The Oxygen Release Instrument: Space Mission Reactive Oxygen Species Measurements for Habitability Characterization, Biosignature Preservation Potential Assessment, and Evaluation of Human Health Hazards
Abstract
1. Introduction
2. Principle of Operation of the OxR (for Oxygen Release) Instrument
3. Enzyme-Based ROS Specificity of the OxR Instrument
4. OxR Assay Simulation Verification on Mars-Analog Regolith
5. The Potential of the OxR Assay for a Field-Deployable Instrument
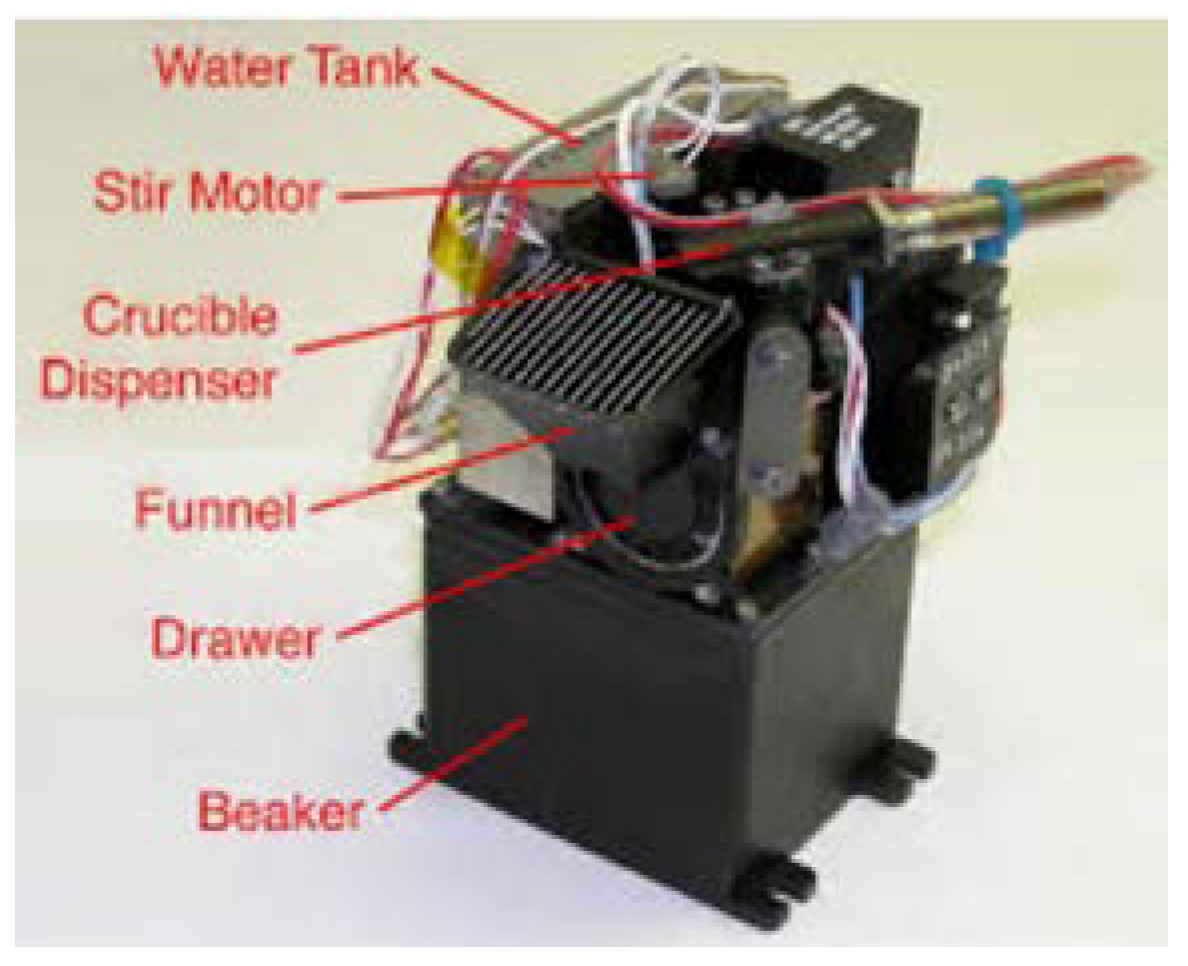
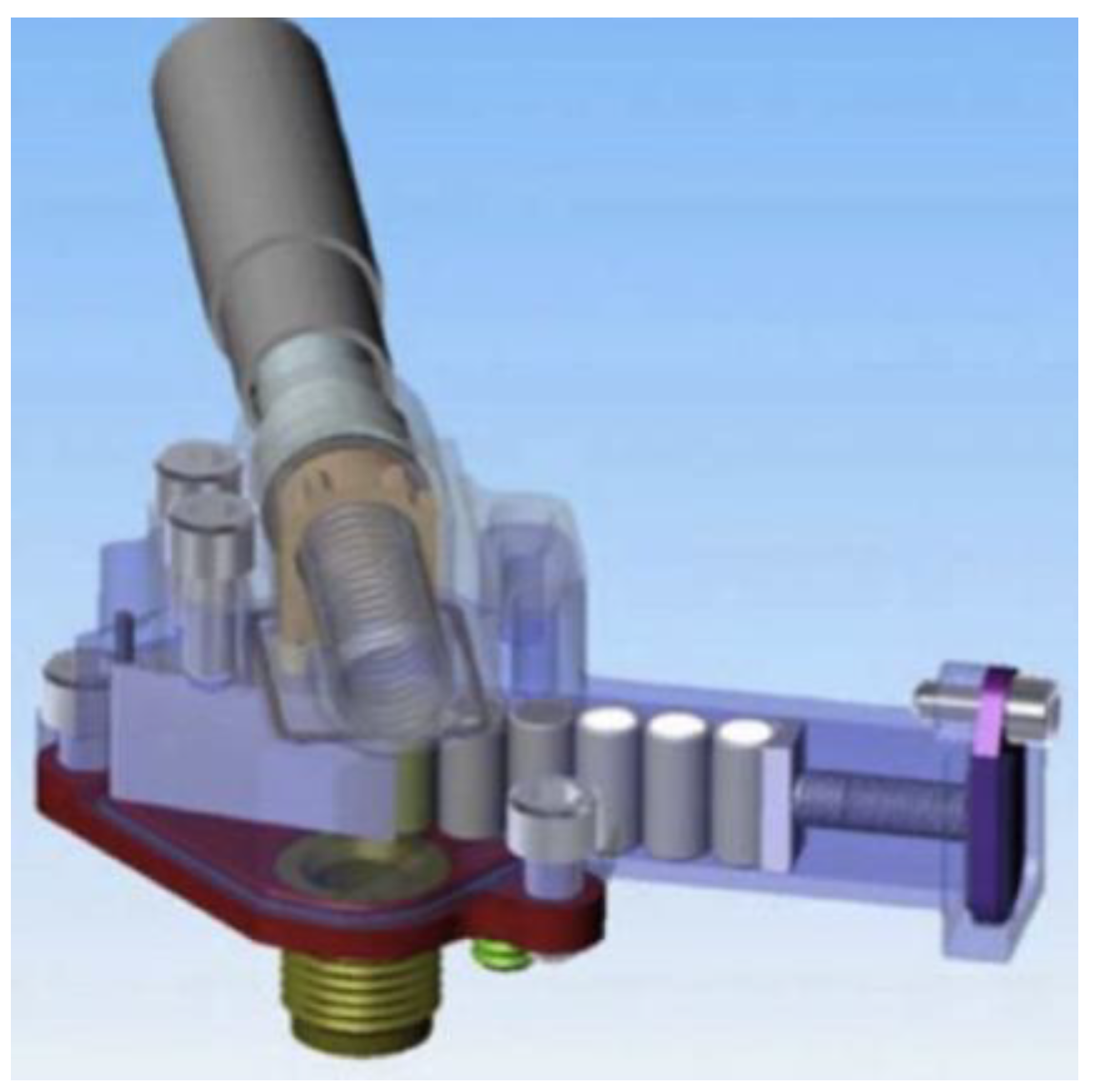
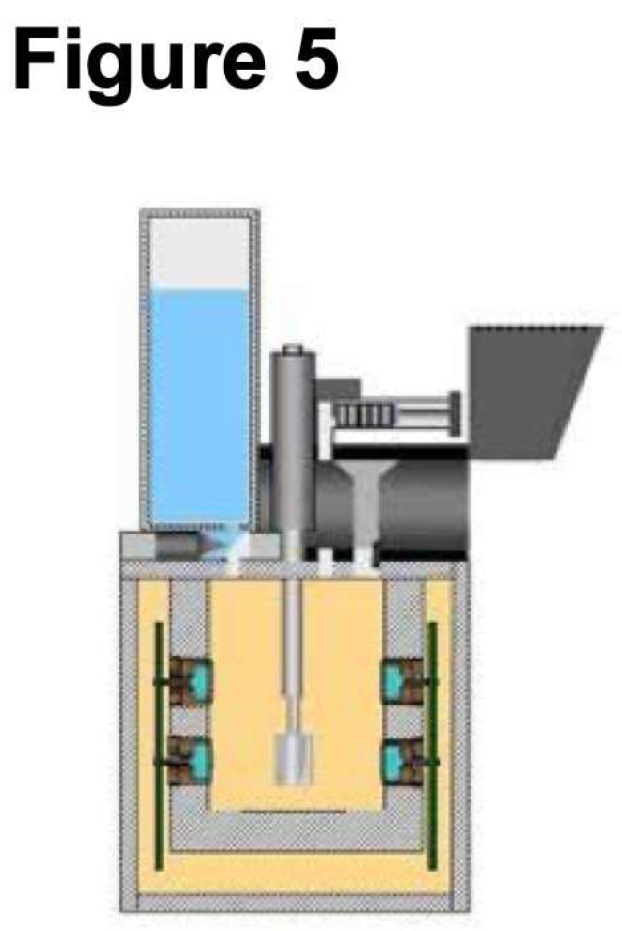
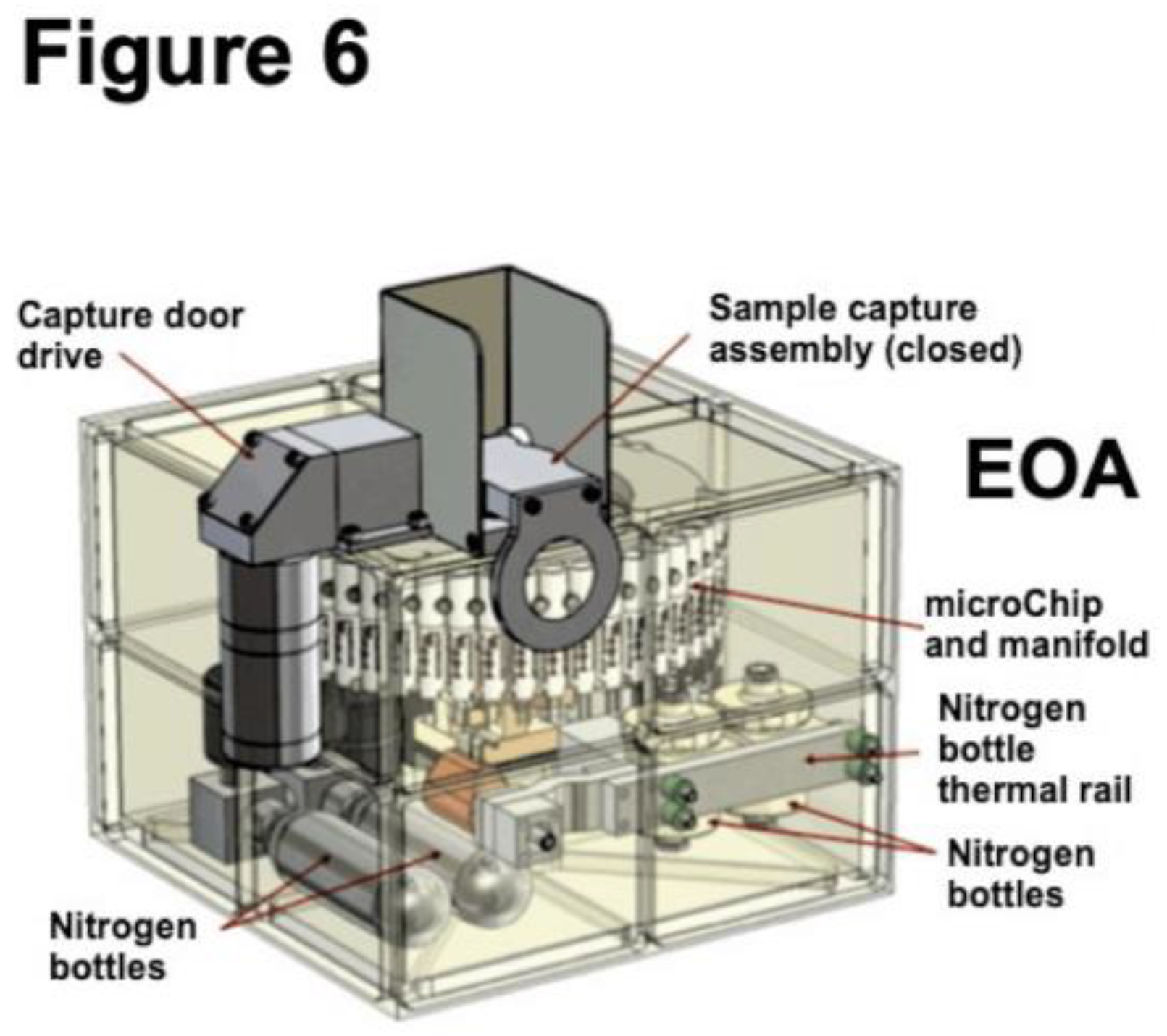
6. Implementation of the OxR Instrument
7. Studies with the OxR Instrument
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Georgiou, C.D.; Sun, H.J.; McKay, C.P.; Grintzalis, K.; Papapostolou, I.; Zisimopoulos, D.; Panagiotidis, K.; Zhang, G.; Koutsopoulou, E.; Christidis, G.E.; et al. Evidence for photochemical production of reactive oxygen species in desert soils. Nat. Commun. 2015, 6, 7100. [Google Scholar] [CrossRef] [PubMed]
- Barker, E.S. Detection of molecular oxygen in the Martian atmosphere. Nature 1972, 238, 447–448. [Google Scholar] [CrossRef]
- Feldman, P.D.; Morrison, D. The Apollo 17 Ultraviolet Spectrometer: Lunar atmosphere measurements revisited. Geophys. Res. Lett. 1991, 18, 2105–2109. [Google Scholar] [CrossRef]
- Stern, A.S. The Lunar atmosphere: History, status, current problems, and context. Rev. Geophys. 1999, 37, 453–491. [Google Scholar] [CrossRef]
- Hall, D.T.; Strobel, D.F.; Feldman, P.D.; Mcgrath, M.A.; Weaver, H.A. Detection of an oxygen atmosphere on Jupiter’s moon Europa. Nature 1995, 373, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.E.; Luhmann, J.G.; Tokar, R.L.; Bouhram, M.; Berthelier, J.J.; Sittler, E.C.; Cooper, J.F.; Hill, T.W.; Smith, H.T.; Michael, M.; et al. Production, ionization and redistribution of O2 in Saturn’s ring atmosphere. Icarus 2006, 180, 393–402. [Google Scholar] [CrossRef]
- Goldsmith, P.F.; Liseau, R.; Bell, T.A.; Black, J.H.; Chen, J.-H.; Hollenbach, D.; Kaufman, M.J.; Li, D.; Lis, D.C.; Melnick, G.; et al. Herschel measurements of molecular oxygen in Orion. Astrophys. J. 2011, 737, 96. [Google Scholar] [CrossRef]
- Yao, Y.; Shushkov, P.; Miller, T.F., III; Giapis, K.P. Direct dioxygen evolution in collisions of carbon dioxide with surfaces. Nat. Commun. 2019, 10, 2294. [Google Scholar] [CrossRef]
- Schwadron, N.A.; Baker, T.; Blake, B.; Case, A.W.; Cooper, J.F.; Golightly, M.; Jordan, A.; Joyce, C.; Kasper, J.; Kozarev, K.; et al. Lunar radiation environment and space weathering from the Cosmic Ray Telescope for the effects of radiation (CRaTER). J. Geophys. Res. 2012, 117, E00H13. [Google Scholar] [CrossRef]
- Turci, F.; Corazzari, I.; Alberto, G.; Martra, G.; Fubini, B. Free-radical chemistry as a means to evaluate Lunar dust health hazard in view of future missions to the moon. Astrobiology 2015, 15, 371–380. [Google Scholar] [CrossRef]
- Hendrix, D.A.; Port, S.T.; Hurowitz, J.A.; Schoonen, M.A. Measurement of OH * generation by pulverized minerals using Electron Spin Resonance spectroscopy and implications for the reactivity of planetary regolith. GeoHealth 2019, 3, 28–42. [Google Scholar] [CrossRef]
- Hurowitz, J.A.; Tosca, N.J.; McLennan, S.M.; Schoonen, M.A.A. Production of hydrogen peroxide in Martian and Lunar soils. Earth Planet. Sci. Lett. 2007, 255, 41–52. [Google Scholar] [CrossRef]
- Linnarsson, D.; Carpenter, J.; Fubini, B.; Gerde, P.; Karlsson, L.L.; Loftus, D.J.; Prisk, G.K.; Staufer, U.; Tranfield, E.M.; van Westrenen, W. Toxicity of Lunar dust. Planet. Space Sci. 2012, 74, 57–71. [Google Scholar] [CrossRef]
- Wallace, W.T.; Phillips, C.J.; Jeevarajan, A.S.; Chen, B.; Taylor, L.A. Nanophase iron-enhanced chemical reactivity of ground Lunar soil. Earth Planet. Sci. Lett. 2010, 295, 571–577. [Google Scholar] [CrossRef]
- Bak, E.N.; Zafirov, K.; Merrison, J.P.; Jensen, S.J.K.; Nørnberg, P.; Gunnlaugsson, H.P.; Finster, K. Production of reactive oxygen species from abraded silicates. Implications for the reactivity of the Martian soil. Earth Planet Sci. Lett. 2017, 473, 113–121. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ogata, T.; Sato, M. Mechano-radicals produced from ground quartz and quartz glass. Powder Technol. 1995, 85, 269–274. [Google Scholar] [CrossRef]
- Fubini, B.; Hubbard, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) generation by silica in inflammation and fibrosis. Free Rad. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Caston, R.; Luc, K.; Hendrix, D.; Hurowitz, J.A.; Demple, B. Assessing toxicity and nuclear and mitochondrial DNA damage caused by exposure of mammalian cells to Lunar regolith simulants. GeoHealth 2018, 2, 139–148. [Google Scholar] [CrossRef]
- Cooper, J.F.; Cooper, P.D.; Sittler, E.C.; Sturner, S.J.; Rymer, A.M. Old faithful model for radiolytic gas-driven cryovolcanism at Enceladus. Planet. Space Sci. 2009, 57, 1607–1620. [Google Scholar] [CrossRef]
- Sittler, E.C.; Ali, A.; Cooper, J.F.; Hartle, R.E.; Johnson, R.E.; Coates, A.J.; Young, D.T. Heavy ion formation in Titan’s ionosphere: Magnetospheric introduction of free oxygen and a source of Titan’s aerosols? Planet. Space Sci. 2009, 57, 1547–1557. [Google Scholar] [CrossRef]
- Gaidos, E.J.; Nealson, K.H.; Kirschvink, J.L. Life in Ice-Covered Oceans. Science 1999, 284, 1631–1633. [Google Scholar] [CrossRef] [PubMed]
- Hand, K.P.; Carlson, R.W.; Chyba, C.F. Energy, chemical disequilibrium, and geological constraints on Europa. Astrobiology 2007, 7, 1006–1022. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.P. The Viking biological experiments on Mars. Icarus 1978, 34, 666–674. [Google Scholar] [CrossRef]
- Zent, A.P.; McKay, C.P. The chemical reactivity of the Martian soil and implications for future missions. Icarus 1994, 108, 146–457. [Google Scholar] [CrossRef]
- Quinn, R.C.; Martucci, H.F.; Miller, S.R.; Bryson, C.E.; Grunthaner, F.J.; Grunthaner, P.J. Perchlorate radiolysis on Mars and the origin of Martian soil reactivity. Astrobiology 2013, 13, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Oyama, V.I.; Berdahl, B.J.; Carle, G.C. Preliminary findings of the Viking Gas Exchange Experiment and a model for Martian surface chemistry. Nature 1977, 265, 110–114. [Google Scholar] [CrossRef]
- Klein, H.P.; Horowitz, N.H.; Levin, G.V.; Oyama, V.I.; Lederberg, J.; Rich, A.; Hubbard, J.S.; Hobby, G.L.; Straat, P.A.; Berdahl, B.J.; et al. The Viking biological investigation: Preliminary results. Science 1976, 194, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.V.; Straat, P.A. Viking Labeled Release biology experiment: Interim results. Science 1976, 194, 1322–1329. [Google Scholar] [CrossRef]
- Yen, A.S.; Kim, S.S.; Hecht, M.H.; Frant, M.S.; Murray, B. Evidence that the reactivity of the Martian soil is due to superoxide ions. Science 2000, 289, 1909–1912. [Google Scholar] [CrossRef]
- Hecht, M.H.; Kounaves, S.P.; Quinn, R.C.; West, S.J.; Young, S.M.M.; Ming, D.W.; Catling, D.C.; Clark, B.C.; Boynton, W.V.; Hoffman, J.; et al. Detection of perchlorate and the soluble chemistry of Martian soil at the Phoenix Lander site. Science 2009, 325, 64–67. [Google Scholar] [CrossRef]
- Navarro-González, R.; Vargas, E.; de la Rosa, J.; Raga, A.C.; McKay, C.P. Reanalysis of the Viking results suggests perchlorate and organics at mid-latitudes on Mars. J. Geophys. Res. 2010, 115, E12010. [Google Scholar]
- Glavin, D.P.; Freissinet, C.; Miller, K.E.; Eigenbrode, J.L.; Brunner, A.E.; Buch, A.; Sutter, B.; Archer, P.D.J.; Atreya, S.K.; Brinckerhoff, W.B.; et al. Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by SAM at the Rocknest aeolian deposit in Gale Crater. J. Geophs. Res. 2013, 118, 1–19. [Google Scholar]
- Prince, L.A.; Johnson, E.R. The radiation-induced decomposition of the alkali and alkaline earth perchlorates. I. Product yields and stoichiometry. J. Phys. Chem. 1965, 69, 359–377. [Google Scholar] [CrossRef]
- Georgiou, C.D.; Zisimopoulos, D.; Kalaitzopoulou, E.; Quinn, R.C. Radiation driven formation of reactive oxygen species in oxychlorine containing Mars surface analogues. Astrobiology 2017, 17, 319–336. [Google Scholar] [CrossRef]
- Lasne, J.; Noblet, A.; Szopa, C.; Navarro-González, R.; Cabane, M.; Poch, O.; Stalport, F.; François, P.; Atreya, S.K.; Coll, P. Oxidants at the surface of Mars: A review in light of recent exploration results. Astrobiology 2016, 16, 977–996. [Google Scholar] [CrossRef]
- McKay, C.P.; Grunthaner, F.J.; Lane, A.L.; Herring, M.; Bartman, R.K.; Ksendzov, A.; Manning, C.M.; Lamb, J.L.; Williams, R.M.; Ricco, A.J.; et al. The Mars Oxidant Experiment (MOX) for Mars’ 96. Planet. Space Sci. 1998, 46, 769–777. [Google Scholar] [CrossRef]
- Sharma, R.K. Peroxides, peracides and their salts. In Inorganic Reaction Mechanisms; Discovery Publishing House: New Delhi, India, 2007; pp. 132–173. [Google Scholar]
- Lunsford, J.H. ERS of adsorbed oxygen species. Catal. Rev. 1973, 8, 135–157. [Google Scholar] [CrossRef]
- Dyrek, K.; Che, M. EPR as a tool to investigate the transition metal chemistry on oxide surfaces. Chem. Rev. 1997, 97, 305–331. [Google Scholar] [CrossRef]
- Makarov, S.Z.; Ladelnova, L.V. The peroxides of titanium, zirconium, and cerium formed in the reaction of their hydroxides with hydrogen peroxide. Rus. Chem. Bull. 1961, 10, 889–893. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Hecht, M.H.; Kapit, J.; Gospodinova, K.; DeFlores, L.; Quinn, R.C.; Boynton, W.V.; Clark, B.C.; Catling, D.C.; Hredzak, P.; et al. Wet chemistry experiments on the 2007 Phoenix Mars Scout Lander mission: Data analysis and results. J. Geophys. Res. 2010, 115, E00E10. [Google Scholar] [CrossRef]
- Quinn, R.C.; Chittenden, J.D.; Kounaves, S.P.; Hecht, M.H. The oxidation-reduction potential of aqueous soil solutions at the Mars Phoenix landing site. Geophys. Res. Lett. 2011, 38, L14202. [Google Scholar] [CrossRef]
- Dukes, C.A.; Baragiola, R.A. The Lunar surface-exosphere connection: Measurement of secondary-ions from Apollo soils. Icarus 2015, 255, 51–57. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; p. 904. [Google Scholar]
- NASA. Forward to the Moon: NASA’s STRATEGIC Plan for Lunar Exploration. 23 May 2019. Available online: https://www.nasa.gov/sites/default/files/atoms/files/america_to_the_moon_2024_artemis_20190523.pdf (accessed on 20 August 2019).
- Allen, C.C.; Morris, R.V.; LJager, K.M.; Golden, D.C.; Lindstrom, D.J.; Lindstrom, M.M.; Lockwood, J.P. Martian Regolith Simulant JSC Mars-1. In Proceedings of the Lunar and Planetary Science XXIX: Papers Presented to the Twenty-Ninth Lunar and Planetary Science Conference, Houston, TX, USA, 16–20 March 1998. [Google Scholar]
- Peters, G.H.; Abbey, W.; Bearman, G.H.; Mungas, G.S.; Smith, J.A.; Anderson, R.C.; Douglas, S.; Beegle, L.W. Mojave Mars simulant—Characterization of a new geologic Mars analog. Icarus 2008, 197, 470–479. [Google Scholar] [CrossRef]
- Slyuta, E.N. Physical and mechanical properties of the Lunar soil. Solar System Res. 2014, 48, 330–353. [Google Scholar] [CrossRef]
- Georgiou, C.D.; Zisimopoulos, D.; Panagiotidis, K.; Grintzalis, K.; Papapostolou, I.; Quinn, R.C.; McKay, C.P.; Sun, H. Martian superoxide and peroxide O2 Release (OR) assay: A new technology for terrestrial and planetary applications. Astrobiology 2016, 16, 126–142. [Google Scholar] [CrossRef]
- Kelly, K.J.; Sandoval, R.M.; Dunn, K.W.; Molitoris, B.A.; Dagher, P.C. A novel method to determine specificity and sensitivity of the Tunel reaction in the quantitation of apoptosis. Am. J. Physiol. Cell Physiol. 2003, 284, C1309–C1318. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. Über die katalyse des hydroperoxydes. Naturwissenschaften 1932, 20, 948–950. [Google Scholar] [CrossRef]
- Gray, B.; Carmichael, A.J. Kinetics of superoxide scavenging by dismutase enzymes and manganese mimics determined by Electron Spin Resonance. Biochem. J. 1992, 281, 795–802. [Google Scholar] [CrossRef]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef]
- Mishra, B.; Priyadarsini, K.I.; Bhide, M.K.; Kadam, R.M.; Mohan, H. Reactions of superoxide radicals with curcumin: Probable mechanisms by Optical Spectroscopy and EPR. Free Radic. Res. 2004, 38, 355–362. [Google Scholar] [CrossRef]
- Afanasev, B.; Kuprianova, N.; Polozova, N. Kinetics and mechanism of the reactions of the superoxide ion in solutions. I. Absorption spectra and interaction with solvents and cations. Int. J. Chem. Kinet. 1983, 15, 1045–1056. [Google Scholar] [CrossRef]
- Do, S.-H.; Batchelor, B.; Lee, H.-K.; Kong, S.-H. Hydrogen peroxide decomposition on manganese oxide (pyrolusite): Kinetics, intermediates, and mechanism. Chemosphere 2009, 75, 8–12. [Google Scholar] [CrossRef]
- Venkatachalapathy, R.; Davila, G.P.; Prakash, J. Catalytic decomposition of hydrogen peroxide in alkaline solutions. Electrochem. Commun. 1999, 1, 614–617. [Google Scholar] [CrossRef]
- MacKenzie, A.; Martin, W. Loss of endothelium-derived nitric oxide in rabbit aorta by oxidant stress: Restoration by superoxide dismutase mimetics. Br. J. Pharmacol. 1998, 124, 719–728. [Google Scholar] [CrossRef]
- Mok, L.S.J.; Paisley, K.; Martin, W. Inhibition of nitrergic neurotransmission in the bovine retractor penis muscle by an oxidant stress: Effects of superoxide dismutase mimetics. Br. J. Pharmacol. 1998, 124, 111–118. [Google Scholar] [CrossRef]
- Behar, D.; Czapski, G.; Rabani, J.; Dorfman, L.M.; Schwarz, H.A. The acid dissociation constant and decay kinetics of the perhydroxyl radical. J. Phys. Chem. 1970, 74, 3209–3213. [Google Scholar] [CrossRef]
- Hughes, G.; Willis, C. Scavenger studies in the radiolysis of aqueous ferricyanide solutions at high pH. Discuss. Faraday Soc. 1963, 36, 223–231. [Google Scholar] [CrossRef]
- Woodbury, W.; Spencer, A.K.; Stahmann, M.A. An improved procedure using ferricyanide for detecting catalase isozymes. Anal. Biochem. 1971, 44, 301–305. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Hecht, M.H.; West, S.J.; Morookian, J.-M.; Young, S.M.M.; Quinn, R.C.; Grunthaner, P.; Wen, X.; Weilert, M.; Cable, C.A.; et al. The MECA Wet Chemistry Laboratory on the 2007 Phoenix Mars Scout Lander. J. Geophys. Res. 2009, 114, E00A19. [Google Scholar] [CrossRef]
- Duca, Z.A.; Tan, G.K.; Cantrell, T.; van Enige, M.; Dorn, M.; Cato, M.; Speller, N.C.; Pital, A.; Mathies, R.A.; Stockton, A.M. Operation of pneumatically-actuated membrane-based microdevices for in situ analysis of extraterrestrial organic molecules after prolonged storage and in multiple orientations with respect to Earth’s gravitational field. Sens. Actuators B Chem. 2018, 272, 229–235. [Google Scholar] [CrossRef]
- Mathies, R.A.; Razu, M.E.; Kim, J.; Stockton, A.B.; Turin, P.; Butterworth, A. Feasibility of detecting bioorganic compounds in Enceladus plumes with the Enceladus Organic Analyzer (EOA). Astrobiology 2017, 17, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Stockton, A.M.; Jensen, E.C.; Mathies, R.A. Pneumatically actuated microvalve circuits for programmable automation of chemical and biochemical analysis. Lab Chip 2016, 16, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Linshiz, G.; Jensen, E.; Stawski, N.; Bi, C.; Elsbree, N.; Jiao, H.; Kim, J.; Mathies, R.; Keasling, J.D.; Hillson, N.J. End-to-end automated microfluidic platform for synthetic biology: From design to functional analysis. J. Biol. Eng. 2016, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; Mathies, R.A. Forensic typing of single cells using droplet microfluidics. In Microfluidic Methods for Molecular Biology; Lu, C., Verbridge, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 71–94. [Google Scholar]
- Jung, Y.K.; Kim, J.; Mathies, R.A. Microfluidic linear hydrogel array for multiplexed single nucleotide polymorphism (snp) detection. Anal. Chem. 2015, 87, 3165–3170. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, C.D.; Deamer, D.W. Lipids as universal biomarkers of extraterrestrial life. Astrobiology 2014, 14, 541–549. [Google Scholar] [CrossRef]
- Georgiou, C.D. Functional properties of amino acid side chains as biomarkers of extraterrestrial life. Astrobiology 2018, 18, 1479–1496. [Google Scholar] [CrossRef] [PubMed]
- NASA. NASA’s Dragonfly Will Fly around Titan Looking for Origins, Signs of Life. 27 June 2019. Available online: https://www.nasa.gov/press-release/nasas-dragonfly-will-fly-around-titan-looking-for-origins-signs-of-life (accessed on 20 August 2019).
- Deamer, W.D.; Georgiou, C.D. Hydrothermal conditions and the origin of cellular life. Astrobiology 2015, 15, 1091–1095. [Google Scholar] [CrossRef]
- Horwell, C.J.; Fenoglio, I.; Vala Ragnarsdottir, K.; Sparks, R.S.; Fubini, B. Surface reactivity of volcanic ash from the eruption of Soufriere Hills volcano, Montserrat, West Indies with implications for health hazards. Environ. Res. 2003, 93, 202–215. [Google Scholar] [CrossRef]
- Schoonen, M.A.A.; Harrington, A.D.; Laffers, R.; Strongin, D.R. Role of hydrogen peroxide and hydroxyl radical in pyrite oxidation by molecular oxygen. Geochim. Cosmochim. Acta 2010, 74, 4971–4987. [Google Scholar] [CrossRef]
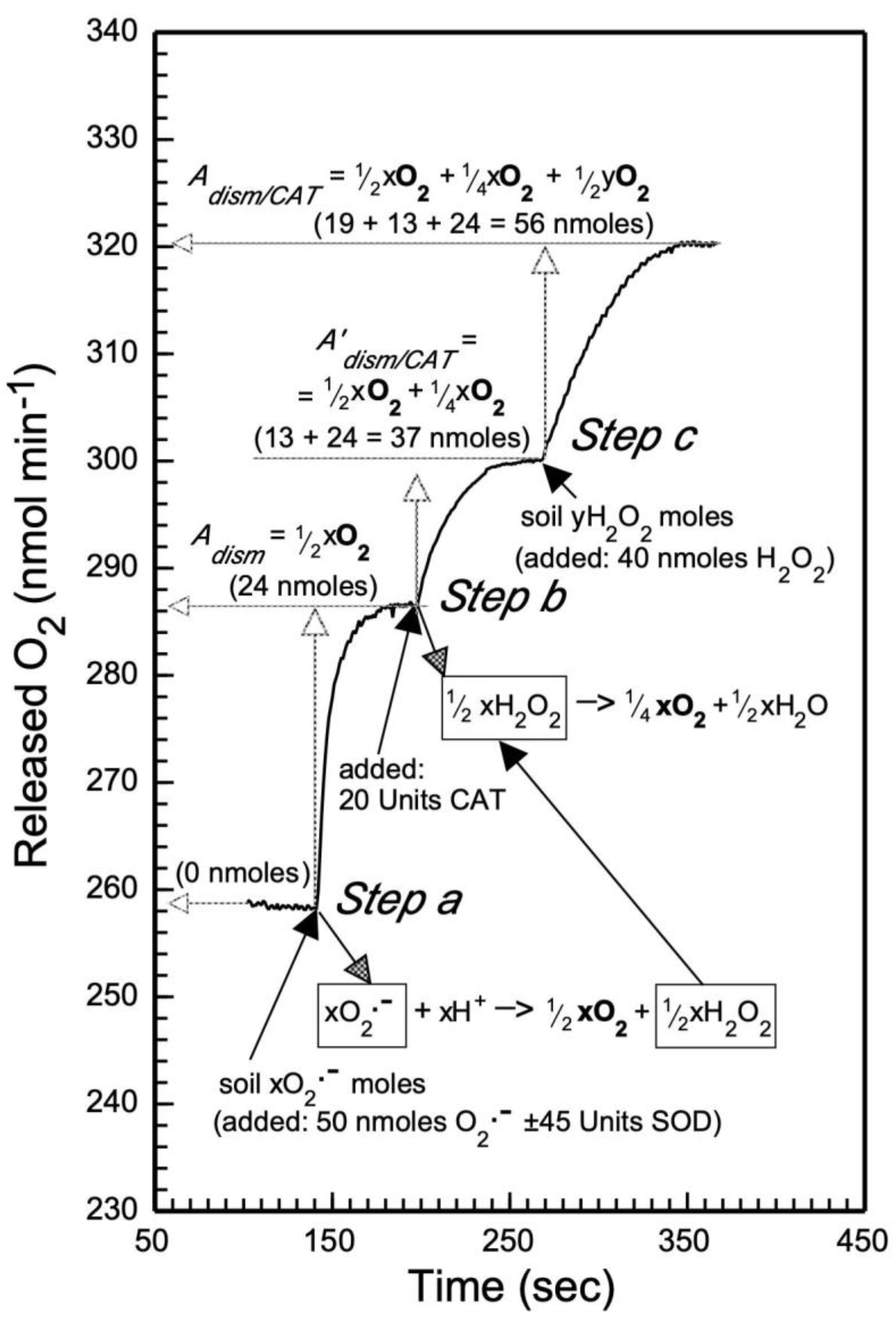
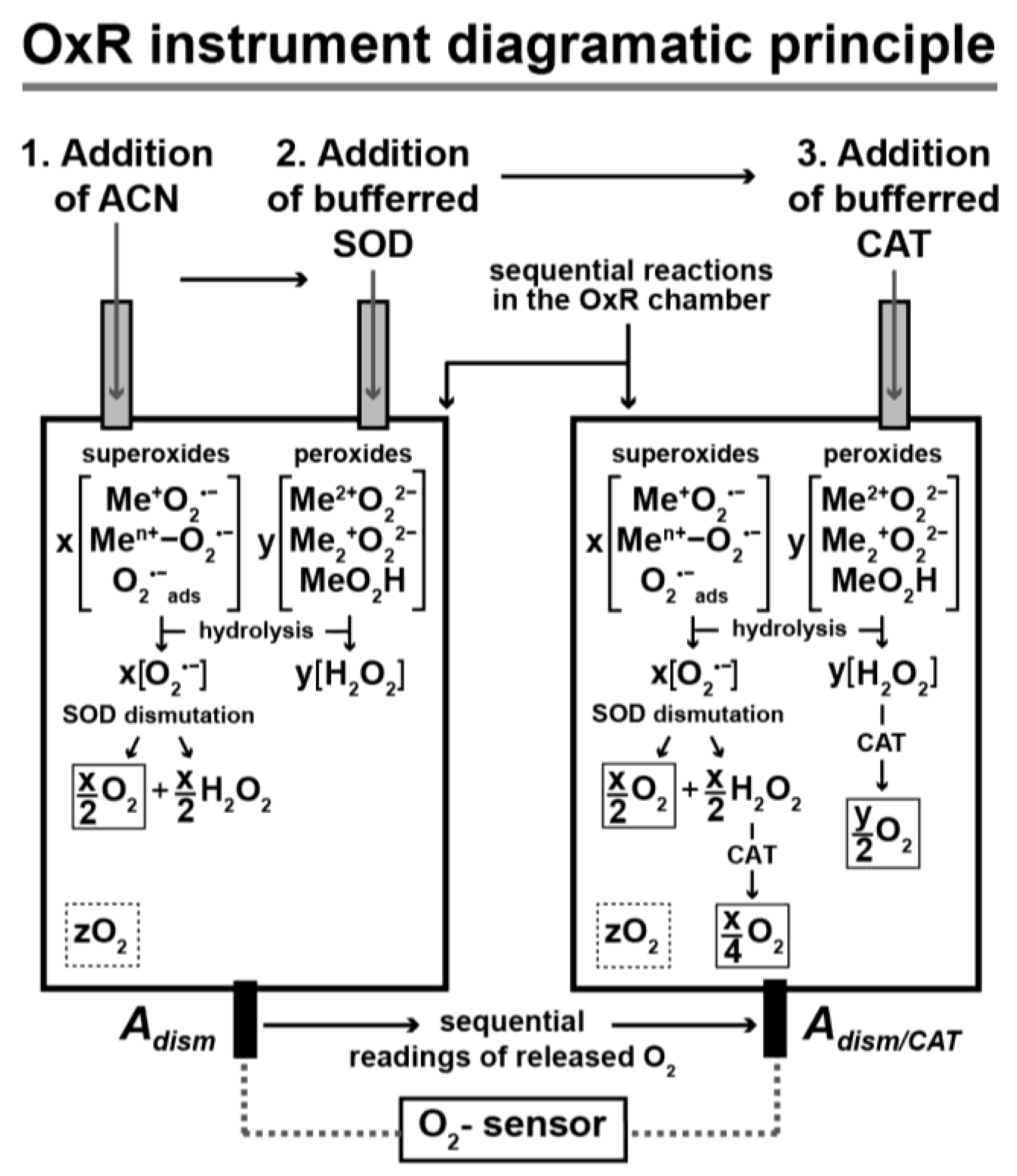
| Metal (Me) Salts of O2•− Release O2 (↑) and H2O2 by the Following Reactions [37,44] | ||
|---|---|---|
| Adsorbed O2•− | 2 O2•−ads + 2 H2O → 2 OH− + H2O2 + O2↑ | (1) |
| Metal salts of O2•− | 2 Me+ O2•− + 2 H2O → 2 Me+OH− + H2O2 + O2↑ | (2) |
| Metal-O2•− complexes | 2 Men+− O2•− + 2 H2O → 2 Men+ + 2 OH− + H2O2 + O2↑ | (3) |
| Metal Peroxides/Hydroperoxides Release H2O2 by the Following Reactions [37] | ||
| Me-salts of O22− | Me+2O22− + 2 H2O → 2 Me+OH− + H2O2 | (4) |
Me2+O22− + 2 H2O → Me2+(OH−)2 + H2O2 | (5) | |
| Me-hydroperoxides (MeO2H) | MeOOH + H2O → MeOH + H2O2 | (6) |
| I. Metal O2•− (O2•−ads, Me+ O2•−, Men+− O2•−); additional details for their hydrolysis/dismutation are presented in reactions 1–3, and in Figure 1 step a. | Step 1. Metal O2•− (e.g., Me+ O2•−) dissociation reaction: Me+ O2•− (in H2O) → O2•− + Me+ Note: Stock solution of stable O2•− is obtained by dissociation of Me+ O2•− (e.g., KO2) in anhydrous acetonitrile (ACN). Step 2. Release of O2 (and H2O2) via SOD-catalyzed dismutation of O2•− (from I, step 1): 2 O2•− + 2 H2O → 2 OH− + H2O2 + O2 ↑(same as reaction 1) Note: The spontaneous dismutation of O2•− by H2O has a rate constant ~2x105 M-1 s-1, while that with SOD is 32,000-fold faster; 6.4x109 M-1 s-1 [52]. Step 3. Base (MeOH) formation: Me+ + OH− → MeOH | |
| II. Metal O22− (Me+2O22−, Me2+O22−, MeOOH); additional details for their hydrolysis are presented in reactions 4–6. | Step 1. Dissociation reaction of metal O22− (e.g., Me+2O22¯): Me+2O22− (in H2O) → O22− + 2 Me+ Step 2. Hydrolysis reaction of O22− (from II, step 1): O22− + 2 H2O → 2 OH− + H2O2 (same as reaction 4) Step 3. Base (MeOH) formation: 2 Me2+ + 2 OH− → 2 MeOH | |
| III. H2O2 released by the hydrolysis of metal O2•− and O22−; additional details are shown in Figure 1 step b. | Release of O2 via CAT-catalyzed decomposition of H2O2 [44], resulting from I, step 2, and/or II, step 2: | |
| 2 H2O2 → 2 H2O + O2↑ | (7) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiou, C.D.; McKay, C.P.; Quinn, R.C.; Kalaitzopoulou, E.; Papadea, P.; Skipitari, M. The Oxygen Release Instrument: Space Mission Reactive Oxygen Species Measurements for Habitability Characterization, Biosignature Preservation Potential Assessment, and Evaluation of Human Health Hazards. Life 2019, 9, 70. https://doi.org/10.3390/life9030070
Georgiou CD, McKay CP, Quinn RC, Kalaitzopoulou E, Papadea P, Skipitari M. The Oxygen Release Instrument: Space Mission Reactive Oxygen Species Measurements for Habitability Characterization, Biosignature Preservation Potential Assessment, and Evaluation of Human Health Hazards. Life. 2019; 9(3):70. https://doi.org/10.3390/life9030070
Chicago/Turabian StyleGeorgiou, Christos D., Christopher P. McKay, Richard C. Quinn, Electra Kalaitzopoulou, Polyxeni Papadea, and Marianna Skipitari. 2019. "The Oxygen Release Instrument: Space Mission Reactive Oxygen Species Measurements for Habitability Characterization, Biosignature Preservation Potential Assessment, and Evaluation of Human Health Hazards" Life 9, no. 3: 70. https://doi.org/10.3390/life9030070
APA StyleGeorgiou, C. D., McKay, C. P., Quinn, R. C., Kalaitzopoulou, E., Papadea, P., & Skipitari, M. (2019). The Oxygen Release Instrument: Space Mission Reactive Oxygen Species Measurements for Habitability Characterization, Biosignature Preservation Potential Assessment, and Evaluation of Human Health Hazards. Life, 9(3), 70. https://doi.org/10.3390/life9030070







