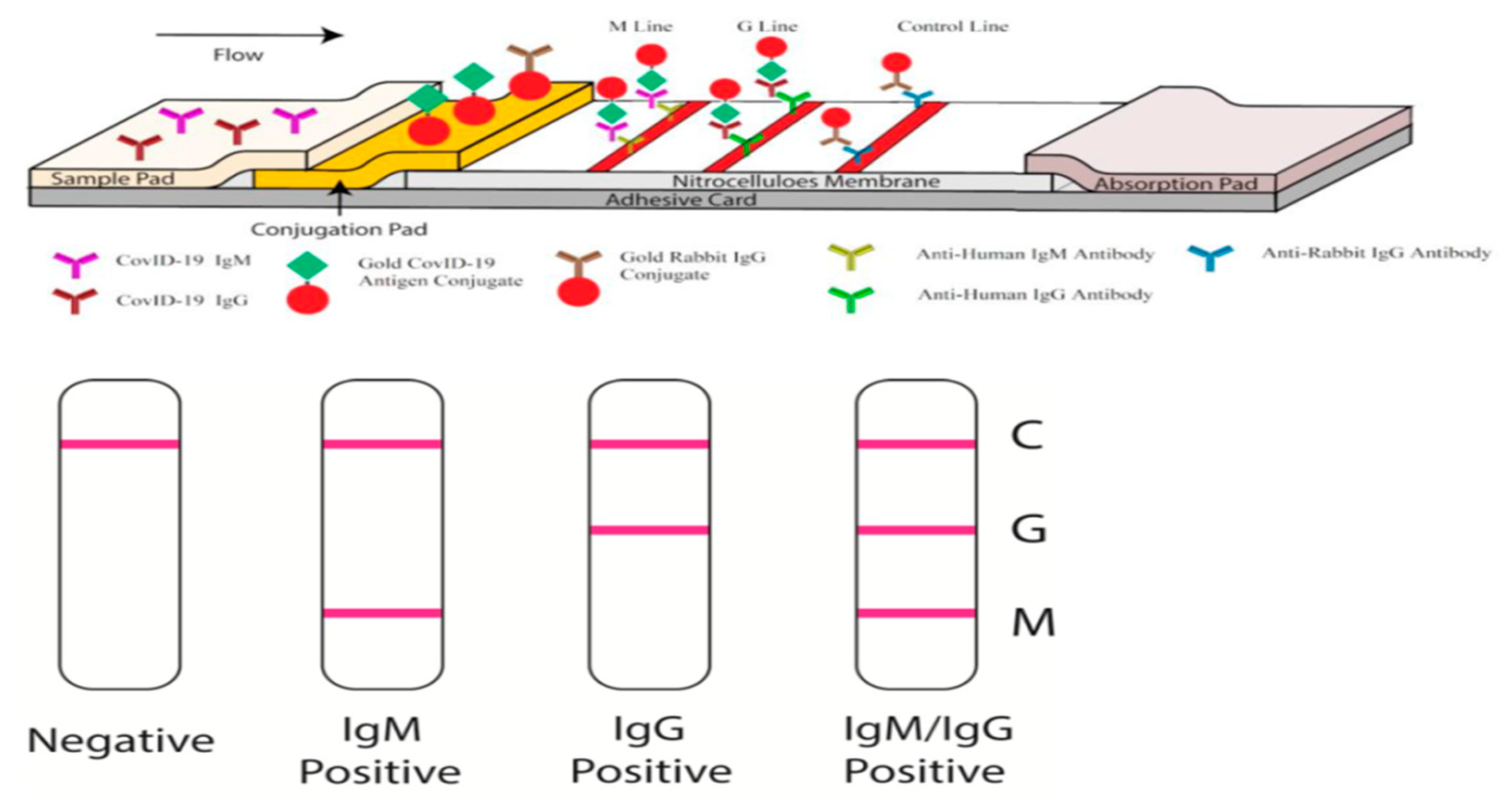
4.3. Antibody Detection LFAs
4.4. Precautionary Measures to Avoid Erroneous Results with Antibody Detection LFA Test
- A new operator uses the kit;
- A new lot of test kits is used;
- A new shipment of kits is used;
- The temperature used during storage of the kit falls outside of 2–30 °C;
- The temperature of the test area falls outside of 15–30 °C;
- To verify a higher than expected frequency of positive or negative results;
- To investigate the cause of repeated invalid results;
- A new test environment is used (e.g., natural light vs. artificial light).
4.5. Detection or Reporter Methods Used in LFAs
4.6. Comparison Between ELISA and LFA
4.7. Take Away From Serological Test Results
4.8. Potential Pitfalls in COVID-19 Testing
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. In Coronaviruses: Methods and Protocols; Humana Press: New York, NY, USA, 2015; ISBN 9781493924387. [Google Scholar]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 1–14. [Google Scholar] [CrossRef]
- Corman, V.; Bleicker, T.; Brunink, S.; Drosten, C. Diagnostic Detection of Wuhan Coronavirus 2019 by Real-Time RTPCR. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/wuhan-virus-assay-v1991527e5122341d99287a1b17c111902.pdf (accessed on 21 October 2020).
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA J. Am. Med. Assoc. 2020, 2–3. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412. [Google Scholar] [CrossRef]
- See, A.; Toh, S.T. Respiratory sampling for severe acute respiratory syndrome coronavirus 2: An Overview. Head Neck 2020, 42, 1652–1656. [Google Scholar] [CrossRef]
- Pan, D.; Sze, S.; Rogers, B.; Bron, J.; Bird, P.W.; Holmes, C.W.; Tang, J.W. Serial simultaneously self-swabbed samples from multiple sites show similarly decreasing SARS-CoV-2 loads in COVID-19 cases of differing clinical severity. J. Infect. 2020, 19–21. [Google Scholar] [CrossRef]
- Loeffelholz, M.J.; Tang, Y.W. Laboratory diagnosis of emerging human coronavirus infections–the state of the art. Emerg. Microbes Infect. 2020, 9, 747–756. [Google Scholar] [CrossRef]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Leung, E.C.; Chow, V.C.; Lee, M.K.; Lai, R.W. Deep throat saliva as an alternative diagnostic specimen type for the detection of SARS-CoV-2. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.K.C.; Chen, Z.; Lui, G.; Ling, L.; Li, T.; Wong, M.C.S.; Ng, R.W.Y.; Tso, E.Y.K.; Ho, T.; Fung, K.S.C.; et al. Prospective Study Comparing Deep Throat Saliva With Other Respiratory Tract Specimens in the Diagnosis of Novel Coronavirus Disease 2019. J. Infect. Dis. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Turner, F.; Slepnev, V.; Bacelar, A.; Deming, L.; Kodeboyina, S.; Klausner, J.D. Self-Collected Oral Fluid and Nasal Swabs Demonstrate Comparable Sensitivity to Clinician Collected Nasopharyngeal Swabs for Covid-19 Detection. medRxiv 2020. [Google Scholar] [CrossRef]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef]
- Abdollahi, A.; Shakoori, A.; Khoshnevis, H.; Arabzadeh, M.; Manshadi, S.A.D.; Mohammadnejad, E.; Ghasemi, D.; Aboksari, M.S.; Alizadeh, S.; Mehrtash, V.; et al. Comparison of patient-collected and lab technician-collected nasopharyngeal and oropharyngeal swabs for detection of COVID-19 by RT-PCR. Iran. J. Pathol. 2020, 15, 313–319. [Google Scholar] [CrossRef]
- Guest, J.L.; Sullivan, P.S.; Valentine-Graves, M.; Valencia, R.; Adam, E.; Luisi, N.; Nakano, M.; Guarner, J.; del Rio, C.; Sailey, C.; et al. Suitability and Sufficiency of Telehealth Clinician-Observed, Participant-Collected Samples for SARS-CoV-2 Testing: The iCollect Cohort Pilot Study. JMIR Public Health Surveill. 2020, 6, e19731. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Shen, C.; Wang, F.; Yuan, J.; Li, J.; Zhang, M.; Wang, Z.; Xing, L.; Wei, J.; et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv 2020. [Google Scholar] [CrossRef]
- CDC Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html (accessed on 21 October 2020).
- Karthik, K.; Aravindh Babu, R.P.; Dhama, K.; Chitra, M.A.; Kalaiselvi, G.; Alagesan Senthilkumar, T.M.; Raj, G.D. Biosafety Concerns During the Collection, Transportation, and Processing of COVID-19 Samples for Diagnosis. Arch. Med. Res. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Qiu, T.; Zeng, Y.; Wang, Y.; Zheng, S.; Chen, X.; Chen, Y. Comparative evaluation of three preprocessing methods for extraction and detection of influenza A virus nucleic acids from sputum. Front. Med. 2018, 5, 7–10. [Google Scholar] [CrossRef]
- Sung, H.; Yong, D.; Ki, C.S.; Kim, J.S.; Seong, M.W.; Lee, H.; Kim, M.N. Comparative evaluation of three homogenization methods for isolating middle east respiratory syndrome coronavirus nucleic acids from sputum samples for real-time reverse transcription PCR. Ann. Lab. Med. 2016, 36, 457–462. [Google Scholar] [CrossRef]
- Bruce, E.A.; Huang, M.; Perchetti, G.A.; Tighe, S.; Laaguiby, P.; Hoffman, J.J.; Gerrard, D.L.; Nalla, A.K.; Wei, Y.; Greninger, A.L.; et al. Direct RT-qPCR detection of SARS-CoV-2 RNA from patient nasopharyngeal swabs without an rna extraction step. bioRxiv Prepr. 2020, 1–14. [Google Scholar] [CrossRef]
- Lübke, N.; Senff, T.; Scherger, S.; Hauka, S.; Andrée, M.; Adams, O.; Timm, J.; Walker, A. Extraction-free SARS-CoV-2 detection by rapid RT-qPCR universal for all primary respiratory materials. J. Clin. Virol. 2020. [Google Scholar] [CrossRef]
- D’Cruz, R.J.; Currier, A.W.; Sampson, V.B. Laboratory Testing Methods for Novel Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2). Front. Cell Dev. Biol. 2020, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.A. Getting The Message with RT-PCR. Scientist 1998, 12, 20–22. [Google Scholar]
- Bustin, S.A.; Nolan, T. Pitfalls of quantitative real- time reverse-transcription polymerase chain reaction. J. Biomol. Tech. 2004, 15, 155–166. [Google Scholar] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.P.; Papenburg, J.; Desjardins, M.; Kanjilal, S.; Quach, C.; Libman, M.; Dittrich, S.; Yansouni, C.P. Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus-2: A Narrative Review. Ann. Intern. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vogels, C.B.F.; Brito, A.F.; Wyllie, A.L.; Fauver, J.R.; Ott, I.M.; Kalinich, C.C.; Petrone, M.E.; Landry, M.-L.; Foxman, E.F.; Grubaugh, N.D. Analytical sensitivity and efficiency comparisons of SARS-COV-2 qRT-PCR assays. medRxiv 2020. [Google Scholar] [CrossRef]
- Nalla, A.K.; Casto, A.M.; Huang, M.-L.W.; Perchetti, G.A.; Sampoleo, R.; Shrestha, L.; Wei, Y.; Zhu, H.; Jerome, K.R.; Greninger, A.L. Comparative Performance of SARS-CoV-2 Detection Assays using Seven Different Primer/Probe Sets and One Assay Kit. J. Clin. Microbiol. 2020. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.-M.; Lee, B. Insufficient Sensitivity of RNA Dependent RNA Polymerase Gene of SARS-CoV-2 Viral Genome as Confirmatory Test using Korean COVID-19 Cases. Preprints 2020, 1–4. [Google Scholar] [CrossRef]
- CDC. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel; 2020. Available online: https://www.cdc.gov/csels/dls/locs/2020/information_about_emergency_use_authorization_for_2019_novel_coronavirus_real_time_rt-pcr_diagnostic_panel.html (accessed on 21 October 2020).
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Lee, S.H.; Baek, Y.H.; Kim, Y.H.; Choi, Y.K.; Song, M.S.; Ahn, J.Y. One-pot reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) for detecting MERS-CoV. Front. Microbiol. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Cui, J.; Huang, L.; Du, B.; Chen, L.; Xue, G.; Li, S.; Zhang, W.; Zhao, L.; Sun, Y.; et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Baek, Y.H.; Um, J.; Antigua, K.J.C.; Park, J.-H.; Kim, Y.; Oh, S.; Kim, Y.; Choi, W.-S.; Kim, S.G.; Jeong, J.H.; et al. Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerg. Microbes Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wu, S.; Hao, X.; Dong, X.; Mao, L.; Pelechano, V.; Chen, W.-H.; Yin, X. Rapid detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. Clin. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Stoddart, D.; Harrington, E.D.; Beaulaurier, J.; Ly, L.; Reid, S.W.; Turner, D.J.; Juul, S. LamPORE: Rapid, accurate and highly scalable molecular screening for SARS-CoV-2 infection, based on nanopore sequencing. medRxiv Prepr. 2020, 2020. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012. [Google Scholar] [CrossRef]
- Myhrvold, C.; Freije, C.A.; Gootenberg, J.S.; Abudayyeh, O.O.; Metsky, H.C.; Durbin, A.F.; Kellner, M.J.; Tan, A.L.; Paul, L.M.; Parham, L.A.; et al. Field-deployable viral diagnostics using CRISPR-Cas13. Science 2018, 360, 444–448. [Google Scholar] [CrossRef]
- Joung, J.; Adha, A.; Segel, M.; Li, J.; Walker, B.D.; Greninger, A.L.; Jerome, K.R. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. medRxiv 2020. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-gonzalez, A.; et al. CRISPR—Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Mishra, A.; Paul, D.; Ansari, A.H.; Azhar, M.; Kumar, M.; Rauthan, R.; Sharma, N.; Aich, M.; Sinha, D.; et al. Francisella novicida Cas9 interrogates genomic DNA with very high specificity and can be used for mammalian genome editing. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.; Phutela, R.; Ansari, A.H.; Sinha, D.; Sharma, N.; Kumar, M.; Aich, M.; Sharma, S.; Rauthan, R.; Singhal, K.; et al. Rapid, field-deployable nucleobase detection and identification using FnCas9. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ackerman, C.M.; Myhrvold, C.; Thakku, S.G.; Freije, C.A.; Metsky, H.C.; Yang, D.K.; Ye, S.H.; Boehm, C.K.; Kosoko-Thoroddsen, T.-S.F.; Kehe, J.; et al. Massively multiplexed nucleic acid detection using Cas13. Nature 2020. [Google Scholar] [CrossRef]
- Nikam, C.; Kazi, M.; Nair, C.; Jaggannath, M.; Manoj, M.M.; Vinaya, R.V.; Shetty, A.; Rodrigues, C. Evaluation of the Indian TrueNAT micro RT-PCR device with GeneXpert for case detection of pulmonary tuberculosis. Int. J. Mycobacteriol. 2014, 3, 205–210. [Google Scholar] [CrossRef]
- Natesan, S.; Bhatia, R.; Sundararajan, A.; Dhama, K.; Malik, Y.S.; Vora, K. Ramping up of SARS CoV-2 testing for the diagnosis of COVID-19 to better manage the next phase of pandemic and reduce the mortality in India. VirusDisease 2020. [Google Scholar] [CrossRef]
- Yelin, I.; Aharony, N.; Shaer-Tamar, E.; Argoetti, A.; Messer, E.; Berenbaum, D.; Shafran, E.; Kuzli, A.; Gandali, N.; Hashimshony, T.; et al. Evaluation of COVID-19 RT-qPCR test in multi-sample pools. medRxiv 2020. [Google Scholar] [CrossRef]
- Lohse, S.; Pfuhl, T.; Berkó-Göttel, B.; Rissland, J.; Geißler, T.; Gärtner, B.; Becker, S.L.; Schneitler, S.; Smola, S. Pooling of samples for testing for SARS-CoV-2 in asymptomatic people. Lancet Infect. Dis. 2020, 3099, 2019–2020. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Klochendler, A.; Seidel, M.; Sido, T.; Gurel-Gurevich, O.; Yassour, M.; Meshorer, E.; Benedek, G.; Fogel, I.; Oiknine-Djian, E.; et al. Large-scale implementation of pooled RNA extraction and RT-PCR for SARS-CoV-2 detection. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Abdalhamid, B.; Bilder, C.R.; McCutchen, E.L.; Hinrichs, S.H.; Koepsell, S.A.; Iwen, P.C. Assessment of Specimen Pooling to Conserve SARS CoV-2 Testing Resources. Am. J. Clin. Pathol. 2020, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shmuel, A.; Brosh-Nissimov, T.; Glinert, I.; Bar-David, E.; Sittner, A.; Poni, R.; Cohen, R.; Achdout, H.; Tamir, H.; Yahalom-Ronen, Y.; et al. Detection and infectivity potential of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) environmental contamination in isolation units and quarantine facilities. Clin. Microbiol. Infect. 2020, 2, 4–8. [Google Scholar] [CrossRef]
- Ding, Z.; Qian, H.; Xu, B.; Huang, Y.; Miao, T.; Yen, H.L.; Xiao, S.; Cui, L.; Wu, X.; Shao, W.; et al. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci. Total Environ. 2020, 753, 141710. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Lertxundi, U.; Barcelo, D. Early SARS-CoV-2 outbreak detection by sewage-based epidemiology. Sci. Total Environ. 2020, 732, 139298. [Google Scholar] [CrossRef] [PubMed]
- WHO. “Immunity Passports” in the Context of COVID-19. 2020. Available online: https://www.who.int/news-room/commentaries/detail/immunity-passports-in-the-context-of-covid-19 (accessed on 21 October 2020).
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Nag, P.; Sadani, K.; Mukherji, S. Optical Fiber Sensors for Rapid Screening of COVID-19. Trans. Indian Natl. Acad. Eng. 2020, 5, 233–236. [Google Scholar] [CrossRef]
- Murugan, D.; Bhatia, H.; Sai, V.V.R.; Satija, J. P-FAB: A Fiber-Optic Biosensor Device for Rapid Detection of COVID-19. Trans. Indian Natl. Acad. Eng. 2020, 5, 211–215. [Google Scholar] [CrossRef]
- Tripathi, S.; Agrawal, A. Blood Plasma Microfluidic Device: Aiming for the Detection of COVID-19 Antibodies Using an On-Chip ELISA Platform. Trans. Indian Natl. Acad. Eng. 2020, 5, 217–220. [Google Scholar] [CrossRef]
- Jääskeläinen, A.J.; Kekäläinen, E.; Kallio-Kokko, H.; Mannonen, L.; Kortela, E.; Vapalahti, O.; Kurkela, S.; Lappalainen, M. Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Eurosurveillance 2020, 25. [Google Scholar] [CrossRef]
- Adams, E.R.; Anand, R.; Andersson, M.I.; Auckland, K.; Baillie, J.K.; Barnes, E.; Bell, J.; Berry, T.; Bibi, S.; Carroll, M.; et al. Evaluation of antibody testing for SARS-Cov-2 using ELISA and lateral flow immunoassays. medRxiv 2020. [Google Scholar] [CrossRef]
- Graham, D.A.; Mawhinney, K.A.; McShane, J.; Connor, T.J.; Adair, B.M.; Merza, M. Standardization of enzyme-linked immunosorbent assays (ELISAs) for quantitative estimation of antibodies specific for infectious bovine rhinotracheitis virus, respiratory syncytial virus, parainfluenza-3 virus, and bovine viral diarrhea virus. J. Vet. Diagn. Investig. 1997, 9, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lu, D.; Zhang, M.; Che, J.; Yin, Z.; Zhang, S.; Zhang, W.; Bo, X.; Ding, Y.; Wang, S. Double-antigen sandwich ELISA for detection of antibodies to SARS-associated coronavirus in human serum. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, L.; Zhang, M.; Hu, Y.; Yang, Q.; Guo, J.; Dai, Y.; Xu, Y.; Cai, Y.; Chen, X.; et al. Evaluations of serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak. medRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K. In vitro diagnostic assays for COVID-19: Recent advances and emerging trends. Diagnostics 2020, 10, 202. [Google Scholar] [CrossRef]
- Taylor, J. New York SARS-CoV Microsphere Immunoassay for Antibody Detection. Available online: https://www.fda.gov/media/137540/download (accessed on 21 October 2020).
- Kimpston-Burkgren, K.; Mora-Díaz, J.C.; Roby, P.; Bjustrom-Kraft, J.; Main, R.; Bosse, R.; Giménez-Lirola, L.G. Characterization of the humoral immune response to porcine epidemic diarrhea virus infection under experimental and field conditions using an AlphaLISA platform. Pathogens 2020, 9, 233. [Google Scholar] [CrossRef]
- Perera, R.A.; Mok, C.K.; Tsang, O.T.; Lv, H.; Ko, R.L.; Wu, N.C.; Yuan, M.; Leung, W.S.; Chan, J.M.; Chik, T.S.; et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Eurosurveillance 2020, 25. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Zhang, X.; Hasoksuz, M.; Nagesha, H.S.; Haynes, L.M.; Fang, Y.; Lu, S.; Saif, L.J. Two-Way Antigenic Cross-Reactivity between Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Group 1 Animal CoVs Is Mediated through an Antigenic Site in the N-Terminal Region of the SARS-CoV Nucleoprotein. J. Virol. 2007, 81, 13365–13377. [Google Scholar] [CrossRef]
- Yong, S.E.F.; Anderson, D.E.; Wei, W.E.; Pang, J.; Chia, W.N.; Tan, C.W.; Teoh, Y.L.; Rajendram, P.; Toh, M.P.H.S.; Poh, C.; et al. Connecting clusters of COVID-19: An epidemiological and serological investigation. Lancet Infect. Dis. 2020, 20. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Sajid, M.; Kawde, A.N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Bendavid, E.; Mulaney, B.; Sood, N.; Shah, S.; Ling, E.; Bromley-Dulfano, R.; Lai, C.; Weissberg, Z.; Saavedra, R.; Tedrow, J.; et al. COVID-19 Antibody Seroprevalence in Santa Clara County, California. medRxiv 2020. [Google Scholar] [CrossRef]
- Lisboa Bastos, M.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.-P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic accuracy of serological tests for covid-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Feng, Y.; Mo, X.; Zheng, P.; Wang, Q.; Li, P.; Peng, P.; Liu, X.; Chen, Z.; Huang, H.; et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 940–948. [Google Scholar] [CrossRef]
- Krüttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.; Imöhl, M.; Kleines, M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J. Clin. Virol. 2020, 128, 104394. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, I.; Gruson, D.; Kabamba, B.; Dahma, H.; Van den Wijngaert, S.; Reza, S.; Carbone, V.; Vandenberg, O.; Gulbis, B.; Wolff, F.; et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020, 128, 104413. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Diao, B.; Ren, F.; Wang, Y.; Ding, J.; Huang, Q. Diagnostic Indexes of a Rapid IgG/IgM Combined Antibody Test for SARS-CoV-2. medRxiv 2020. [Google Scholar] [CrossRef]
- Lee, N.Y.; Li, C.W.; Tsai, H.P.; Chen, P.L.; Syue, L.S.; Li, M.C.; Tsai, C.S.; Lo, C.L.; Hsueh, P.R.; Ko, W.C. A case of COVID-19 and pneumonia returning from Macau in Taiwan: Clinical course and anti-SARS-CoV-2 IgG dynamic. J. Microbiol. Immunol. Infect. 2020. [Google Scholar] [CrossRef]
- Gorse, G.J.; Donovan, M.M.; Patel, G.B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J. Med. Virol. 2020, 92, 512–517. [Google Scholar] [CrossRef]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease 2019 Patients. Emerg. Infect. Dis. 2020, 26. [Google Scholar] [CrossRef]
- Nishiura, H.; Jung, S.; Linton, N.M.; Kinoshita, R.; Yang, Y.; Hayashi, K.; Kobayashi, T.; Yuan, B.; Akhmetzhanov, A.R. The Extent of Transmission of Novel Coronavirus in Wuhan, China, 2020. J. Clin. Med. 2020, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, Q.N.; Wang, F.L.; Ma, X.M.; Wang, X.Y.; Zhang, X.G.; Zhang, Z.F. Characteristics of asymptomatic patients with SARS-CoV-2 infection in Jinan, China. Microbes Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, K.; Chowell, G. Transmission potential of the novel coronavirus (COVID-19) onboard the diamond Princess Cruises Ship, 2020. Infect. Dis. Model. 2020. [Google Scholar] [CrossRef] [PubMed]
- Omori, R.; Mizumoto, K.; Chowell, G. Changes in testing rates could mask the novel coronavirus disease (COVID-19) growth rate. Int. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Hong, K.H.; Lee, S.W.; Kim, T.S.; Huh, H.J.; Lee, J.; Kim, S.Y.; Park, J.-S.; Kim, G.J.; Sung, H.; Roh, K.H.; et al. Guidelines for Laboratory Diagnosis of Coronavirus Disease 2019 (COVID-19) in Korea. Ann. Lab. Med. 2020, 40, 351–360. [Google Scholar] [CrossRef]
- Xu, H.; Yan, L.; Qiu, C.M.; Jiao, B.; Chen, Y.; Tan, X.; Chen, Z.; Ai, L.; Xiao, Y.; Luo, A.; et al. Analysis and Prediction of False Negative Results for SARS-CoV-2 Detection with Pharyngeal Swab Specimen in COVID-19 Patients: A Retrospective Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Wikramaratna, P.; Paton, R.S.; Ghafari, M.; Kingdom, U. Estimating false-negative detection rate of SARS-CoV-2 by RT-PCR. medRxiv 2020, 413. [Google Scholar] [CrossRef]
- Kucirka, L.M.; Lauer, S.A.; Laeyendecker, O.; Boon, D.; Lessler, J. Variation in False Negative Rate of RT-PCR Based SARS-CoV-2 Tests by Time Since Exposure. medRxiv Prepr. 2020, 1–9. [Google Scholar] [CrossRef]
- Yedidag, E.N.; Koffron, A.J.; Mueller, K.H.; Kaplan, B.; Kaufman, D.B.; Fryer, J.P.; Stuart, F.P.; Asecassis, M. Acyclovir triphosphate inhibits the diagnostic polymerase chain reaction for cytomegalovirus. Transplantation 1996. [Google Scholar] [CrossRef]
| No | Test Name | SARS-CoV-2 Target Genes | Control Assay | Assay Type | Sensitivity (Limit of Detection) | Regulatory Status | Company |
|---|---|---|---|---|---|---|---|
| 1 | RealStar SARS-CoV-2 RT-PCR Kit 1.0 | E and S | Internal control | Multiplex | NA | FDA EUA, CE-IVD | Altona Diagnostics GmbH, Hamburg, Germany |
| 2 | TaqPath COVID-19 RT-PCR | ORF1ab, N, S | MS2 phage internal control | Multiplex | NA | US FDA EUA, CE-IVD | Applied Biosystems - Thermo Fisher, CA, USA |
| 3 | 2019-nCoV: Real-Time Fluorescent RT-PCR kit | ORF1ab | β-Actin | Multiplex | 150 viral copies/mL from thrat swab samples | NMPA Certified/CE Marked/FDA Approved/PMDA Approved | BGI Genomics Co. Ltd., Shenzhen, China |
| 4 | SARS-CoV-2 R-GENE test | N, RdRp and E | Internal control | Multiplex | 380 copies/mL | CE-IVD | bioMérieux SA, Marcy-l’Étoile, France |
| 5 | CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel | N1, N2, RP | Rnase P | Singleplex | 1 to 3.2 copies/μL | FDA EUA | Center for Disease Control, Atlanta, GA, USA |
| 6 | EURORealTime SARS-CoV-2 | ORF1ab and N | internal extraction control | Multiplex | 1 copy/μL nucleic acid eluate | CE-IVD | EUROIMMUN AG, Lubeck, Germany |
| 7 | SARS-CoV-2 Real-time RT-PCR Assay | ORF1ab and N | MS2 phage internal control | Multiplex | 20 copies/mL | FDA EUA | PerkinElmer Inc, Austin, TX, USA |
| 8 | geneSig- Real Time PCR Coronavirus COVID-19 | RdRp | internal extraction control | Multiplex | 0.58 copies/μL of SARS-CoV-2 viral RNA | US FDA EUA - CE-IVD - WHO EUL | Primerdesign Ltd., Chandler’s Ford, UK |
| 9 | Lyra SARS-CoV-2 Assay | Nsp pp1ab | MS2 phage internal control | Multiplex | 8.00E-01 genomic RNA copies/μL | FDA EUA, CE | Quidel Corporation, San Diego, CA, USA |
| 10 | cobas SARS-CoV-2 Test | ORF-1a/b & E | MS2 phage internal control | Using cobas 6800 system, multiplex | 689.3 copies/mL | FDA EUA, CE-IVD | Roche Diagnostics Mannheim, Germany |
| 11 | Allplex 2019-nCoV Assay | E, RdRP & N | MS2 phage control | Multiplex | 1250 to 4167 copies/mL | US, FDA EUA, - Korea MFDS EUA - Singapore HSA - CE-IVD | Seegene Inc, Seoul, Republic of Korea |
| 12 | SARS-CoV-2 RealTime PCR Kit | N & E | Rnase P | Multiplex | 50 copies/tube | CE-IVD | Vircell SL, Granada, Spain |
| 1. ELISAs Including Automated Immunoassays (IAs) | |||||||||
| No | Name of Test | Assay Type | Capture Antigen | Target Analyte | Specificity | Sensitivity | Regulatory Status | Assay Read out | Company/Location |
| 1 | Wantai SARS-CoV-2 IgM ELISA | antibody capture ELISA | RBD domain | IgM | 100% | 86.8% | CE-IVD | Microplate reader | Beijing Wantai Biological Pharmacy Enterprise Co., Ltd. |
| 2 | Wantai SARS-CoV-2 Ab ELISA | double antigen | RBD domain | Total Ab | 100% | 94.5% | CE-IVD | Microplate reader | Beijing Wantai Biological Pharmacy Enterprise Co., Ltd. |
| 3 | EDI Novel Coronavirus COVID-19 IgG ELISA kit | indirect ELISA | Not provided | IgG | Not provided | Not provided | CE-IVD | Microplate reader | Epitope Diagnostics, Inc. |
| 4 | EDI Novel Coronavirus COVID-19 IgM ELISA kit | indirect ELISA | Not provided | IgM | Not provided | Not provided | CE-IVD | Microplate reader | Epitope Diagnostics, Inc. |
| 5 | Anti-SARS-CoV-2 ELISA (IgA) | indirect ELISA | S1 with RBD region | IgA | 92% | 98.6% | CE-IVD | Microplate reader | EUROIMMUN AG |
| 6 | Anti-SARS-CoV-2 ELISA (IgG) | indirect ELISA | S1 domain of spike | IgG | 99.6% | 94.4% | CE-IVD; USA; Brazil | Microplate reader | EUROIMMUN AG |
| 7 | 2019 Novel Coronavirus IgG Test (ELISA) | indirect ELISA | Not provided | IgG | Not provided | Not provided | RUO | Microplate reader | Guangzhou Darui Biotechnology Co., Ltd. |
| 8 | 2019 Novel Coronavirus IgM Test (ELISA) | indirect ELISA | Not provided | IgM | Not provided | Not provided | RUO | Microplate reader | Guangzhou Darui Biotechnology Co., Ltd. |
| 9 | VITROS® SARS-CoV-2 Antibody kit | micro well, Chemiluminescence | Not provided | IgG | 100% | 90% | RUO | VITROS ECi/ECiQ/3600 | Ortho-Clinical Diagnostics, Inc |
| 10 | COVID-19 ELISA IgG Antibody Test | Indirect ELISA | Not provided | IgG | 100% | 92.5% | EUA 4/15/2020 | Microplate reader | Mount Sinai Laboratory |
| 11 | LIAISON® SARS-CoV-2 IgG | DiaSorin | Magnetic beads Chemiluminescence | S1 and S2 spike domain | IgG | 97.4 | 98.5 | RUO | Dedicated equipment | DiaSorin Inc. |
| 12 | Platelia SARS-CoV-2 Total Ab Assay | indirect ELISA | nucleoprotein | All Ig | 99.56 | 100 | EUA 4/29/2020 | Microplate reader | Bio-Rad Co., Ltd. |
| 13 | Elecsys anti-SARS-CoV-2 serology test | Electro-chemiluminescence immunoassay (ECLIA) | Not provided | IgG | 99.8 | 100 | RUO | cobas e analyser series | Roche Co., Ltd. |
| 14 | New York SARS-CoV Microsphere Immunoassay | luminex | nucleocapsid of SARS-CoV-1 | IgG | 79.3 | 96.7 | RUO | Luminex reader | Wadsworth Center, New York State Department of Health |
| 15 | Abbott Alinity i ARS-CoV-2 IgG | Chemilumunscnece | Nucleocapsid | IgG | 100 | 99.9 | EUA 05/11/2020 | ARCHITECT and Alinity systems | Abbott Laboratories |
| 16 | Diazyme DZ-LITE SARS-CoV-2 IgG, IgM CLIA Kits | chemiluminescence | Not provided | IgG/IgM | 97.3 | 91.2 | Not provided | Not provided | Diazyme Laboratories |
| 17 | NovaLisa® SARS-CoV-2 IgG | indirect ELISA | Not provided | IgG | Not provided | Not provided | CE-IVD CE mark 4/2020 | Microplate reader | Gold Standard Diagnostics/Eurofins Technologies |
| 18 | ErbaLisa COVID-19 ELISA kits | indirect ELISA | Not provided | IgG | 98.1 | 98.3 | CE mark 4/2020 | Microplate reader | Erba Mannheim |
| 19 | UBI SARS-CoV-2 ELISA | indirect ELISA | Not provided | NA | NA | CE mark 4/2020 | Microplate reader | United Biomedical | |
| 2. Antigen (Ag)-based LFAs | |||||||||
| Name of Test | Assay Type | Capture Antibody | Target analyte | Specificity | Sensitivity | Regulatory status | Assay Read out | Company/location | |
| 1 | COVID-19 Ag Respi-Strip | gold conjugate | Not provided | Not provided | 100 | 60 | CE-IVD | Visual | Coris BioConcept |
| 2 | BIOCREDIT COVID-19 Ag | gold conjugate | Not provided | Not provided | Not provided | Not provided | CE-IVD | Visual | RapiGEN, Inc. |
| 3 | STANDARD F COVID-19 Ag FIA | time-resolved fluorescence europium | Not provided | Not provided | Not provided | Not provided | CE-IVD | Reader | SD BIOSENSOR, INC. |
| 4 | STANDARD Q COVID-19 Ag Test | gold conjugate | Not provided | Not provided | Not provided | Not provided | CE-IVD | Visual | SD BIOSENSOR, INC. |
| 5 | BIOEASY 2019-nCoV Ag Fluorescence Rapid Test Kit (time-resolved fluorescence) | time-resolved fluorescence | Not provided | Not provided | Not provided | Not provided | CE-IVD | Reader | Shenzhen Bioeasy Biotechnology Co., Ltd. |
| 6 | Sofia 2 SARS Antigen FIA | time-resolved fluorescence | Not provided | Not provided | Not provided | Not provided | EUA 5/8/2020 | Reader | Quidel Co., Ltd. |
| 7 | ichromaTM COVID-19 Ag test | time-resolved fluorescence | Not provided | Not provided | 97 | 95.8 | CE-IVD | Reader | Boditech Co., Ltd. |
| 8 | 2019-Novel Coronavirus (2019-nCoV) Antigen Rapid Test Kit (FIA) | fluorescence | Not provided | Not provided | Not provided | Not provided | CE-IVD | Reader | Bioeasy Co., Ltd. |
| 3. Antibody based LFAs | |||||||||
| No | Name of Test | Assay Type | Capture Antibody | Target analyte | Specificity | Sensitivity | Regulatory status | Assay Read out | Company/location |
| 1 | 2019-nCoV IgG/IgM Antibody Determination Kit | gold conjugate | Not provided | IgM/IgG | NA | NA | CE-IVD | Reader required | Beijing Diagreat Biotechnologies Co., Ltd. |
| 2 | Tigsun COVID-19 Combo IgM/IgG Rapid Test (lateral flow) | gold conjugate | Not provided | IgM/IgG | NA | NA | CE-IVD; India | Visual | Beijing Tigsun Diagnostics Co., Ltd. |
| 3 | Wantai SARS-CoV-2 Ab Rapid Test | gold conjugate | Not provided | Total Ab | NA | NA | Australia | Visual | Beijing Wantai Biological Pharmacy Enterprise Co., Ltd. |
| 4 | COVID-19 IgM-IgG Combined Antibody Rapid Test | gold conjugate | Not provided | IgM/IgG | NA | NA | CE-IVD; India | Visual | BioMedomics, Inc. |
| 5 | iChroma COVID-19 Ab | time-resolved fluorescence | Not provided | IgM/IgG | 96.7 | 95.8 | RUO | Reader required | Boditech Inc. |
| 6 | Rapid Response COVID-19 IgG/IgM Test Cassette (whole blood/serum/plasma) | gold conjugate | Not provided | IgM/IgG | NA | NA | RUO | Visual | BTNX, Inc. |
| 7 | Cellex qSARS-CoV-2 IgG/IgM Cassette Rapid Test | gold conjugate | Not provided | IgM/IgG | NA | NA | CE-IVD; USA | Visual | Cellex, Inc. |
| 8 | COVID-19 IgM/IgG Ab Test | gold conjugate | Not provided | IgM/IgG | NA | NA | CE-IVD | Visual | Core Technology Co., Ltd. |
| 9 | 2019-nCoV IgG/IgM Rapid Test | gold conjugate | Not provided | IgM/IgG | 92 | NA | CE-IVD | Visual | Dynamiker Biotechnology Co., Ltd. |
| 10 | GenBody COVID-19 IgM/IgG | gold conjugate | Not provided | IgM/IgG | 97.5 | 95.2 | CE-IVD; Australia; Brazil | 95.2 | GenBody Inc. |
| 11 | RightSign COVID-19 IgG/IgM Rapid Test | gold conjugate | Not provided | IgM/IgG | NA | NA | CE-IVD | Visual | Hangzhou Biotest Biotech Co., Ltd. |
| 12 | PerfectPOC Novel Corona Virus (SARS-CoV-2) IgM/IgG Rapid Test Kit | gold conjugate | Not provided | IgM/IgG | 95.7 | NA | CE-IVD | Visual | Jiangsu Bioperfectus Technology Co., Ltd. |
| 13 | HIGHTOP COVID-19 IgM/IgG Ab Rapid Test Kit | gold conjugate | Not provided | IgM/IgG | NA | NA | CE-IVD | Visual | Qingdao Hightop Biotech Co., Ltd. |
| 14 | BIOCREDIT COVID-19 IgG+IgM Duo | gold conjugate | Not provided | IgM/IgG | NA | NA | CE-IVD | Visual | RapiGEN Co., Ltd. |
| 15 | STANDARDTM Q COVID-19 IgM/IgG Combo Test | gold conjugate | Not provided | IgM/IgG | 95.09 | 94.3 | CE-IVD; Brazil | Visual | SD BIOSENSOR, INC |
| 16 | BIOEASY 2019-nCoV Ab (IgG/IgM) GICA Rapid Test Kit | gold conjugate | Not provided | IgM/IgG | NA | NA | CE-IVD | Visual | Bioeasy Biotechnology Co., Ltd. |
| 17 | VivaDiagTM COVID-19 IgM/IgG Rapid Test | gold conjugate | Not provided | IgM/IgG | NA | NA | CE-IVD | Visual | VivaChek Biotech (Hangzhou) Co., Ltd. |
| 18 | Diagnostic Kit for IgG Antibody to Corona Virus (nCoV-2019) | gold conjugate | Not provided | IgG + IgM | NA | NA | CE-IVD; China | Visual | Zhuhai Livzon Diagnostics, Inc. |
| 19 | COVID-19 IgG/IgM Rapid Test | gold conjugate | Not provided | IgG + IgM | NA | NA | CE-IVD | Visual | Assure Tech Co., Ltd. |
| 20 | Novel Coronavirus IgM/IgG Combo Rapid Test | gold conjugate | Not provided | IgG + IgM | NA | NA | EUA submission pending | Visual | Decombio Biotechnology Co., Ltd. |
| 21 | SARS-CoV-2 IgG/IgM Antibody Detection Kit | gold conjugate | Not provided | IgG + IgM | NA | NA | CE mark 4/2020 | Visual | Beroni Group Co., Ltd. |
| 22 | 2019-nCoV IgG/IgM Detection Kit (Colloidal Gold) | gold conjugate | Not provided | IgG + IgM | NA | NA | CE mark 4/2020 | Visual | Biolidics Co., Ltd. |
| 23 | COVID-19 IgM-IgG Rapid Test | gold conjugate | Not provided | IgG + IgM | NA | NA | EUA submission pending | Visual | BioMedomics Co., Ltd. |
| 24 | AccuRapid SARS-CoV-2 IgM/IgG Test Kit (Lateral Flow Immunoassay | gold conjugate | Not provided | IgG + IgM | NA | NA | EUA submission pending | Visual | Eachy Biopharmaceuticals Co., Ltd. |
| 25 | One Step SARS-CoV-2 (COVID-19) IgG/IgM Test | gold conjugate | Not provided | IgG + IgM | NA | NA | CE mark 5/2020 | Visual | Hangzhou Testsea biotechnology Co., LTD |
| 26 | COVID-19 IgG/IgM Rapid Test | gold conjugate | Not provided | IgG + IgM | 97.5 | 96.7 | CE mark 5/2020 | Visual | Healgen Scientific, LLC |
| 27 | SARS-CoV-2 IgM/IgG Antibody Rapid Test Kit | gold conjugate | Not provided | IgG + IgM | 98.7 | 93.3 | CE mark 5/2020 | Visual | Nanjing Liming Bio-products Co., Ltd. |
| 28 | COVID-19 (SARS-CoV-2) IgG Antibody Detection Kit | gold conjugate | Not provided | IgG + IgM | 100 | 97.5 | CE mark 5/2020 | Visual | Nirmidas Biotech Co., Ltd. |
| 29 | PCL COVID-19 IgG/IgM Rapid Gold | gold conjugate | Not provided | IgG+ IgM | NA | NA | CE mark 5/2020 | Visual | Vitrex Medical A/S |
| 30 | MosaiQ COVID-19 Antibody Microarray | fluorescence | Not provided | IgG/IgM | 99.8 | 100 | CE mark 5/2020 | Reader | Quotient Limited Co., Ltd. |
| 31 | SureScreen COVID-19 IgM/IgG Rapid Test Cassette | gold conjugate | Not provided | IgG+ IgM | 99 | 91 | CE mark 2020 | Visual | SureScreen Diagnostics Co., Ltd. |
| 32 | SARS-CoV-2 IgG/IgM Antibody Detection Kit | gold conjugate | Not provided | IgG+ IgM | NA | NA | CE mark 2020 | Visual | Tianjin Beroni Biotechnology Co., Ltd. |
| 33 | Diagnostic Kit for IgM/IgG Antibody to Coronavirus (SARS-CoV-2) | gold conjugate | Not provided | IgG+ IgM | NA | NA | CE mark 2020 | Visual | Zhuhai Livzon Diagnostics Co., Ltd. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Premraj, A.; Aleyas, A.G.; Nautiyal, B.; Rasool, T.J. Nucleic Acid and Immunological Diagnostics for SARS-CoV-2: Processes, Platforms and Pitfalls. Diagnostics 2020, 10, 866. https://doi.org/10.3390/diagnostics10110866
Premraj A, Aleyas AG, Nautiyal B, Rasool TJ. Nucleic Acid and Immunological Diagnostics for SARS-CoV-2: Processes, Platforms and Pitfalls. Diagnostics. 2020; 10(11):866. https://doi.org/10.3390/diagnostics10110866
Chicago/Turabian StylePremraj, Avinash, Abi George Aleyas, Binita Nautiyal, and Thaha J Rasool. 2020. "Nucleic Acid and Immunological Diagnostics for SARS-CoV-2: Processes, Platforms and Pitfalls" Diagnostics 10, no. 11: 866. https://doi.org/10.3390/diagnostics10110866
APA StylePremraj, A., Aleyas, A. G., Nautiyal, B., & Rasool, T. J. (2020). Nucleic Acid and Immunological Diagnostics for SARS-CoV-2: Processes, Platforms and Pitfalls. Diagnostics, 10(11), 866. https://doi.org/10.3390/diagnostics10110866





