Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review
Abstract
1. Introduction
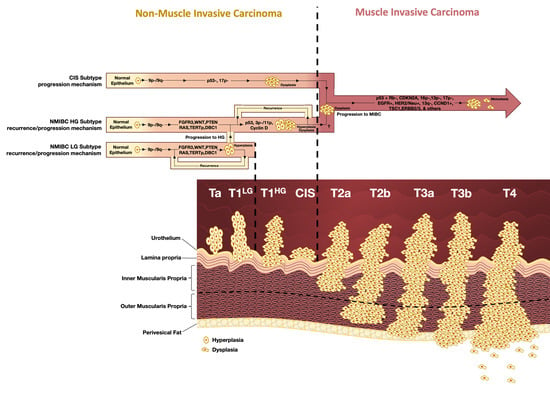
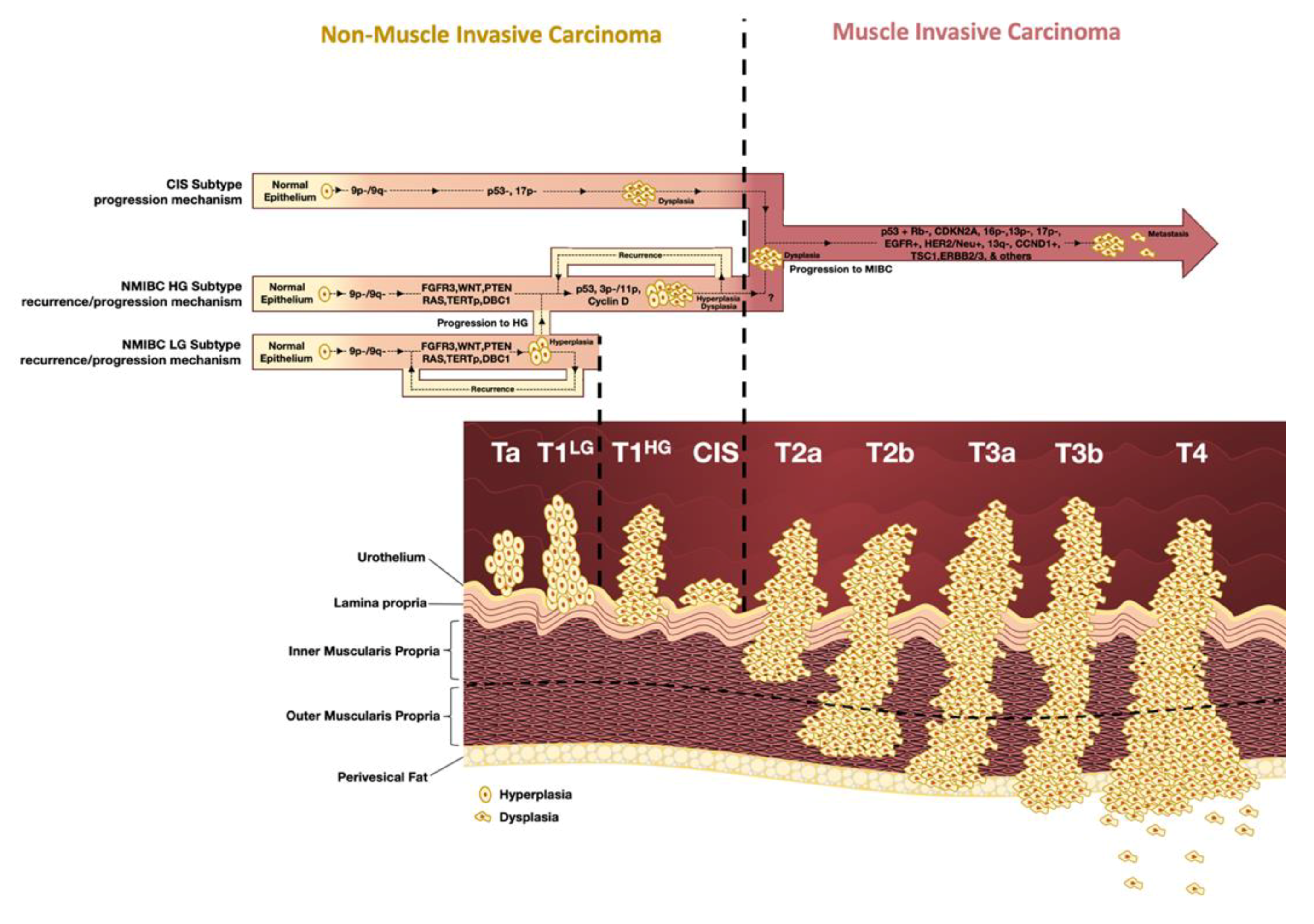
1.1. Classification, Staging, and Grading
1.2. Clinical Presentation and Management
1.3. Molecular Subtypes of Bladder Cancer
2. Method of Search
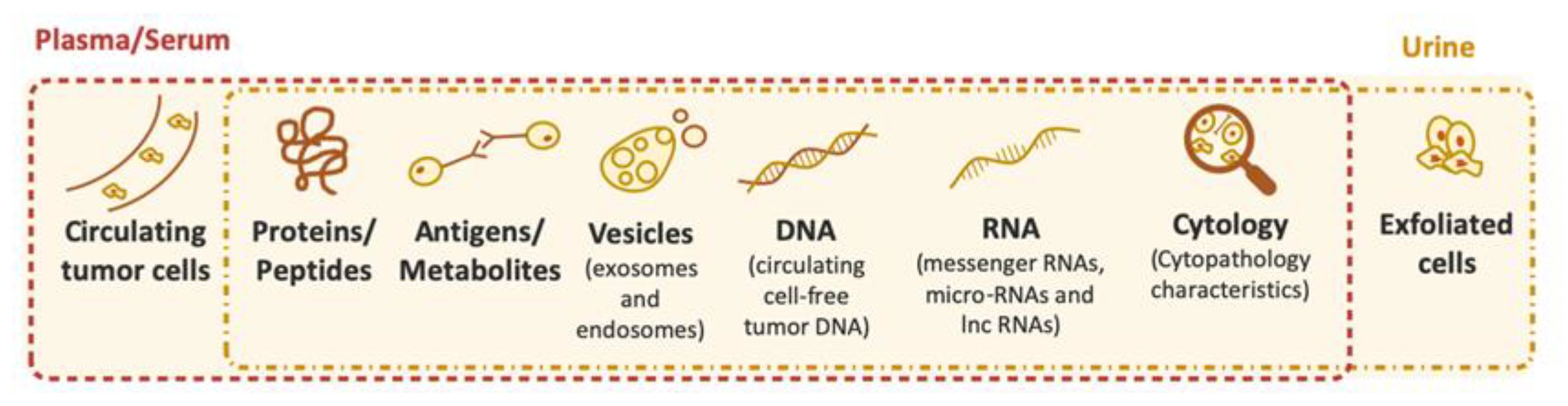
3. Biomarkers for Bladder Cancer Diagnosis and Follow-up (Currently Available FDA-Approved Tests and Kits)
3.1. NMP-22 Protein Test
3.2. BTA Stat and BTA-TRAK
3.3. UroVysion
3.4. ImmunoCyt/uCyt+
3.5. Uromonitor and Uromonitor-V2
3.6. UroSEEK
3.7. EpiCheck
4. Other Biomarkers for Bladder Cancer Diagnosis and Follow-up
4.1. TERTp Mutations and Hypermethylation
4.2. miRNA Biomarkers
4.3. lncRNAs Biomarkers
4.4. Extracellular Vesicles
5. Predictive Biomarkers for Therapy in Bladder Cancer Patients
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Observatory, T.G.C. Bladder. Available online: http://gco.iarc.fr/today/data/factsheets/cancers/30-Bladder-fact-sheet.pdf (accessed on 12 September 2019).
- Observatory, T.G.C. World. Available online: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (accessed on 12 September 2019).
- Vinay Kumar, A.K.A.; Jon, C. Aster. In Robbins and Cotran Pathologic Basis of Disease, 9th ed.; Elsevier Health Sciences: Kidlington, UK, 2014. [Google Scholar]
- Christensen, C.H.; Rostron, B.; Cosgrove, C.; Altekruse, S.F.; Hartman, A.M.; Gibson, J.T.; Apelberg, B.; Inoue-Choi, M.; Freedman, N.D. Association of Cigarette, Cigar, and Pipe Use With Mortality Risk in the US Population. JAMA Intern. Med. 2018, 178, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, A.; Citarella, F.; Croghan, I.; Tonini, G. The effects of cigarette smoking extracts on cell cycle and tumor spread: Novel evidence. Future Sci. OA 2019, 5, FSO394. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, R.; Lv, C.; Zhong, Z.; Zhang, L.; Zhu, L.; Tang, Y.; Zhao, X. A chronic obstructive pulmonary disease negatively influences the prognosis of patients with bladder urothelial carcinoma via hypoxia inducible factor-1alpha. Int. J. Clin. Exp. Med. 2014, 7, 3344–3353. [Google Scholar] [PubMed]
- Pezzuto, A.; Stellato, M.; Catania, G.; Mazzara, C.; Tonini, S.; Caricato, M.; Crucitti, P.; Tonini, G. Short-term benefit of smoking cessation along with glycopirronium on lung function and respiratory symptoms in mild COPD patients: A retrospective study. J. Breath Res. 2018, 12, 046007. [Google Scholar] [CrossRef]
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Comperat, E.M.; Burger, M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Roupret, M.; van Rhijn, B.W.G.; Shariat, S.F.; Sylvester, R.J.; Zigeuner, R.; et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur. Urol. Focus 2019, 5, 457–466. [Google Scholar] [CrossRef]
- Babjuk, M.; Bohle, A.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Hernandez, V.; Kaasinen, E.; Palou, J.; Roupret, M.; et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef]
- Xylinas, E.; Kluth, L.A.; Rieken, M.; Karakiewicz, P.I.; Lotan, Y.; Shariat, S.F. Urine markers for detection and surveillance of bladder cancer. Urol. Oncol. 2014, 32, 222–229. [Google Scholar] [CrossRef]
- Bellmunt, J.; Orsola, A.; Leow, J.J.; Wiegel, T.; De Santis, M.; Horwich, A.; Group, E.G.W. Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol 2014, 25 (Suppl. 3), iii40–iii48. [Google Scholar] [CrossRef]
- Alfred Witjes, J.; Lebret, T.; Comperat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernandez, V.; Espinos, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef]
- Sonpavde, G.; Goldman, B.H.; Speights, V.O.; Lerner, S.P.; Wood, D.P.; Vogelzang, N.J.; Trump, D.L.; Natale, R.B.; Grossman, H.B.; Crawford, E.D. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer 2009, 115, 4104–4109. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Cleves, A.; Wilt, T.J.; Mason, M.; Kynaston, H.G.; Shelley, M. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database Syst. Rev. 2012, 1, CD009294. [Google Scholar] [CrossRef] [PubMed]
- Messing, E.M.; Tangen, C.M.; Lerner, S.P.; Sahasrabudhe, D.M.; Koppie, T.M.; Wood, D.P., Jr.; Mack, P.C.; Svatek, R.S.; Evans, C.P.; Hafez, K.S.; et al. Effect of Intravesical Instillation of Gemcitabine vs.Saline Immediately Following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial. JAMA 2018, 319, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Bosschieter, J.; Nieuwenhuijzen, J.A.; van Ginkel, T.; Vis, A.N.; Witte, B.; Newling, D.; Beckers, G.M.A.; van Moorselaar, R.J.A. Value of an Immediate Intravesical Instillation of Mitomycin C in Patients with Non-muscle-invasive Bladder Cancer: A Prospective Multicentre Randomised Study in 2243 patients. Eur. Urol. 2018, 73, 226–232. [Google Scholar] [CrossRef]
- Bohle, A.; Jocham, D.; Bock, P.R. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: A formal meta-analysis of comparative studies on recurrence and toxicity. J. Urol. 2003, 169, 90–95. [Google Scholar] [CrossRef]
- Shelley, M.D.; Wilt, T.J.; Court, J.; Coles, B.; Kynaston, H.; Mason, M.D. Intravesical bacillus Calmette-Guerin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: A meta-analysis of randomized trials. BJU Int. 2004, 93, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Comperat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Bohle, A.; Bock, P.R. Intravesical bacille Calmette-Guerin versus mitomycin C in superficial bladder cancer: Formal meta-analysis of comparative studies on tumor progression. Urology 2004, 63, 682–686; discussion 686–687. [Google Scholar] [CrossRef] [PubMed]
- Ehdaie, B.; Sylvester, R.; Herr, H.W. Maintenance bacillus Calmette-Guerin treatment of non-muscle-invasive bladder cancer: A critical evaluation of the evidence. Eur. Urol. 2013, 64, 579–585. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der, M.A.; Lamm, D.L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J. Urol. 2002, 168, 1964–1970. [Google Scholar] [CrossRef]
- Malmstrom, P.U.; Sylvester, R.J.; Crawford, D.E.; Friedrich, M.; Krege, S.; Rintala, E.; Solsona, E.; Di Stasi, S.M.; Witjes, J.A. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur. Urol. 2009, 56, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Network, N.C.C. Bladder Cancer. Available online: http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (accessed on 25 September 2019).
- Ploussard, G.; Shariat, S.F.; Dragomir, A.; Kluth, L.A.; Xylinas, E.; Masson-Lecomte, A.; Rieken, M.; Rink, M.; Matsumoto, K.; Kikuchi, E.; et al. Conditional survival after radical cystectomy for bladder cancer: Evidence for a patient changing risk profile over time. Eur. Urol. 2014, 66, 361–370. [Google Scholar] [CrossRef]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef]
- Milowsky, M.I.; Rumble, R.B.; Booth, C.M.; Gilligan, T.; Eapen, L.J.; Hauke, R.J.; Boumansour, P.; Lee, C.T. Guideline on Muscle-Invasive and Metastatic Bladder Cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J. Clin. Oncol. 2016, 34, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; deVere White, R.W.; Sarosdy, M.F.; Wood, D.P., Jr.; Raghavan, D.; et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Resch, I.; Shariat, S.F.; Gust, K.M. PD-1 and PD-L1 inhibitors after platinum-based chemotherapy or in first-line therapy in cisplatin-ineligible patients: Dramatic improvement of prognosis and overall survival after decades of hopelessness in patients with metastatic urothelial cancer. Memo 2018, 11, 43–46. [Google Scholar] [CrossRef]
- Nadal, R.; Bellmunt, J. Management of metastatic bladder cancer. Cancer Treat. Rev. 2019, 76, 10–21. [Google Scholar] [CrossRef]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Martin-Doyle, W.; Kwiatkowski, D.J. Molecular biology of bladder cancer. Hematol./Oncol. Clin. North. Am. 2015, 29, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Kompier, L.C.; Lurkin, I.; van der Aa, M.N.; van Rhijn, B.W.; van der Kwast, T.H.; Zwarthoff, E.C. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS ONE 2010, 5, e13821. [Google Scholar] [CrossRef] [PubMed]
- Jebar, A.H.; Hurst, C.D.; Tomlinson, D.C.; Johnston, C.; Taylor, C.F.; Knowles, M.A. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene 2005, 24, 5218–5225. [Google Scholar] [CrossRef] [PubMed]
- Billerey, C.; Chopin, D.; Aubriot-Lorton, M.H.; Ricol, D.; Gil Diez de Medina, S.; Van Rhijn, B.; Bralet, M.P.; Lefrere-Belda, M.A.; Lahaye, J.B.; Abbou, C.C.; et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am. J. Pathol. 2001, 158, 1955–1959. [Google Scholar] [CrossRef]
- Tan, T.Z.; Rouanne, M.; Tan, K.T.; Huang, R.Y.; Thiery, J.P. Molecular Subtypes of Urothelial Bladder Cancer: Results from a Meta-cohort Analysis of 2411 Tumors. Eur. Urol. 2019, 75, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.L. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef]
- Kamat, A.M.; Karam, J.A.; Grossman, H.B.; Kader, A.K.; Munsell, M.; Dinney, C.P. Prospective trial to identify optimal bladder cancer surveillance protocol: Reducing costs while maximizing sensitivity. BJU Int. 2011, 108, 1119–1123. [Google Scholar] [CrossRef]
- Yeung, C.; Dinh, T.; Lee, J. The health economics of bladder cancer: An updated review of the published literature. Pharmacoeconomics 2014, 32, 1093–1104. [Google Scholar] [CrossRef]
- Blick, C.G.; Nazir, S.A.; Mallett, S.; Turney, B.W.; Onwu, N.N.; Roberts, I.S.; Crew, J.P.; Cowan, N.C. Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cystoscopy and voided urine cytology: Results for 778 patients from a hospital haematuria clinic. BJU Int. 2012, 110, 84–94. [Google Scholar] [CrossRef]
- Tabayoyong, W.; Kamat, A.M. Current Use and Promise of Urinary Markers for Urothelial Cancer. Curr. Urol. Rep. 2018, 19, 96. [Google Scholar] [CrossRef]
- Soria, F.; Droller, M.J.; Lotan, Y.; Gontero, P.; D’Andrea, D.; Gust, K.M.; Roupret, M.; Babjuk, M.; Palou, J.; Shariat, S.F. An up-to-date catalog of available urinary biomarkers for the surveillance of non-muscle invasive bladder cancer. World J. Urol. 2018, 36, 1981–1995. [Google Scholar] [CrossRef]
- Moonen, P.M.; Kiemeney, L.A.; Witjes, J.A. Urinary NMP22 BladderChek test in the diagnosis of superficial bladder cancer. Eur. Urol. 2005, 48, 951–956; discussion 956. [Google Scholar] [CrossRef]
- Dogan, C.; Pelit, E.S.; Yildirim, A.; Zemheri, I.E.; Canakci, C.; Basok, E.K.; Caskurlu, T. The value of the NMP22 test for superficial bladder cancer diagnosis and follow-up. Turk. J. Urol. 2013, 39, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kinders, R.; Jones, T.; Root, R.; Bruce, C.; Murchison, H.; Corey, M.; Williams, L.; Enfield, D.; Hass, G.M. Complement factor H or a related protein is a marker for transitional cell cancer of the bladder. Clin. Cancer Res. 1998, 4, 2511–2520. [Google Scholar] [PubMed]
- Oehr, P. Proteomics as a tool for detection of nuclear matrix proteins and new biomarkers for screening of early tumors stage. Anticancer Res. 2003, 23, 805–812. [Google Scholar]
- Raitanen, M.P. The role of BTA stat Test in follow-up of patients with bladder cancer: Results from FinnBladder studies. World J. Urol. 2008, 26, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Banos, J.L.; del Henar Rebollo Rodrigo, M.; Antolin Juarez, F.M.; Garcia, B.M. Usefulness of the BTA STAT Test for the diagnosis of bladder cancer. Urology 2001, 57, 685–689. [Google Scholar] [CrossRef]
- Thomas, L.; Leyh, H.; Marberger, M.; Bombardieri, E.; Bassi, P.; Pagano, F.; Pansadoro, V.; Sternberg, C.N.; Boccon-Gibod, L.; Ravery, V.; et al. Multicenter trial of the quantitative BTA TRAK assay in the detection of bladder cancer. Clin. Chem. 1999, 45, 472–477. [Google Scholar]
- Kim, T.J.; Moon, H.W.; Kang, S.; Yang, J.; Hong, S.H.; Lee, J.Y.; Ha, U.S. Urovysion FISH Could Be Effective and Useful Method to Confirm the Identity of Cultured Circulating Tumor Cells from Bladder Cancer Patients. J. Cancer 2019, 10, 3259–3266. [Google Scholar] [CrossRef]
- Dimashkieh, H.; Wolff, D.J.; Smith, T.M.; Houser, P.M.; Nietert, P.J.; Yang, J. Evaluation of urovysion and cytology for bladder cancer detection: A study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol. 2013, 121, 591–597. [Google Scholar] [CrossRef]
- Lavery, H.J.; Zaharieva, B.; McFaddin, A.; Heerema, N.; Pohar, K.S. A prospective comparison of UroVysion FISH and urine cytology in bladder cancer detection. BMC Cancer 2017, 17, 247. [Google Scholar] [CrossRef]
- Schmitz-Drager, C.; Bonberg, N.; Pesch, B.; Todenhofer, T.; Sahin, S.; Behrens, T.; Bruning, T.; Schmitz-Drager, B.J. Replacing cystoscopy by urine markers in the follow-up of patients with low-risk non-muscle-invasive bladder cancer?-An International Bladder Cancer Network project. Urol. Oncol. 2016, 34, 452–459. [Google Scholar] [CrossRef]
- Kassouf, W.; Traboulsi, S.L.; Schmitz-Drager, B.; Palou, J.; Witjes, J.A.; van Rhijn, B.W.; Grossman, H.B.; Kiemeney, L.A.; Goebell, P.J.; Kamat, A.M. Follow-up in non-muscle-invasive bladder cancer-International Bladder Cancer Network recommendations. Urol. Oncol. 2016, 34, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.; Prazeres, H.; Sampaio, C.; Peralta, P.; Conceição, P.; Sismeiro, A.; Leão, R.; Gomes, A.; Furriel, F.; Oliveira, C.; et al. Validation of a novel, sensitive and specific urine-based test for recurrence surveillance of patients with non-muscle invasive bladder cancer in a comprehensive multicenter study. Front. Genet. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Han, C.; Hao, L.; Zang, G. ImmunoCyt test compared to cytology in the diagnosis of bladder cancer: A meta-analysis. Oncol. Lett. 2016, 12, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Mowatt, G.; Zhu, S.; Kilonzo, M.; Boachie, C.; Fraser, C.; Griffiths, T.R.L.; N’Dow, J.; Nabi, G.; Cook, J.; Vale, L. Systematic review of the clinical and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Available online: https://aura.abdn.ac.uk/bitstream/handle/2164/731/Mowatt2010.pdf?sequence=1 (accessed on 12 January 2020).
- Zhang, Z.; Fan, W.; Deng, Q.; Tang, S.; Wang, P.; Xu, P.; Wang, J.; Yu, M. The prognostic and diagnostic value of circulating tumor cells in bladder cancer and upper tract urothelial carcinoma: A meta-analysis of 30 published studies. Oncotarget 2017, 8, 59527. [Google Scholar] [CrossRef] [PubMed]
- Hajdinjak, T. UroVysion FISH Test for Detecting Urothelial Cancers: Meta-Analysis of Diagnostic Accuracy and Comparison with Urinary Cytology Testing; Elsevier: Amsterdam, The Netherlands, 2008; pp. 646–651. [Google Scholar]
- Glas, A.S.; Lijmer, J.G.; Prins, M.H.; Bonsel, G.J.; Bossuyt, P.M.M. The diagnostic odds ratio: A single indicator of test performance. J. Clin. Epidemiol. 2003, 56, 1129–1135. [Google Scholar] [CrossRef]
- O’Sullivan, P.; Sharples, K.; Dalphin, M.; Davidson, P.; Gilling, P.; Cambridge, L.; Harvey, J.; Toro, T.; Giles, N.; Luxmanan, C. A multigene urine test for the detection and stratification of bladder cancer in patients presenting with hematuria. J. Urol. 2012, 188, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Van Valenberg, F.J.P.; Bridge, J.A.; Mayne, D.; Beqaj, S.; Sexton, W.J.; Lotan, Y.; Weizer, A.Z.; Jansz, G.K.; Stenzl, A.; Danella, J.F. Validation of a mRNA-based urine test for bladder cancer detection in patients with hematuria. Eur. Urol. Suppl. 2017, 16, e190–e191. [Google Scholar] [CrossRef]
- Witjes, J.A.; Morote, J.; Cornel, E.B.; Gakis, G.; van Valenberg, F.J.P.; Lozano, F.; Sternberg, I.A.; Willemsen, E.; Hegemann, M.L.; Paitan, Y. Performance of the Bladder EpiCheck™ Methylation Test for patients under surveillance for non–muscle-invasive bladder cancer: Results of a multicenter, prospective, blinded clinical trial. Eur. Urol. Oncol. 2018, 1, 307–313. [Google Scholar] [CrossRef]
- Springer, S.U.; Chen, C.H.; Rodriguez Pena, M.D.C.; Li, L.; Douville, C.; Wang, Y.; Cohen, J.D.; Taheri, D.; Silliman, N.; Schaefer, J.; et al. Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. eLife 2018, 7, e32134. [Google Scholar] [CrossRef]
- Rodriguez Pena, M.D.C.; Springer, S.U.; Taheri, D.; Li, L.; Tregnago, A.C.; Eich, M.L.; Eltoum, I.A.; VandenBussche, C.J.; Papadopoulos, N.; Kinzler, K.W.; et al. Performance of novel non-invasive urine assay UroSEEK in cohorts of equivocal urine cytology. Virchows Arch. Int. J. Pathol. 2019. [Google Scholar] [CrossRef]
- Trenti, E.; D’Elia, C.; Mian, C.; Schwienbacher, C.; Hanspeter, E.; Pycha, A.; Kafka, M.; Degener, S.; Danuser, H.; Roth, S.; et al. Diagnostic predictive value of the Bladder EpiCheck test in the follow-up of patients with non-muscle-invasive bladder cancer. Cancer Cytopathol. 2019, 127, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Kurtis, B.; Zhuge, J.; Ojaimi, C.; Ye, F.; Cai, D.; Zhang, D.; Fallon, J.T.; Zhong, M. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann. Diagn. Pathol. 2016, 21, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kinde, I.; Munari, E.; Faraj, S.F.; Hruban, R.H.; Schoenberg, M.; Bivalacqua, T.; Allaf, M.; Springer, S.; Wang, Y.; Diaz, L.A., Jr.; et al. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res. 2013, 73, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, P.S.; Hosen, I.; de Verdier, P.J.; Fallah, M.; Heidenreich, B.; Ryk, C.; Wiklund, N.P.; Steineck, G.; Schadendorf, D.; Hemminki, K.; et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl. Acad. Sci. USA 2013, 110, 17426–17431. [Google Scholar] [CrossRef] [PubMed]
- Allory, Y.; Beukers, W.; Sagrera, A.; Flandez, M.; Marques, M.; Marquez, M.; van der Keur, K.A.; Dyrskjot, L.; Lurkin, I.; Vermeij, M.; et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: High frequency across stages, detection in urine, and lack of association with outcome. Eur. Urol. 2014, 65, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.D.; Platt, F.M.; Knowles, M.A. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur. Urol. 2014, 65, 367–369. [Google Scholar] [CrossRef]
- Hosen, I.; Rachakonda, P.S.; Heidenreich, B.; de Verdier, P.J.; Ryk, C.; Steineck, G.; Hemminki, K.; Kumar, R. Mutations in TERT promoter and FGFR3 and telomere length in bladder cancer. Int. J. Cancer 2015, 137, 1621–1629. [Google Scholar] [CrossRef]
- Netto, G.J. Molecular biomarkers in urothelial carcinoma of the bladder: Are we there yet? Nat. Rev. Urol. 2011, 9, 41–51. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef]
- Vinagre, J.; Pinto, V.; Celestino, R.; Reis, M.; Populo, H.; Boaventura, P.; Melo, M.; Catarino, T.; Lima, J.; Lopes, J.M.; et al. Telomerase promoter mutations in cancer: An emerging molecular biomarker? Virchows Arch. Int. J. Pathol. 2014, 465, 119–133. [Google Scholar] [CrossRef]
- Descotes, F.; Kara, N.; Decaussin-Petrucci, M.; Piaton, E.; Geiguer, F.; Rodriguez-Lafrasse, C.; Terrier, J.E.; Lopez, J.; Ruffion, A. Non-invasive prediction of recurrence in bladder cancer by detecting somatic TERT promoter mutations in urine. Br. J. Cancer 2017, 117, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; Leao, R.; Komosa, M.; Gallo, M.; Zhang, C.H.; Lipman, T.; Remke, M.; Heidari, A.; Nunes, N.M.; Apolonio, J.D.; et al. DNA hypermethylation within TERT promoter upregulates TERT expression in cancer. J. Clin. Investig. 2019, 129, 223–229. [Google Scholar] [CrossRef]
- Leao, R.; Lee, D.; Figueiredo, A.; Hermanns, T.; Wild, P.; Komosa, M.; Lau, I.; Mistry, M.; Nunes, N.M.; Price, A.J.; et al. Combined genetic and epigenetic alterations of the TERT promoter affect clinical and biological behavior of bladder cancer. Int. J. Cancer 2019, 144, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Hanke, M.; Hoefig, K.; Merz, H.; Feller, A.C.; Kausch, I.; Jocham, D.; Warnecke, J.M.; Sczakiel, G. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol. Oncol. 2010, 28, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Du, L.; Wang, L.; Li, J.; Liu, Y.; Zheng, G.; Qu, A.; Zhang, X.; Pan, H.; Yang, Y.; et al. Serum microRNA expression signatures identified from genome-wide microRNA profiling serve as novel noninvasive biomarkers for diagnosis and recurrence of bladder cancer. Int. J. Cancer 2015, 136, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. MiR-101 acts as a novel bio-marker in the diagnosis of bladder carcinoma. Medicine 2019, 98, e16051. [Google Scholar] [CrossRef]
- Blanca, A.; Sanchez-Gonzalez, A.; Requena, M.J.; Carrasco-Valiente, J.; Gomez-Gomez, E.; Cheng, L.; Cimadamore, A.; Montironi, R.; Lopez-Beltran, A. Expression of miR-100 and miR-138 as prognostic biomarkers in non-muscle-invasive bladder cancer. Apmis Acta Pathol. Microbiol. Et Immunol. Scand. 2019, 127, 545–553. [Google Scholar] [CrossRef]
- Fang, Z.; Dai, W.; Wang, X.; Chen, W.; Shen, C.; Ye, G.; Li, L. Circulating miR-205: A promising biomarker for the detection and prognosis evaluation of bladder cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 8075–8082. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liu, X.; Fang, A.; Wang, J.; Yang, Y.; Wang, L.; Du, L.; Wang, C. Direct quantitative detection for cell-free miR-155 in urine: A potential role in diagnosis and prognosis for non-muscle invasive bladder cancer. Oncotarget 2016, 7, 3255–3266. [Google Scholar] [CrossRef]
- Shindo, T.; Shimizu, T.; Nojima, M.; Niinuma, T.; Maruyama, R.; Kitajima, H.; Kai, M.; Itoh, N.; Suzuki, H.; Masumori, N. Evaluation of Urinary DNA Methylation as a Marker for Recurrent Bladder Cancer: A 2-Center Prospective Study. Urology 2018, 113, 71–78. [Google Scholar] [CrossRef]
- Jiang, F.; Qi, W.; Wang, Y.; Wang, W.; Fan, L. lncRNA PEG10 promotes cell survival, invasion and migration by sponging miR-134 in human bladder cancer. Biomed. Pharmacother. 2019, 114, 108814. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Mao, J.; Hu, P.; Chen, Q.; Ding, W.; Pu, R. lncRNA TUC338 is a potential diagnostic biomarker for bladder cancer. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Safwat, M.; Matboli, M.; Zaghloul, A.; El-Sawalhi, M.; Shaheen, A. Measurement of Urinary Level of a Specific Competing endogenous RNA network (FOS and RCAN mRNA/miR-324-5p, miR-4738-3p, /lncRNA miR-497-HG) Enables Diagnosis of Bladder Cancer. Urol. Oncol. 2019, 37, 292.e219–292.e292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.R.; Ortiz-Bonilla, C.J.; Lee, Y.F. Extracellular Vesicles in Bladder Cancer: Biomarkers and Beyond. Int. J. Mol. Sci. 2018, 19, 2822. [Google Scholar] [CrossRef]
- Liang, L.G.; Kong, M.Q.; Zhou, S.; Sheng, Y.F.; Wang, P.; Yu, T.; Inci, F.; Kuo, W.P.; Li, L.J.; Demirci, U.; et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 2017, 7, 46224. [Google Scholar] [CrossRef]
- Kluth, L.A.; Black, P.C.; Bochner, B.H.; Catto, J.; Lerner, S.P.; Stenzl, A.; Sylvester, R.; Vickers, A.J.; Xylinas, E.; Shariat, S.F. Prognostic and Prediction Tools in Bladder Cancer: A Comprehensive Review of the Literature. Eur. Urol. 2015, 68, 238–253. [Google Scholar] [CrossRef]
- Zuiverloon, T.C.; Nieuweboer, A.J.; Vekony, H.; Kirkels, W.J.; Bangma, C.H.; Zwarthoff, E.C. Markers predicting response to bacillus Calmette-Guerin immunotherapy in high-risk bladder cancer patients: A systematic review. Eur. Urol. 2012, 61, 128–145. [Google Scholar] [CrossRef]
- Funt, S.A.; Rosenberg, J.E. Systemic, perioperative management of muscle-invasive bladder cancer and future horizons. Nat. Rev. Clin. Oncol. 2017, 14, 221–234. [Google Scholar] [CrossRef]
- Xylinas, E.; Kent, M.; Kluth, L.; Pycha, A.; Comploj, E.; Svatek, R.S.; Lotan, Y.; Trinh, Q.D.; Karakiewicz, P.I.; Holmang, S.; et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br. J. Cancer 2013, 109, 1460–1466. [Google Scholar] [CrossRef]
- Kamat, A.M.; Briggman, J.; Urbauer, D.L.; Svatek, R.; Nogueras Gonzalez, G.M.; Anderson, R.; Grossman, H.B.; Prat, F.; Dinney, C.P. Cytokine Panel for Response to Intravesical Therapy (CyPRIT): Nomogram of Changes in Urinary Cytokine Levels Predicts Patient Response to Bacillus Calmette-Guerin. Eur. Urol. 2016, 69, 197–200. [Google Scholar] [CrossRef]
- Wankowicz, S.A.M.; Werner, L.; Orsola, A.; Novak, J.; Bowden, M.; Choueiri, T.K.; de Torres, I.; Morote, J.; Freeman, G.J.; Signoretti, S.; et al. Differential Expression of PD-L1 in High Grade T1 vs.Muscle Invasive Bladder Carcinoma and its Prognostic Implications. J. Urol. 2017, 198, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Luftenegger, W.; Ackermann, D.K.; Futterlieb, A.; Kraft, R.; Minder, C.E.; Nadelhaft, P.; Studer, U.E. Intravesical versus intravesical plus intradermal bacillus Calmette-Guerin: A prospective randomized study in patients with recurrent superficial bladder tumors. J. Urol. 1996, 155, 483–487. [Google Scholar] [CrossRef]
- Saint, F.; Salomon, L.; Quintela, R.; Cicco, A.; Hoznek, A.; Abbou, C.C.; Chopin, D.K. Do prognostic parameters of remission versus relapse after Bacillus Calmette-Guerin (BCG) immunotherapy exist?. analysis of a quarter century of literature. Eur. Urol. 2003, 43, 351–360; discussion 360–351. [Google Scholar] [CrossRef]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salome, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-Pereira, S.; Dartiguenave, F.; Fritschi, A.S.; et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Investig. 2017, 127, 2916–2929. [Google Scholar] [CrossRef] [PubMed]
- Pietzak, E.J.; Bagrodia, A.; Cha, E.K.; Drill, E.N.; Iyer, G.; Isharwal, S.; Ostrovnaya, I.; Baez, P.; Li, Q.; Berger, M.F.; et al. Next-generation Sequencing of Nonmuscle Invasive Bladder Cancer Reveals Potential Biomarkers and Rational Therapeutic Targets. Eur. Urol. 2017, 72, 952–959. [Google Scholar] [CrossRef]
- Hu, T.; Kumar, Y.; Shazia, I.; Duan, S.J.; Li, Y.; Chen, L.; Chen, J.F.; Yin, R.; Kwong, A.; Leung, G.K.; et al. Forward and reverse mutations in stages of cancer development. Hum. Genom. 2018, 12, 40. [Google Scholar] [CrossRef]
- Kumar, Y.; Yang, J.; Hu, T.; Chen, L.; Xu, Z.; Xu, L.; Hu, X.X.; Tang, G.; Wang, J.M.; Li, Y.; et al. Massive interstitial copy-neutral loss-of-heterozygosity as evidence for cancer being a disease of the DNA-damage response. BMC Med. Genom. 2015, 8, 42. [Google Scholar] [CrossRef][Green Version]
- Santis, M.D.; Abdrashitov, R.; Hegele, A.; Kolb, M.; Parker, S.; Redorta, J.P.; Nishiyama, H.; Xiao, F.; Gupta, A.K.; Shore, N.D. A phase III, randomized, open-label, multicenter, global study of durvalumab and bacillus calmette-guérin (BCG) versus BCG alone in high-risk, BCG-naïve non-muscle-invasive bladder cancer (NMIBC) patients (POTOMAC). J. Clin. Oncol. 2019, 37, TPS500. [Google Scholar] [CrossRef]
- Squibb, B.-M. A Study of Nivolumab or Nivolumab Plus Experimental Medication BMS-986205 With or Without Bacillus Calumette-Guerin (BCG) in BCG Unresponsive Bladder Cancer That Has Not Invaded Into the Muscle Wall of the Bladder (CheckMate 9UT). Available online: https://clinicaltrials.gov/ct2/show/NCT03519256 (accessed on 25 September 2019).
| Name (Commercially Available Kits/Procedures) | FDA Approval/CE Mark | Present in EAU Guidelines 2019 | Sample | Starting Material | Technology | Type of biomarker assessed | Purpose | Overall Performance | References |
|---|---|---|---|---|---|---|---|---|---|
| Cytology | Yes | Yes | Urine |  Exfoliated cells Exfoliated cells | Giemsa an H&E staining |  Cell phenotype Cell phenotype | Diagnostic & Surveillance | Sensitivity = 38% Specificity = 98% | Christopher G.T. Blick, et al. 2011 [41] |
| uCyt+ | Yes/NA*1 | No | Urine |  Exfoliated cells Exfoliated cells | Immunofluorescence |  Antigens/ Antigens/Metabolites | Surveillance | Sensitivity = 73% Specificity = 66% | He, H. et al. 2016 [57] |
| NMP22 | Yes/Yes | Yes | Urine |  Exfoliated cells Exfoliated cells | Elisa |  Peptides Peptides | Surveillance | Sensitivity = 40% Specificity = 99% | Mowatt, G. et al. 2010 [58] |
| CellSearch | Yes/Yes | No | Plasma/serum |  CTC’s CTC’s | Immunomagnetic enrichment |  Proteins Proteins | Surveillance | Sensitivity = 35% Specificity = 97% | Zhang, Z. et al. 2017 [59] |
| UroVysion | Yes/Yes | Yes | Urine |  Exfoliated cells Exfoliated cells | FISH |  DNA DNA(Aneuploidies) | Diagnostic | Sensitivity= 72% Specificity = 83% | Hajdinjak, T. et al. 2008 [60] |
| BTA stat/ BTA Track | Yes/NA*1 | No | Urine |  Exfoliated cells Exfoliated cells | Dipstick immunoassay |  Proteins Proteins | Diagnostic & Surveillance | Sensitivity = 70% Specificity = 75% | Glas, A.S. et al. 2003 [61] |
| CxBladder | No/No | No | Urine |  Exfoliated cells Exfoliated cells | RT-qPCR |  RNA (messenger RNA) RNA (messenger RNA) | Diagnostic | Sensitivity = 82% Specificity = 85% | O’Sullivan, P. et al. 2012 [62] |
| Xpert Detection | No/Yes | No | Urine |  Exfoliated cells Exfoliated cells | RT-qPCR |  RNA (messenger RNA) RNA (messenger RNA) | Diagnostic | Sensitivity = 76% Specificity = 85% | Valenberg FJP, V. et al. 2017 [63] |
| Uromonitor | No/Yes | Yes*2 | Urine |  Exfoliated cells Exfoliated cells | Real-time PCR |  DNA DNA(tumor cell DNA) | Surveillance | Sensitivity = 74% Specificity = 93% | Batista, R. et al. 2019 [56] |
| Epicheck | No/Yes | No | Urine |  Exfoliated cells Exfoliated cells | Real-time PCR |  DNA DNA(methylation) | Surveillance | Sensitivity = 68% Specificity = 88% | Witjes, A. et al. 2018 [64] |
| UroSEEK | No/No | No | Urine |  Exfoliated cells Exfoliated cells | Massively parallel sequencing-based assay |  DNA DNA(tumor cell DNA) | Diagnostic & Surveillance | Sensitivity = 95% Specificity = 93% | Springer, S.U. et al. 2018 [65] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, R.; Vinagre, N.; Meireles, S.; Vinagre, J.; Prazeres, H.; Leão, R.; Máximo, V.; Soares, P. Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review. Diagnostics 2020, 10, 39. https://doi.org/10.3390/diagnostics10010039
Batista R, Vinagre N, Meireles S, Vinagre J, Prazeres H, Leão R, Máximo V, Soares P. Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review. Diagnostics. 2020; 10(1):39. https://doi.org/10.3390/diagnostics10010039
Chicago/Turabian StyleBatista, Rui, Nuno Vinagre, Sara Meireles, João Vinagre, Hugo Prazeres, Ricardo Leão, Valdemar Máximo, and Paula Soares. 2020. "Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review" Diagnostics 10, no. 1: 39. https://doi.org/10.3390/diagnostics10010039
APA StyleBatista, R., Vinagre, N., Meireles, S., Vinagre, J., Prazeres, H., Leão, R., Máximo, V., & Soares, P. (2020). Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review. Diagnostics, 10(1), 39. https://doi.org/10.3390/diagnostics10010039