The Influence of Serum Sample Storage Conditions on Selected Laboratory Parameters Related to Oxidative Stress: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
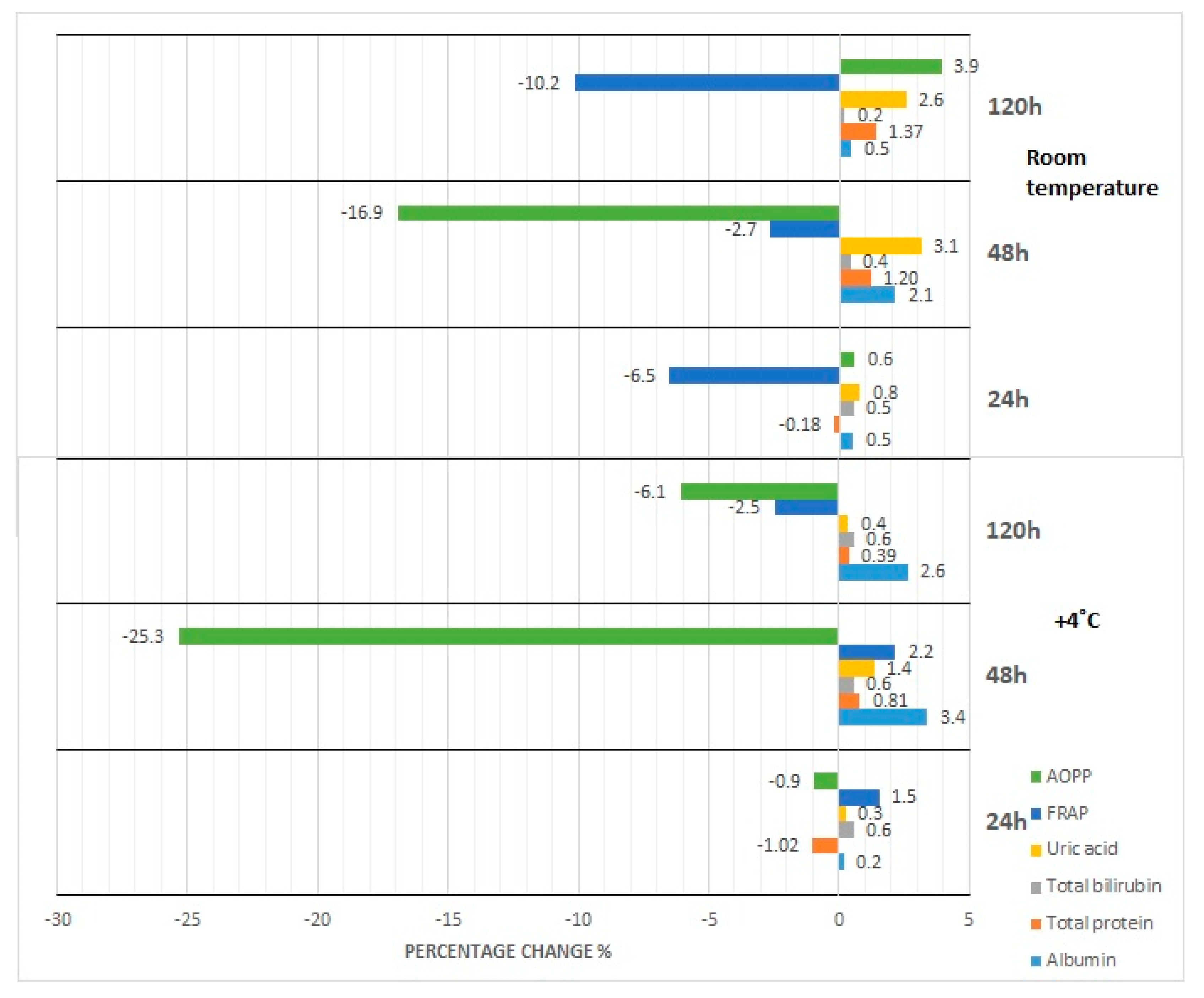
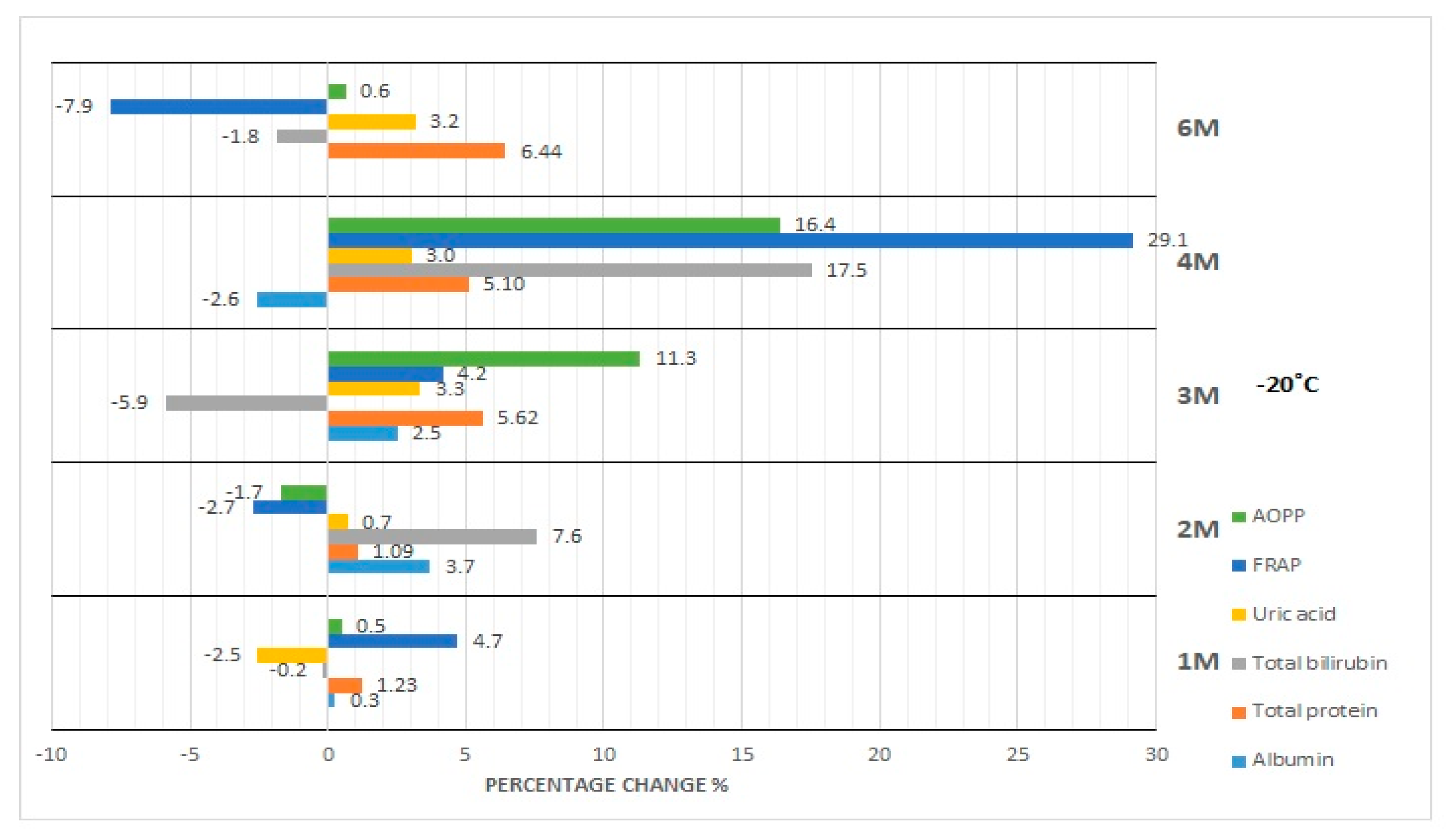
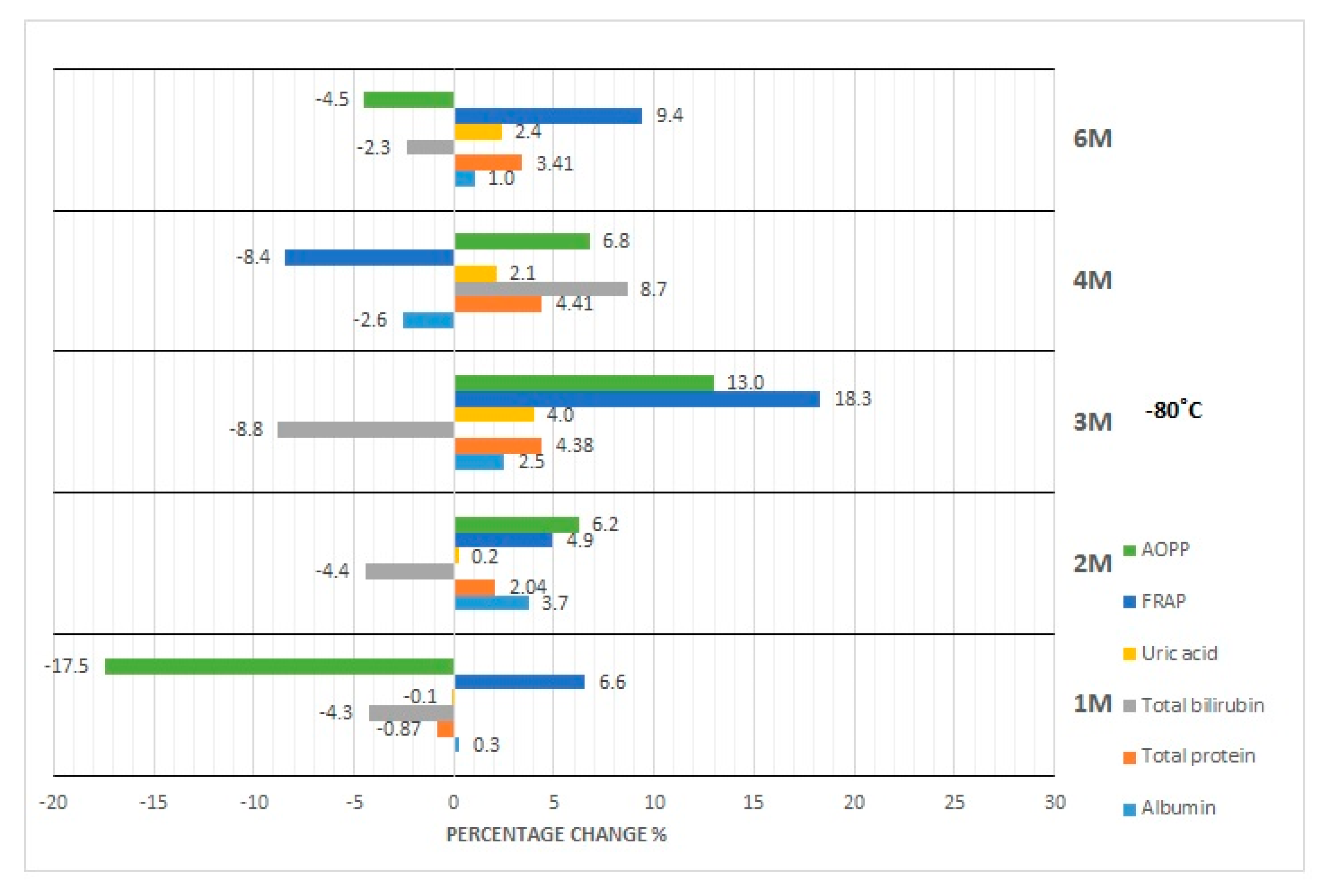
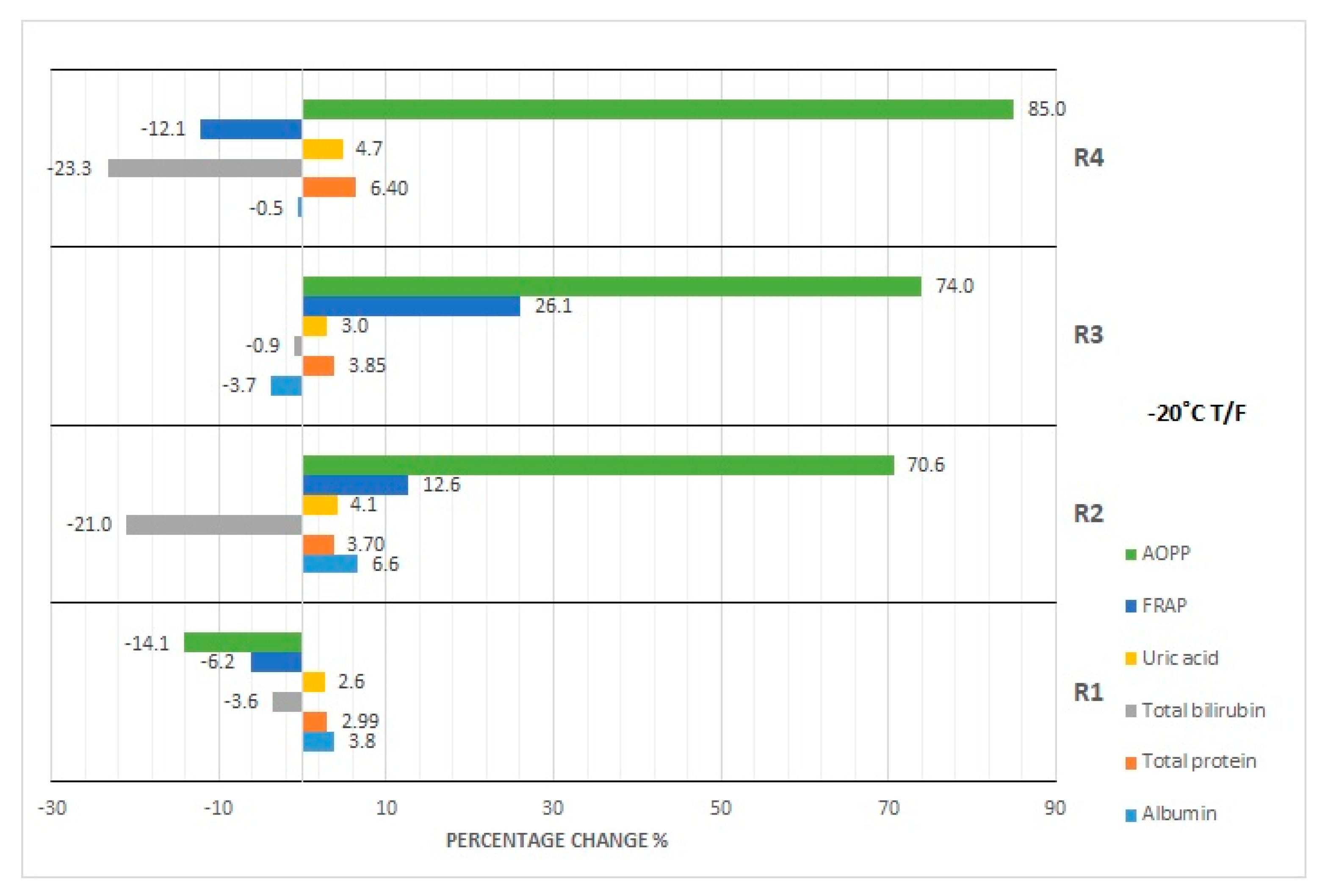
3.1. Albumin Concentration
3.2. Total Protein Concentration
3.3. Bilirubin Concentration
3.4. Uric Acid Concentration
3.5. FRAP Concentration
3.6. AOPP Concentration
4. Discussion
4.1. Effect of Storage at Room Temperature and Refrigeration
4.2. Effect of Storage at −20 °C and −80 °C
4.3. Effect of Repeated Freezing and Thawing
4.4. Limitations
4.5. Strengths
5. Summary
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wians, F.H. Clinical laboratory tests: Which, why, and what do the results mean? Lab. Med. 2009, 40, 105–113. [Google Scholar] [CrossRef]
- Ignatowicz, A. Biobanki—wyłaniające się narzędzie badawcze ku lepszej wiedzy dotyczącej zdrowia ludzkiego i chorób. Biobanks—Emerging research tool in advancing our understanding of human health and diseases. Pomoc Rozw. 2014, 2, 4–12. (In Polish) [Google Scholar]
- Lippi, G.; Becan-McBride, K.; Behúlová, D.; Bowen, R.A.; Church, S.; Delanghe, J.; Grankvist, K.; Kitchen, S.; Nybo, M.; Nauck, M.; et al. Preanalytical quality improvement: In quality we trust. Clin. Chem. Lab. Med. 2013, 51, 229–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plebani, M. The detection and prevention of errors in laboratory medicine. Ann. Clin. Biochem. 2010, 47, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Gernand, W. Wskaźniki jakości w laboratoryjnej diagnostyce medycznej. Quality indicators in laboratory medical diagnostics. Diagn. Lab. 2011, 47, 39–43. (In Polish) [Google Scholar]
- Pietruczuk, M.; Bartoszko-Tyczkowska, A. Laboratory Diagnostics. In Klinikleitfaden Labordiagnostik (Clinical Guide); Neumeister, B., Besenthal, I., Böhm, B.O., Eds.; Elsevier Urban&Partner: Wrocław, Poland, 2013. (In Polish) [Google Scholar]
- Rana, S.V. No preanalytical errors in laboratory testing: A beneficial aspect for patients. Indian J. Clin. Biochem. 2012, 27, 319–321. [Google Scholar] [CrossRef] [Green Version]
- Pahwa, M.B.; Menaka, K.; Minakshi Raj, M.; Singh, V. Effect of storage time and temperature on serum clinical biochemistry analytes. Biochem. Ind. J. 2015, 9, 150–160. [Google Scholar]
- Comstock, G.W.; Burke, A.E.; Norkus, E.P.; Gordon, G.B.; Hoffman, S.C.; Helzlsouer, K.J. Effects of repeated freeze-thaw cycles on concentrations of cholesterol, micronutrients, and hormones in human plasma and serum. Am. J. Epidemiol. 2008, 1, 827–830. [Google Scholar] [CrossRef]
- Jansen, E.H.J.; Beekhof, P.K.; Cremers, J.W.J.M.; Viezeliene, D.; Muzakova, V.; Skalicky, J. Short-term stability of biomarkers of oxidative stress and antioxidant status in human serum. ISRN Biomark. 2013, 2013, 316528. Available online: https://www.hindawi.com/journals/isrn/2013/316528/ (accessed on 30 May 2019). [CrossRef]
- Jansen, E.H.J.; Beekhof, P.; Cremers, J.; Weinberger, B.; Fiegl, S.; Toussaint, O.; Bernhard, J.; Gonos, E.; Capri, M.; Franceschi, C.; et al. Quality control data of physiological and immunological biomarkers measured in serum and plasma. Mech. Ageing Dev. 2015, 151, 54–59. [Google Scholar] [CrossRef]
- Jansen, E.H.J.; Beekhof, P.K.; Viezeliene, D.; Muzakova, V.; Skalicky, J. Long-term stability of oxidative stress biomarkers in human serum. Free Radic. Res. 2017, 51, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Plantier, J.L.; Duretz, V.; Devos, V.; Urbain, R.; Jorieux, S. Comparison of antioxidant properties of different therapeutic albumin preparations. Biologicals 2016, 44, 226–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boon, A.C.; Lam, A.K.; Gopalan, V.; Benzie, I.F.; Briskey, D.; Coombes, J.S.; Fassett, R.G.; Bulmer, A.C. Endogenously elevated bilirubin modulates kidney function and protects from circulating oxidative stress in a rat model of adenine-induced kidney failure. Sci. Rep. 2015, 5, 15482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Settle, T.; Klandorf, H. The Role of Uric Acid as an Antioxidant in Selected Neurodegenerative Disease Pathogenesis: A Short Review. Brain Disord. Ther. 2014, 3, 129. [Google Scholar] [CrossRef] [Green Version]
- Fabbrini, E.; Serafini, M.; Colic Baric, I.C.; Hazen, S.L.; Klein, S. Effect of Plasma Uric Acid on Antioxidant Capacity, Oxidative Stress, and Insulin Sensitivity in Obese Subjects. Diabetes 2014, 63, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Ricos, C.; Alvarez, V.; Cava, F.; Garcia-Lario, J.V.; Hernandez, A.; Jimenez, C.V.; Minchinela, J.; Perich, C.; Simon, M. Current databases on biologic variation: Pros, cons and progress. Scand. J. Clin. Lab. Invest. 1999, 59, 491–500. [Google Scholar]
- Ricos, C.; Alvarez, V.; Cava, F.; Garcia-Lario, J.V.; Hernandez, A.; Jimenez, C.V.; Minchinela, J.; Perich, C.; Simon, M. Desirable Specifications for Total Error, impreCision, and Bias, Derived from Intra- and Inter-Individual Biologic Variation. 2014. Available online: http://www.westgard.com/biodatabase1.htm (accessed on 30 May 2019).
- Theodorsson, E.; Magnusson, B.; Leito, I. Bias in clinical chemistry. Bioanalysis 2014, 6, 2855–2875. [Google Scholar] [CrossRef] [Green Version]
- German Medical Association; Instand e.V. Guidelines of the German Medical Association on quality assurance in medical laboratory testing. GMS Z. Forder. Qualitatssich. Med. Lab. 2015, 6. [Google Scholar] [CrossRef]
- Application: Albumin. Available online: https://assets.thermofisher.com/TFS-Assets/CDD/manuals/Albumin-Reagent-EN.pdf (accessed on 30 May 2019).
- Novotny, A. Basic Exercises in Immunochemistry; Springer: Berlin/Heidelberg, Germany, 1979; pp. 168–169. [Google Scholar]
- Application: Total Protein. Available online: https://assets.thermofisher.com/TFS-Assets/CDD/manuals/JL840890-Total-Protein-Reagent-EN.pdf (accessed on 30 May 2019).
- Application: Bilirubin. Available online: https://assets.thermofisher.com/TFS-Assets/CDD/manuals/Total-Bilirubin-Reagent-EN.pdf (accessed on 30 May 2019).
- Application: Uric Acid. Available online: https://assets.thermofisher.com/TFS-Assets/CDD/manuals/Infinity-Uric-Acid-Liquid-Stable-Reagent-EN.pdf (accessed on 30 May 2019).
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nquyen-Khoa, T.; Nquyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembińska-Kieć, A.; Naskalski, J.W. Laboratory Diagnostics with Elements of Clinical Biochemistry; Diagnostyka Laboratoryjna z Elementami Biochemii Klinicznej; Urban&Partner: Wrocław, Poland, 2005. [Google Scholar]
- Oddoze, C.; Lombard, E.; Portugal, H. Stability study of 81 analytes in human whole blood, in serum and in plasma. Clin. Biochem. 2012, 45, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Dirar, A.M.; Abdallah, D.A.; Abdelsalam, K.E.A. Effect of storage time and temperature on some serum analytes. Int. J. Pathol. 2010, 8, 68–71. [Google Scholar]
- Cuhadar, S.; Atay, A.; Koseoglu, M.; Dirican, A.; Hur, A. Stability studies of common biochemical analytes in serum separator tubes with or without gel barrier subjected to various storage conditions. Biochem. Med. 2012, 22, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Guder, W.G.; Narayanan, S.; Wisser, H.; Zawta, B. Samples: from the Patient to the Laboratory. In The Impact of Preanalytical Variables on the Quality of Laboratory Results; GIT Verlag: Darmstadt, Germany, 1996. [Google Scholar]
- Ministry of Health. Annex No. 3 to the Ordinance of the Minister of Health of March 23, 2006 on Quality Standards for Medical Diagnostic and Microbiological Laboratories. Ministerstwo Zdrowia. Załącznik nr 3 do Rozporządzenia Ministra Zdrowia z dnia 23 marca 2006 r. w sprawie standardów jakości dla medycznych laboratoriów diagnostycznych i mikrobiologicznych. Available online: http://prawo.sejm.gov.pl/isap.nsf/download.xsp/WDU20060610435/O/D20060435.pdf (accessed on 7 June 2019).
- Kift, R.L.; Byrne, C.; Liversidge, R.; Babbington, F.; Binns, J.; Barth, J.H. The effect of storage conditions on sample stability in the routine clinical laboratory. Ann. Clin. Biochem. 2015, 52, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Cuhadar, S.; Koseoglu, M.; Atay, A.; Dirican, A. The effect of storage time and freeze-thaw cycles on the stability of serum samples. Biochem. Med. 2013, 23, 70–77. [Google Scholar] [CrossRef]
- Amin, S.B.; Ahlfors, C. Effect of Storage and Freezing on Unbound Bilirubin Measurement. Clin. Chim. Acta 2008, 396, 56–57. [Google Scholar] [CrossRef] [Green Version]
- Jansen, E.H.; Beekhof, P.K.; Cremers, J.W.; Viezeliene, D.; Muzakova, V.; Skalicky, J. Long-term stability of parameters of antioxidant status in human serum. Free Radic. Res. 2013, 47, 535–540. [Google Scholar] [CrossRef]
- Kachhawa, K.; Kachhawa, P.; Varma, M.; Behera, R.; Agrawal, D.; Kumar, S. Study of the stability of various biochemical analytes in samples stored at different predefined storage conditions at an accredited laboratory of India. J. Lab. Physicians 2017, 9, 11–15. [Google Scholar] [CrossRef]
- Brinc, D.; Chan, M.K.; Venner, A.A.; Pasic, M.D.; Colantonio, D.; Kyriakopolou, L.; Adeli, K. Long-term stability of biochemical markers in pediatric serum specimens stored at −80 °C: A CALIPER Substudy. Clin. Biochem. 2012, 45, 816–826. [Google Scholar] [CrossRef]
- Sanfelice, D.; Temussi, P.A. Cold denaturation as a tool to measure protein stability. Biophys. Chem. 2016, 208, 4–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matteucci, E.; Biasci, E.; Giampietro, O. Advanced oxidation protein products in plasma: Stability during storage and correlation with other clinical characteristics. Acta Diabetol. 2001, 38, 187–189. [Google Scholar] [CrossRef]
- Zeng, Q.; E, L.-L.; Wang, D.-S.; Liu, H.-C. Relationship of advanced oxidative protein products in human saliva and plasma: Age- and gender-related changes and stability during storage. Free Radic. Res. 2012, 46, 1201–1206. [Google Scholar]
- Firuzi, O.; Mladenka, P.; Riccieri, V.; Spadaro, A.; Petrucci, R.; Marrosu, G.; Saso, L. Parameters of oxidative stress status in healthy subjects: Their correlations and stability after sample collection. J. Clin. Lab. Anal. 2006, 20, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.R.J. Prospects for the use of antioxidant therapies. Drugs 1995, 49, 345–361. [Google Scholar] [CrossRef]
| Storage Period | Group I | Procedure | |||
| 24 (h) | RT; 4 °C | Examination of serum | |||
| 48 (h) | |||||
| 120 (h) | |||||
| Storage Period | Group II | Procedure | Group III | Cycle | Procedure |
| 1 (M) | −20 °C; −80 °C | Thawing and examination of serum | −20°; −80 °C | T/F | Thawing, and re-freezing of serum |
| 2 (M) | R1 | Thawing, examination and re-freezing of serum | |||
| 3 (M) | R2 | ||||
| 4 (M) | R3 | ||||
| 6 (M) | R4 |
| Time Temperature | T0 | 24 h RT | 48 h RT | 120 h RT | 24 h FT | 48 h FT | 120 h FT | 1 M −20 °C | 2 M −20 °C | 3 M −20 °C | 4 M −20 °C | 6 M −20 °C | 1 M −80 °C | 2 M −80 °C | 3 M −80 °C | 4 M –80 °C | 6 M −80 °C | R1 −20 °C | R2 −20 °C | R3 −20 °C | R4 −20 °C | R1 −80 °C | R2 −80 °C | R3 −80 °C | R4 −80 °C | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Results | ||||||||||||||||||||||||||
| Albumin (g/dL) (3.5–5.0) * | ||||||||||||||||||||||||||
| Mean concentration | 4.31 | 4.33 | 4.33 | 4.40 | 4.32 | 4.43 | 4.46 | 4.39 | 4.46 | 4.45 | 4.47 | 4.52 | 4.32 | 4.47 | 4.51 | 4.43 | 4.55 | 4.47 | 4.60 | 4.38 | 4.64 | 4.39 | 4.52 | 4.43 | 4.61 | |
| Standard Deviation | 0.30 | 0.39 | 0.33 | 0.30 | 0.34 | 0.41 | 0.38 | 0.34 | 0.49 | 0.37 | 0.39 | 0.39 | 0.44 | 0.39 | 0.38 | 0.29 | 0.43 | 0.44 | 0.42 | 0.33 | 0.51 | 0.36 | 0.38 | 0.30 | 0.53 | |
| Median | 4.28 | 4.31 | 4.25 | 4.35 | 4.28 | 4.38 | 4.36 | 4.31 | 4.32 | 4.41 | 4.37 | 4.39 | 4.27 | 4.40 | 4.41 | 4.38 | 4.39 | 4.39 | 4.47 | 4.34 | 4.46 | 4.30 | 4.42 | 4.39 | 4.43 | |
| Range | 3.81–5.02 | 3.46–5.04 | 3.92–5.08 | 3.98–5.14 | 3.92–5.05 | 3.95–5.34 | 4.02–5.23 | 3.95–5.15 | 3.95–5.62 | 3.78–5.18 | 3.99–5.43 | 4.03–5.27 | 3.75–5.30 | 3.72–5.33 | 3.94–5.25 | 9.96–5.10 | 3.99–5.32 | 3.72–5.33 | 4.04–5.27 | 3.89–5.21 | 4.02–5.79 | 3.78–5.17 | 3.88–5.25 | 4.00–5.16 | 4.07–5.72 | |
| Concentration percentage change (DB = 1.43%) | 0.00 | 0.46 | 0.52 | 2.13 | 0.17 | 2.64 | 3.38 | 1.94 | 3.37 | 3.09 | 3.56 | 4.81 | 0.26 | 3.71 | 4.70 | 2.72 | 5.45 | 3.76 | 6.78 | 1.59 | 7.52 | 1.72 | 4.82 | 2.67 | 6.91 | |
| p value | 0.666 | 0.627 | <0.05 | 0.793 | 0.522 | <0.05 | 0.016 | 0.350 | 0.003 | 0.057 | 0.000 | 0.794 | 0.001 | 0.000 | 0.000 | 0.000 | 0.004 | 0.000 | 0.014 | 0.000 | 0.079 | 0.007 | 0.00 | 0.00 | ||
| Total Protein (g/dL)(6.0–8.0) * | ||||||||||||||||||||||||||
| Mean concentration | 6.79 | 6.78 | 6.89 | 6.87 | 6.72 | 6.85 | 6.82 | 6.87 | 6.86 | 7.15 | 7.14 | 7.23 | 6.73 | 6.93 | 7.09 | 7.09 | 7.04 | 6.99 | 7.04 | 7.05 | 7.22 | 6.84 | 7.01 | 7.17 | 7.08 | |
| Standard Deviation | 0.41 | 0.36 | 0.40 | 0.37 | 0.49 | 0.43 | 0.53 | 0.36 | 0.38 | 0.44 | 0.41 | 0.42 | 0.36 | 0.43 | 0.38 | 0.46 | 0.45 | 0.45 | 0.44 | 0.49 | 0.44 | 0.38 | 0.45 | 0.51 | 0.42 | |
| Median | 6.69 | 6.75 | 6.84 | 6.84 | 6.67 | 6.73 | 6.73 | 6.86 | 6.82 | 7.07 | 7.09 | 7.17 | 6.72 | 6.82 | 7.07 | 7.03 | 7.12 | 6.83 | 6.87 | 6.93 | 7.19 | 6.74 | 6.94 | 7.12 | 7.10 | |
| Range | 6.11–7.83 | 6.24–7.73 | 6.21–7.82 | 6.23–7.86 | 5.89–7.99 | 6.17–7.85 | 6.14–8.14 | 6.18–7.82 | 6.34–7.81 | 6.38–8.25 | 6.56–8.22 | 6.63–8.25 | 5.96–7.50 | 6.36–7.91 | 6.49–8.15 | 6.43–8.31 | 6.39–8.06 | 6.00–8.15 | 6.52–8.25 | 6.44–8.33 | 6.40–8.39 | 6.32–7.95 | 6.43–8.30 | 6.44–8.54 | 6.41–8.21 | |
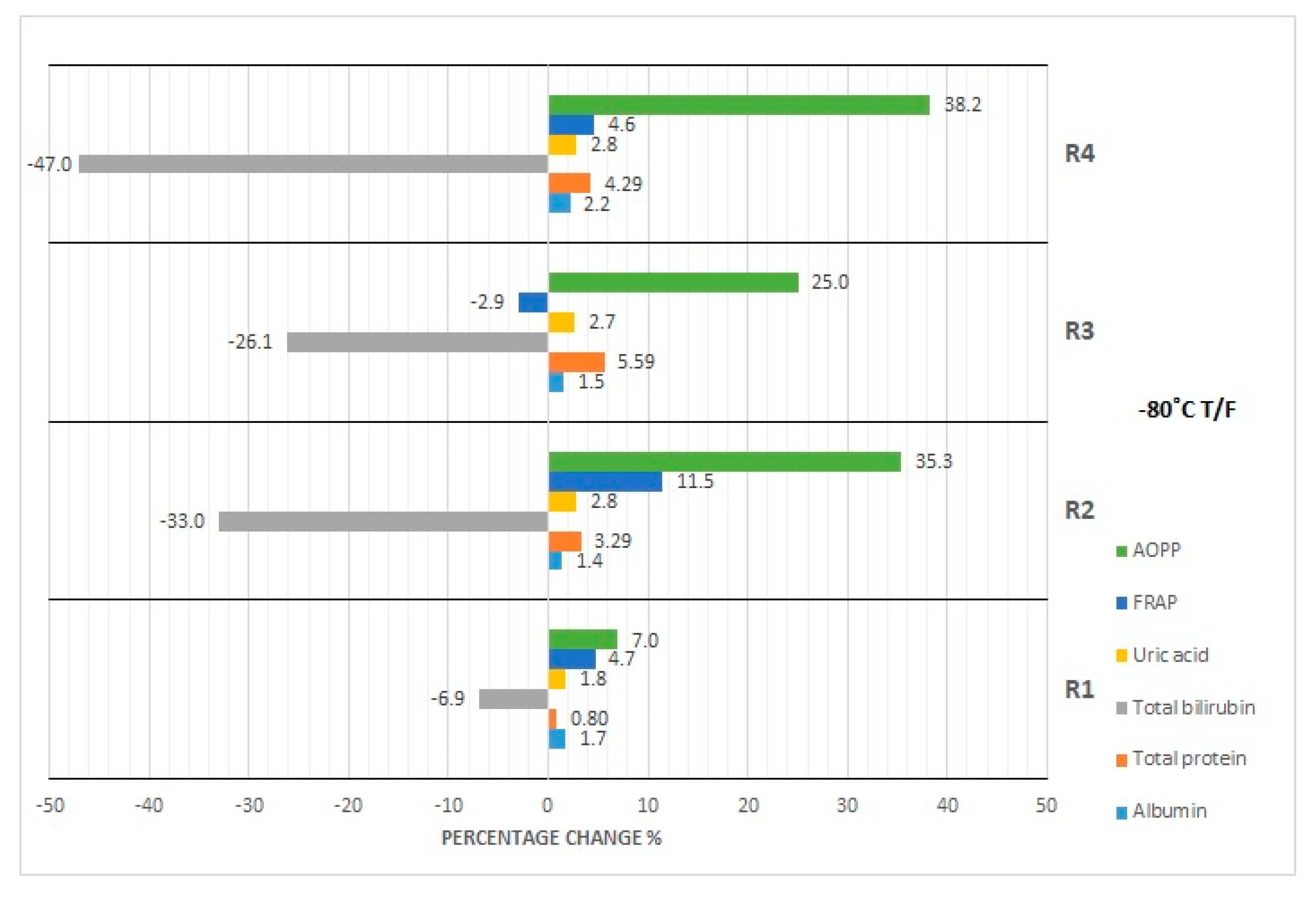
| Concentration percentage change (DB = 1.36%) | 0.0 | −0.18 | 1.37 | 1.20 | −1.02 | 0.81 | 0.39 | 1.23 | 1.09 | 5.32 | 5.11 | 6.44 | −0.83 | 2.04 | 4.38 | 4.41 | 3.68 | 2.99 | 3.68 | 3.85 | 6.40 | 0.73 | 3.29 | 5.59 | 4.29 | |
| p value | 0.808 | 0.067 | 0.04 | 0.091 | 0.186 | 0.852 | 0.073 | 0.575 | 0.000 | 0.004 | 0.000 | 0.184 | 0.056 | 0.000 | 0.000 | 0.002 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | ||
| Bilirubin (mg/dL)(<1.3) * | ||||||||||||||||||||||||||
| Mean concentration | 0.57 | 0.54 | 0.43 | 0.20 | 0.57 | 0.57 | 0.57 | 0.57 | 0.62 | 0.54 | 0.67 | 0.56 | 0.55 | 0.55 | 0.52 | 0.62 | 0.56 | 0.55 | 0.45 | 0.57 | 0.44 | 0.53 | 0.38 | 0.42 | 0.30 | |
| Standard Deviation | 0.17 | 0.16 | 0.12 | 0.06 | 0.17 | 0.17 | 0.17 | 0.16 | 0.16 | 0.18 | 0.19 | 0.18 | 0.16 | 0.16 | 0.17 | 0.18 | 0.18 | 0.16 | 0.16 | 0.16 | 0.17 | 0.15 | 0.13 | 0.12 | 0.10 | |
| Median | 0.58 | 0.54 | 0.41 | 0.20 | 0.57 | 0.57 | 0.56 | 0.57 | 0.61 | 0.54 | 0.68 | 0.57 | 0.55 | 0.57 | 0.52 | 0.61 | 0.58 | 0.54 | 0.44 | 0.54 | 0.42 | 0.53 | 0.37 | 0.40 | 0.27 | |
| Range | 0.26–0.88 | 0.24–0.82 | 0.22–0.64 | 0.12–0.31 | 0.26–0.86 | 0.24–0.87 | 0.25–0.86 | 0.28–0.87 | 0.29–0.92 | 0.21–0.87 | 0.32–1.03 | 0.23–0.89 | 0.23–0.83 | 0.23–0.85 | 0.18–0.84 | 0.27–0.95 | 0.21–0.88 | 0.27–0.83 | 0.180–0.78 | 0.29–0.90 | 0.20–0.73 | 0.26–0.80 | 0.14–0.63 | 0.23–0.65 | 0.13–0.47 | |
| Concentration percentage change (DB = 8.95%) | 00.0 | −6.37 | −25.87 | −64.35 | −0.61 | −1.04 | −1.63 | −0.17 | 7.59 | −5.88 | 17.49 | −1.84 | −4.25 | −4.42 | −8.79 | 8.72 | −1.75 | −3.56 | −20.98 | −0.96 | −23.27 | −6.93 | −32.95 | −26.16 | −47.04 | |
| p value | 0.000 | 0.000 | 0.000 | 0.907 | 0.305 | 0.783 | 0.761 | 0.376 | 0.000 | 0.022 | 0.018 | 0.000 | 0.001 | 0.000 | 0.061 | 0.229 | 0.096 | 0.000 | 0.482 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Uric acid (mg/dL) (women < 5.7; men < 7.0) * | ||||||||||||||||||||||||||
| Mean concentration | 4.72 | 4.76 | 4.85 | 4.87 | 4.74 | 4.79 | 4.74 | 4.61 | 4.75 | 4.88 | 4.87 | 4.88 | 4.72 | 4.74 | 4.91 | 4.82 | 4.85 | 4.85 | 4.92 | 4.87 | 4.94 | 4.81 | 4.85 | 4.83 | 4.85 | |
| Standard deviation | 0.77 | 0.78 | 0.81 | 0.79 | 0.80 | 0.78 | 0.80 | 0.79 | 0.80 | 0.82 | 0.80 | 0.81 | 0.77 | 0.80 | 0.81 | 0.83 | 0.80 | 0.84 | 0.79 | 0.81 | 0.81 | 0.80 | 0.83 | 0.80 | 0.82 | |
| Median | 4.89 | 4.83 | 4.96 | 4.99 | 4.89 | 4.96 | 4.86 | 4.57 | 4.95 | 5.08 | 4.99 | 5.03 | 4.90 | 4.81 | 5.07 | 4.97 | 4.97 | 4.99 | 5.03 | 4.99 | 5.12 | 4.95 | 5.08 | 5.02 | 5.01 | |
| Range | 2.74–6.03 | 2.79–6.00 | 2.81–6.11 | 2.83–6.14 | 2.70–6.01 | 2.74–6.05 | 2.66–6.15 | 2.63–5.85 | 2.71–6.02 | 2.80–6.12 | 2.92–6.09 | 2.83–6.09 | 2.73–5.91 | 2.72–5.95 | 2.83–6.21 | 2.77–6.15 | 2.77–6.14 | 2.69–6.15 | 2.80–6.12 | 2.80–6.16 | 2.97–6.26 | 2.79–6.07 | 2.78–6.12 | 2.75–6.15 | 2.74–6.14 | |
| Concentration percentage change (DB = 4.87%) | 0.00 | 0.78 | 2.56 | 3.13 | 0.26 | 1.35 | 0.35 | −2.52 | 0.71 | 3.34 | 3.03 | 3.18 | −0.07 | 0.23 | 4.02 | 2.10 | 2.69 | 2.61 | 4.13 | 2.99 | 4.66 | 1.76 | 2.79 | 2.69 | 2.80 | |
| p value | 0.297 | 0.311 | 0.000 | 0.750 | 0.077 | 0.815 | 0.008 | 0.777 | 0.000 | 0.116 | 0.001 | 0.921 | 0.848 | 0.000 | 0.451 | 0.000 | 0.364 | 0.000 | 0.000 | 0.383 | 0.009 | 0.009 | 0.003 | 0.007 | ||
| FRAP (mmol/L) | ||||||||||||||||||||||||||
| Mean concentration | 1.14 | 1.11 | 1.07 | 1.03 | 1.16 | 1.17 | 1.12 | 1.19 | 1.11 | 1.19 | 1.48 | 1.05 | 1.22 | 1.20 | 1.35 | 1.04 | 1.25 | 1.07 | 1.29 | 1.44 | 1.00 | 1.19 | 1.27 | 1.11 | 1.19 | |
| Standard deviation | 0.12 | 0.11 | 0.13 | 0.10 | 0.19 | 0.10 | 0.11 | 0.11 | 0.12 | 0.12 | 0.08 | 0.10 | 0.12 | 0.10 | 0.11 | 0.09 | 0.11 | 0.11 | 0.15 | 0.09 | 0.11 | 0.11 | 0.12 | 0.11 | 0.11 | |
| Median | 1.16 | 1.13 | 1.08 | 1.04 | 1.18 | 1.20 | 1.13 | 1.22 | 1.14 | 1.23 | 1.49 | 1.06 | 1.25 | 1.21 | 1.36 | 1.04 | 1.25 | 1.08 | 1.32 | 1.44 | 1.02 | 1.22 | 1.30 | 1.11 | 1.18 | |
| Range | 0.85–1.44 | 0.81–1.26 | 0.80–1.34 | 0.76–1.18 | 0.61–1.38 | 0.92–1.30 | 0.83–1.28 | 0.94–1.36 | 0.75–1.27 | 0.88–1.39 | 1.31–1.62 | 0.78–1.22 | 0.96–1.41 | 0.97–1.37 | 1.12–1.54 | 0.87–1.19 | 0.98–1.42 | 0.80–1.27 | 0.981–1.61 | 1.22–1.61 | 0.74–1.17 | 0.92–1.35 | 1.03–1.50 | 0.85–1.27 | 0.96–1.37 | |
| Concentration percentage change (DB = 10%) | 0.00 | −2.65 | −6.50 | −10.16 | 1.54 | 2.16 | −2.45 | 4.71 | −2.71 | 4.14 | 29.18 | −7.89 | 6.57 | 4.94 | 18.27 | −8.77 | 9.44 | −6.21 | 12.60 | 26.12 | −12.11 | 4.68 | 11.40 | −2.91 | 4.64 | |
| p value | 0.031 | 0.000 | 0.000 | 0.047 | 0.030 | 0.004 | 0.002 | 0.011 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.031 | 0.000 | ||
| AOPP (µmol/L) | ||||||||||||||||||||||||||
| Mean concentration | 58.80 | 59.14 | 61.10 | 48.86 | 58.25 | 43.90 | 55.22 | 59.09 | 57.80 | 65.45 | 68.43 | 59.18 | 48.53 | 62.45 | 66.42 | 62.78 | 56.16 | 50.52 | 100. 32 | 102. 32 | 108. 79 | 62.93 | 79.58 | 73.51 | 81.27 | |
| Standard deviation | 11.48 | 10.74 | 11.15 | 11.29 | 11.50 | 14.46 | 13.74 | 17.21 | 16.29 | 16.20 | 16.11 | 16.33 | 13.78 | 14.95 | 16.63 | 18.74 | 26.61 | 17.21 | 31.03 | 38.16 | 34.45 | 13.88 | 24.42 | 26.61 | 33.69 | |
| Median | 56.21 | 58.92 | 58.39 | 46.99 | 56.44 | 41.77 | 52.06 | 55.52 | 54.91 | 62.57 | 63.51 | 54.19 | 45.57 | 59.79 | 60.73 | 58.88 | 63.49 | 55.52 | 90.89 | 91.67 | 100.61 | 59.89 | 73.13 | 63.49 | 73.44 | |
| Range | 47.23–101.24 | 44.75–93.94 | 49.43–100. 96 | 33.30–86.88 | 44.15–99.50 | 23.97–85.89 | 44.75–107.87 | 44.69–121.99 | 40.46–116.52 | 51.05–125.31 | 53.51–127.84 | 43.08–119.64 | 40.46–116.52 | 48.48–119.50 | 51.08–126.71 | 44.46–130.44 | 37.71–121.92 | 32.86–114.72 | 68.36–161.45 | 62.70–210.51 | 64.40–191.95 | 48.94–109.97 | 52.83–149.05 | 42.60–137.50 | 46.25–173.91 | |
| Concentration percentage change (DB = 10%) | 0.00 | 0.57 | 3.90 | −16.91 | −0.94 | −25.34 | −6.09 | 0.50 | −1.69 | 11.32 | 16.38 | 0.65 | −17.46 | 6.22 | 12.97 | 6.78 | −4.49 | −14.07 | 70.61 | 74.02 | 85.03 | 7.00 | 35.34 | 25.02 | 38.21 | |
| p value | 0.765 | 0.012 | 0.000 | 0.550 | 0.000 | 0.002 | 0.910 | 0.525 | 0.000 | 0.000 | 0.736 | 0.000 | 0.017 | 0.000 | 0.079 | 0.178 | 0.011 | 0.000 | 0.000 | 0.000 | 0.009 | 0.000 | 0.004 | 0.001 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik-Sobecka, L.; Sołkiewicz, K.; Kokot, I.; Kiraga, A.; Płaczkowska, S.; Schlichtinger, A.M.; Kratz, E.M. The Influence of Serum Sample Storage Conditions on Selected Laboratory Parameters Related to Oxidative Stress: A Preliminary Study. Diagnostics 2020, 10, 51. https://doi.org/10.3390/diagnostics10010051
Pawlik-Sobecka L, Sołkiewicz K, Kokot I, Kiraga A, Płaczkowska S, Schlichtinger AM, Kratz EM. The Influence of Serum Sample Storage Conditions on Selected Laboratory Parameters Related to Oxidative Stress: A Preliminary Study. Diagnostics. 2020; 10(1):51. https://doi.org/10.3390/diagnostics10010051
Chicago/Turabian StylePawlik-Sobecka, Lilla, Katarzyna Sołkiewicz, Izabela Kokot, Aleksandra Kiraga, Sylwia Płaczkowska, Agnieszka Matylda Schlichtinger, and Ewa Maria Kratz. 2020. "The Influence of Serum Sample Storage Conditions on Selected Laboratory Parameters Related to Oxidative Stress: A Preliminary Study" Diagnostics 10, no. 1: 51. https://doi.org/10.3390/diagnostics10010051
APA StylePawlik-Sobecka, L., Sołkiewicz, K., Kokot, I., Kiraga, A., Płaczkowska, S., Schlichtinger, A. M., & Kratz, E. M. (2020). The Influence of Serum Sample Storage Conditions on Selected Laboratory Parameters Related to Oxidative Stress: A Preliminary Study. Diagnostics, 10(1), 51. https://doi.org/10.3390/diagnostics10010051





