Methodologies for Analyzing Soluble Organic Compounds in Extraterrestrial Samples: Amino Acids, Amines, Monocarboxylic Acids, Aldehydes, and Ketones
Abstract
1. Extraterrestrial Organic Matter
2. Sample Types
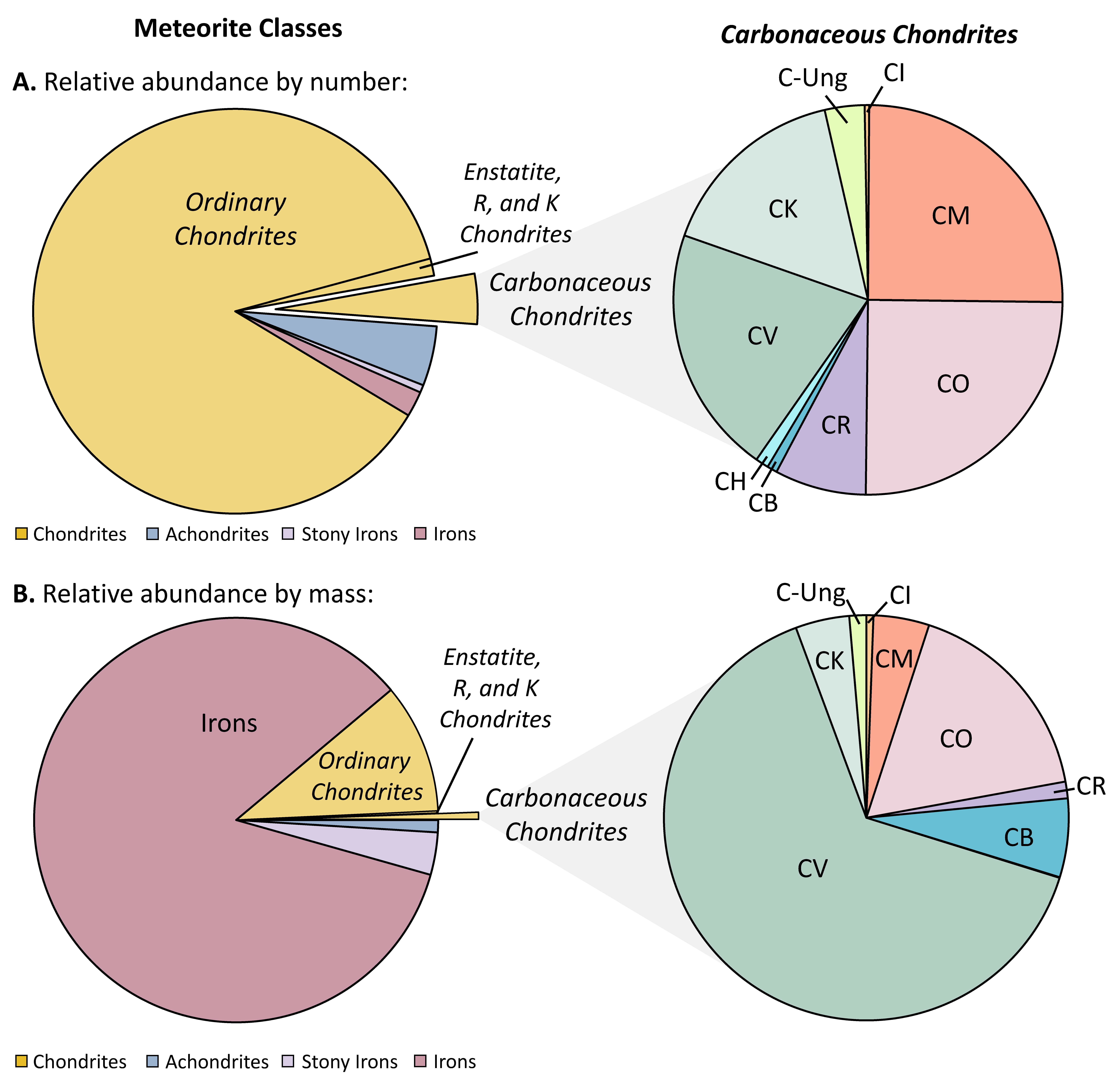
2.1. Meteorites
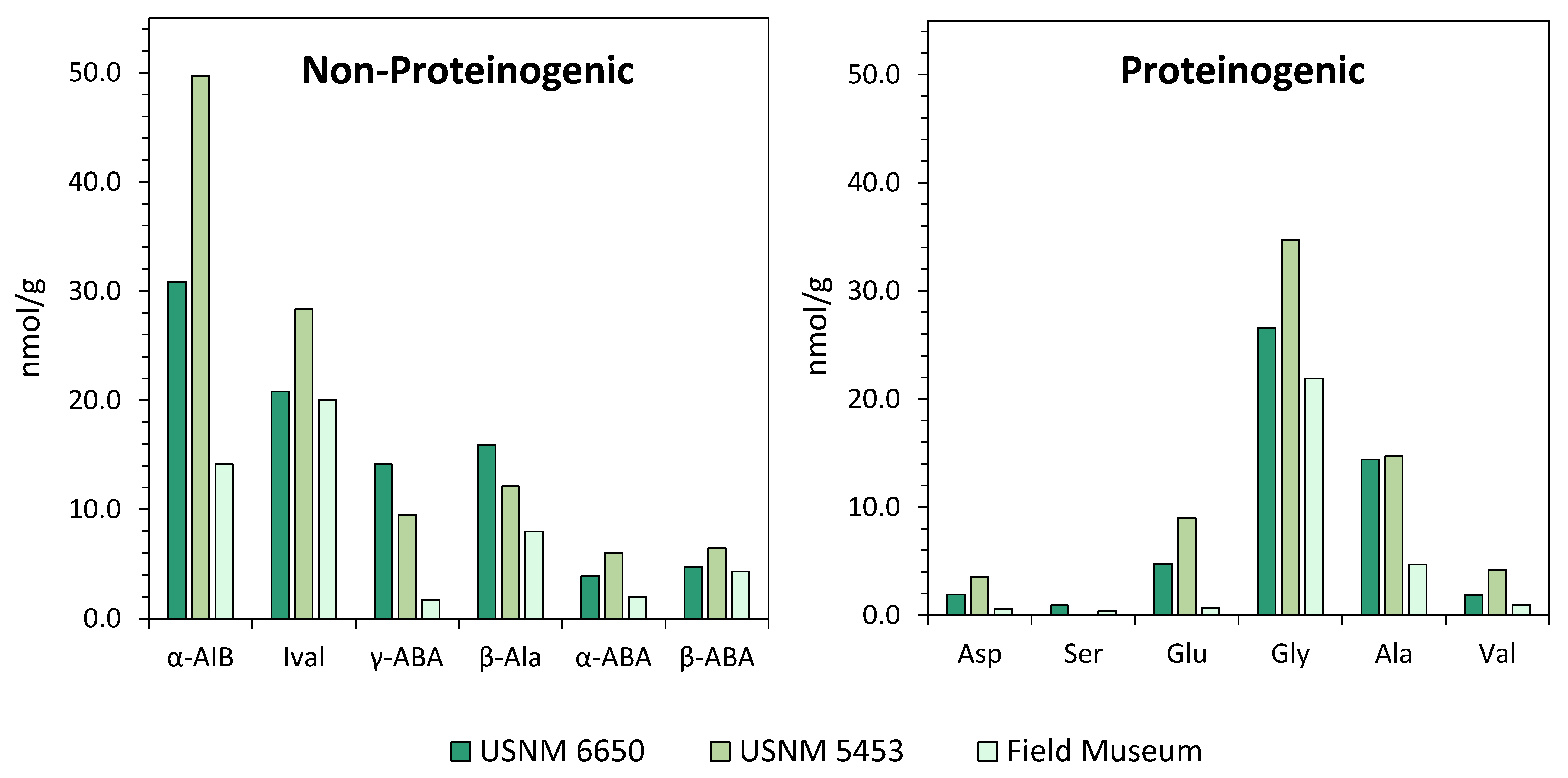
Obtaining Meteorite Samples for Analysis
2.2. Dust and Micrometeorites
2.3. Spaceflight Mission Samples
3. Review Objectives
4. Analysis of Extraterrestrial Organic Compounds: Method Overview
4.1. Mitigation and Monitoring of Sample Contamination
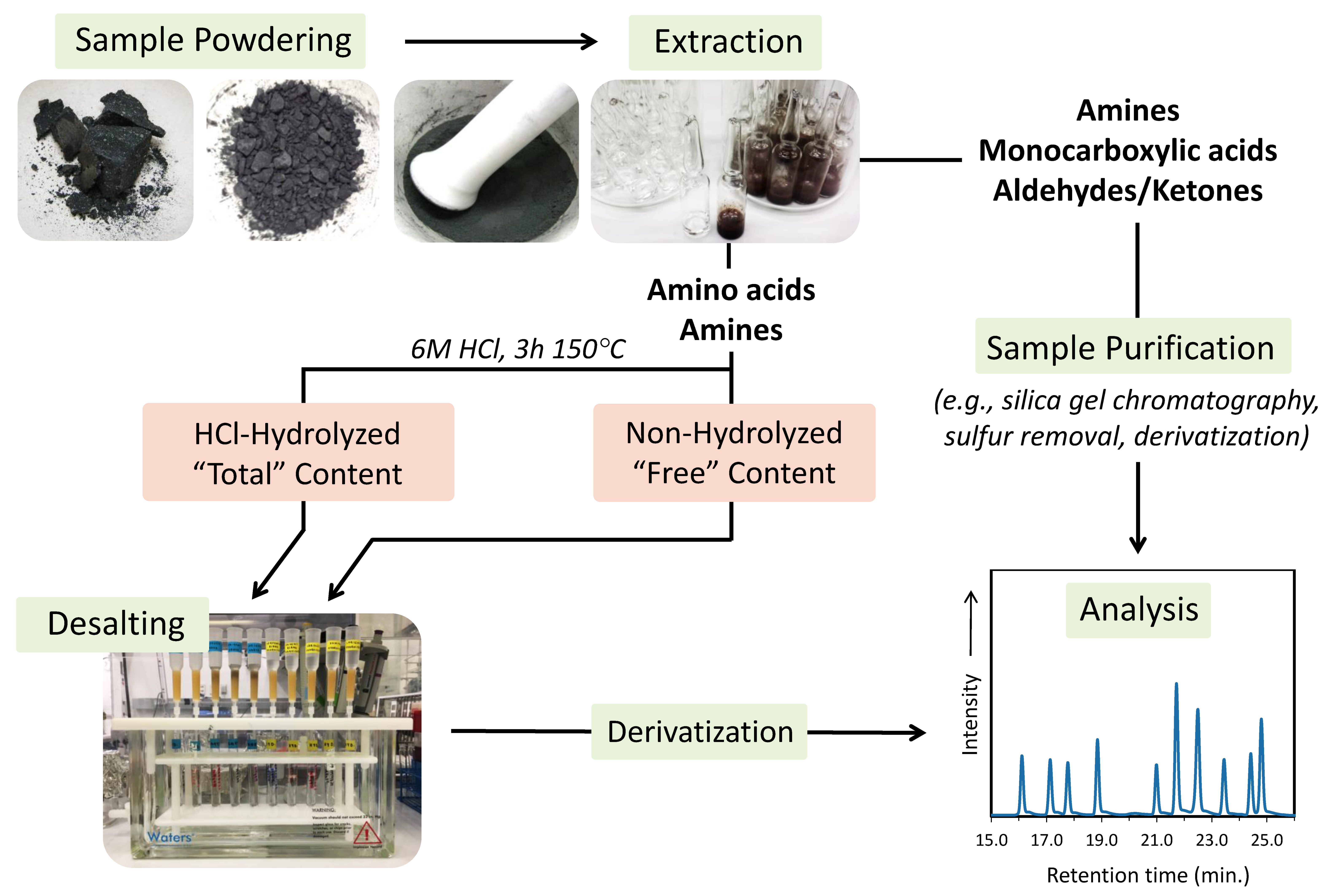
4.2. Typical Sample Extraction Protocols
4.3. Processing and Purifying the Extract
4.3.1. Separating and Concentrating the Supernatant
4.3.2. Acid Hydrolysis
4.3.3. Purification Methods
4.4. Chromatographic Separation and Detection
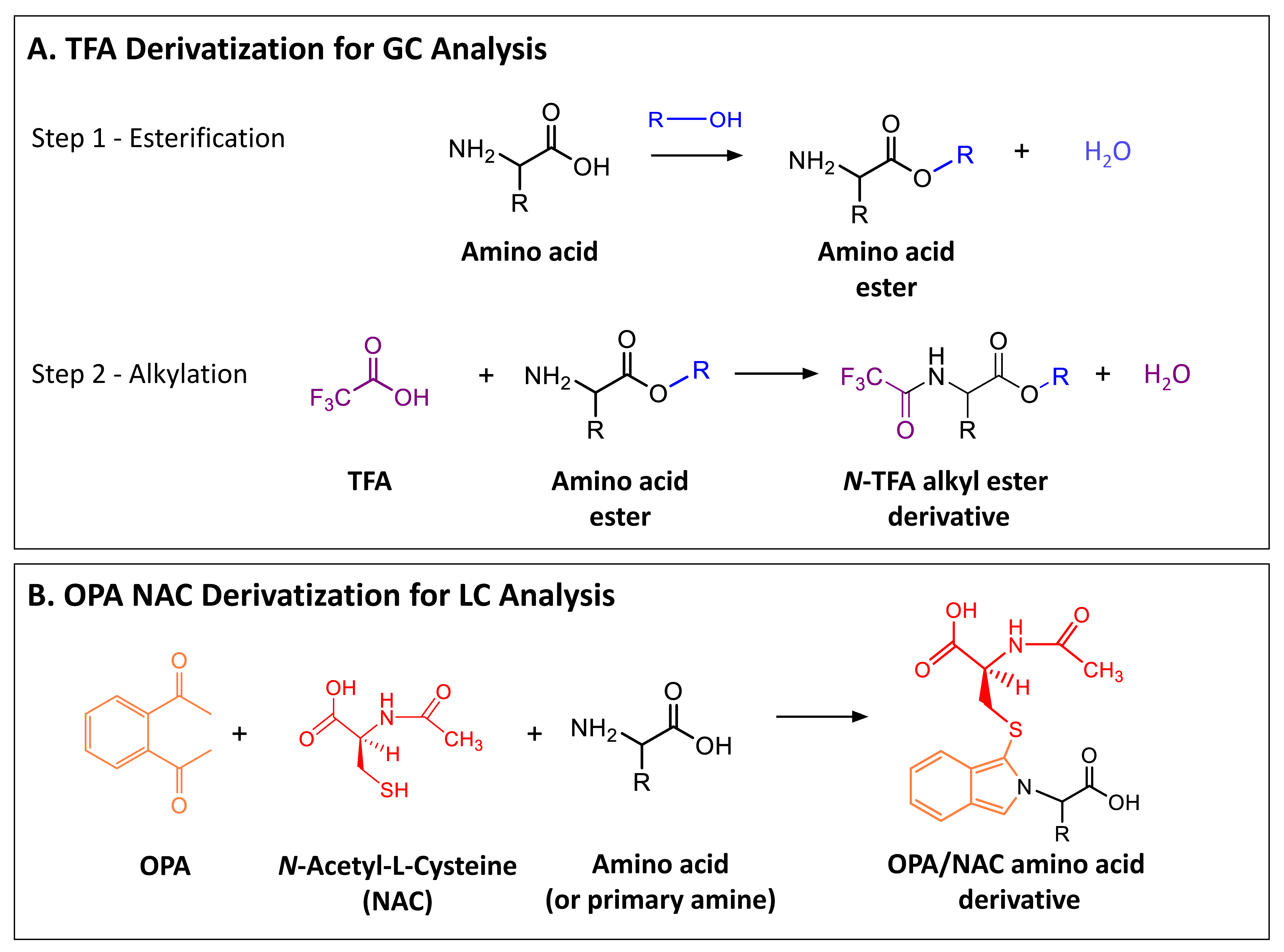
4.4.1. Amino Acids
4.4.2. Amines
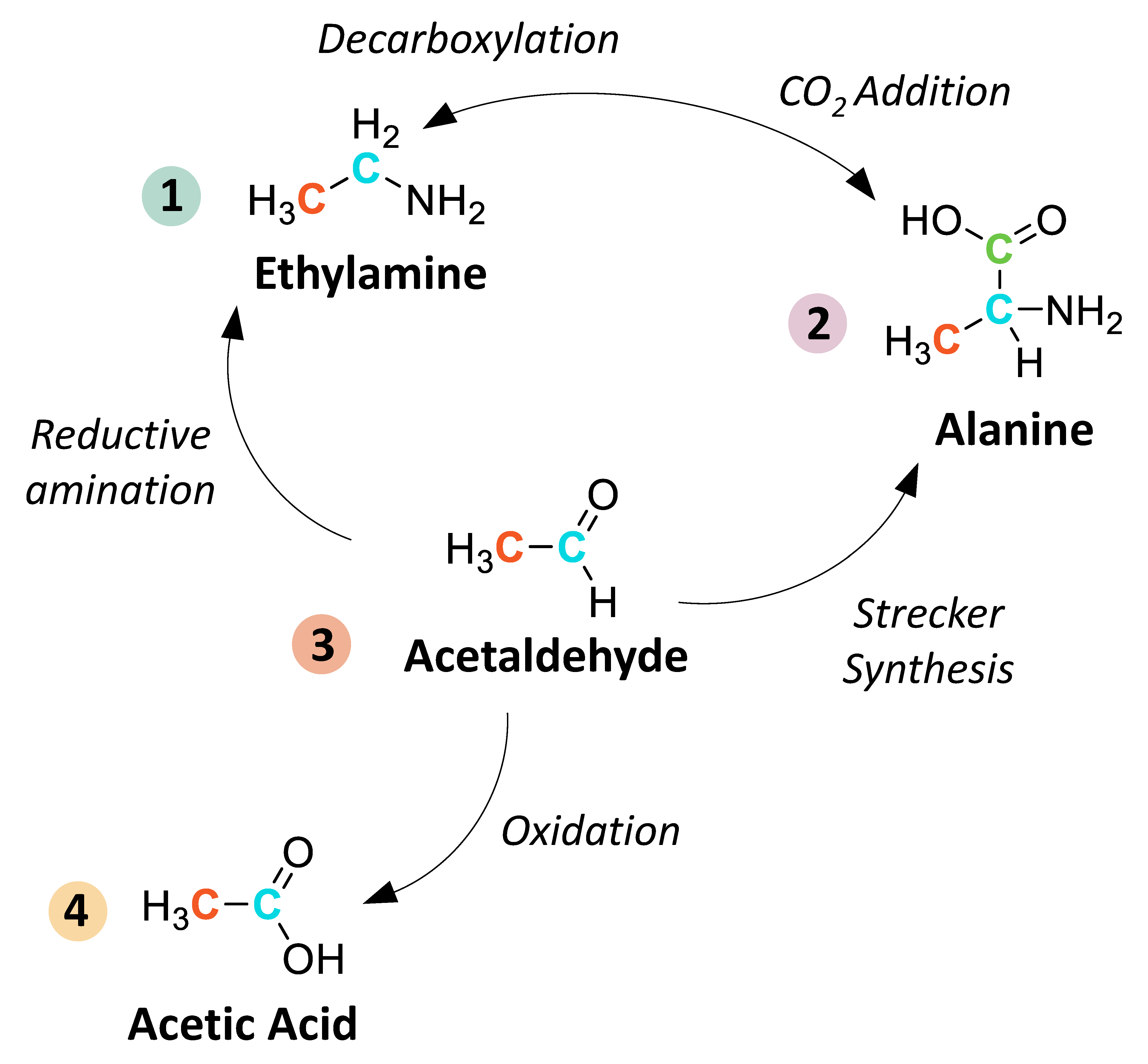
4.4.3. Monocarboxylic Acids
4.4.4. Aldehydes and Ketones
5. Identifying Limitations of Cross-Comparisons between Studies
5.1. Meteorite Sample Heterogeneity
5.2. Influence of Varying Methodologies
5.2.1. Influence of Extraction and Sample Processing
5.2.2. Influence of Derivatization and Chromatographic Techniques
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexander, C.M.O.D.; Fogel, M.; Yabuta, H.; Cody, G.D. The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim. Cosmochim. Acta 2007, 71, 4380–4403. [Google Scholar] [CrossRef]
- Botta, O.; Bada, J.L. Extraterrestrial organic compounds in meteorites. Surv. Geophys. 2002, 23, 411–467. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cooper, G.W.; Flynn, G.J. The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In Meteorites and the Early Solar System II; Lauretta, D.S., Ed.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 625–651. [Google Scholar]
- Sephton, M.A. Organic geochemistry of meteorites. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: New York, NY, USA, 2014; Volume 12, pp. 1–31. [Google Scholar]
- Glavin, D.P.; Alexander, C.M.O.; Aponte, J.C.; Dworkin, J.P.; Elsila, J.E.; Yabuta, H. The origin and evolution of organic matter in carbonaceous chondrites and links to their parent bodies. In Primitive Meteorites and Asteroids; Abreu, N.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–271. [Google Scholar]
- Krot, A.N.; Keil, K.; Scott, E.R.D.; Goodrich, C.A.; Weisberg, M.K. Classification of meteorites and their genetic relationships. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: New York, NY, USA, 2014; Volume 1, pp. 1–63. [Google Scholar]
- Weisberg, M.K.; McCoy, T.J.; Krot, A.N. Systematics and evaluation of meteorite classification. In Meteorites and the Early Solar System II, 2nd ed.; Lauretta, D.S., McSween, H.Y., Jr., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 19–52. [Google Scholar]
- Berzelius, J. Ueber Meteorsteine IV. Meteorstein von Alais. Ann. Phys. Chem. 1834, 33, 113–148. [Google Scholar] [CrossRef]
- Nagy, B.; Meinschein, W.G.; Hennessy, D.J. Mass spectroscopic analysis of the Orgueil meteorite: Evidence for biogenic hydrocarbons. Ann. N. Y. Acad. Sci. 1961, 93, 27–35. [Google Scholar] [CrossRef]
- Mueller, G. The properties and theory of genesis of the carbonaceous complex within the Cold Bokevelt meteorite. Geochim. Cosmochim. Acta 1953, 4, 1–10. [Google Scholar] [CrossRef]
- Briggs, M.H. Organic constituents of meteorites. Nature 1961, 191, 1137–1140. [Google Scholar] [CrossRef]
- Meinschein, W.G. Benzene Extracts of the Orgueil Meteorite. Nature 1963, 197, 833–836. [Google Scholar] [CrossRef]
- Kvenvolden, K.; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C.; Kaplan, I.R.; Moore, C. Evidence for extraterrestrial amino acids and hydrocarbons in the Murchison meteorite. Nature 1970, 228, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Jungclaus, G.A.; Yuen, G.U.; Moore, C.B.; Lawless, J.G. Evidence for the presence of low molecular weight alcohols and carbonyl compounds in the Murchison meteorite. Meteoritics 1976, 11, 231–237. [Google Scholar] [CrossRef]
- Jungclaus, G.; Cronin, J.R.; Moore, C.B.; Yuen, G.U. Aliphatic amines in the Murchison meteorite. Nature 1976, 261, 126–128. [Google Scholar] [CrossRef]
- Cronin, J.R.; Gandy, W.E.; Pizzarello, S. Amino-Acids of the Murchison Meteorite: I. Six Carbon Acyclic Primary Alpha-Amino Alkanoic Acids. J. Mol. Evol. 1981, 17, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfreund, P.; Glavin, D.P.; Botta, O.; Cooper, G.; Bada, J.L. Extraterrestrial amino acids in Orgueil and Ivuna: Tracing the parent body of Cl type carbonaceous chondrites. Proc. Natl. Acad. Sci. USA 2001, 98, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Huang, Y.; Fuller, M. The carbon isotopic distribution of Murchison amino acids. Geochim. Cosmochim. Acta 2004, 68, 4963–4969. [Google Scholar] [CrossRef]
- Righter, K.; Corrigan, C.; McCoy, T.; Harvey, R. 35 Seasons of U.S. Antarctic Meteorites (1976–2010): A Pictorial Guide to the Collection; American Geophysical Union: Washington, DC, USA; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Kojima, H. The history of Japanese Antarctic meteorites. Geol. Soc. Lond. Spec. Publ. 2006, 256, 291–303. [Google Scholar] [CrossRef]
- Meteoritical Bulletin Database. Available online: https://www.lpi.usra.edu/meteor/ (accessed on 30 March 2019).
- Clemett, S.J.; Maechling, C.R.; Zare, R.N.; Swan, P.D.; Walker, R.M. Identification of complex aromatic molecules in individual interplanetary dust particles. Science 1993, 262, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Clemett, S.J.; Chillier, X.D.F.; Gillette, J.S.; Zare, R.N.; Maurette, M.; Engrand, C.; Kurat, G. Observation of indigenous polycyclic aromatic hydrocarbons in ‘giant’ carbonaceous Antarctic micrometeorites. Orig. Life Evol. Biosph. 1998, 28, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, G.; Pizzarello, S.; Taylor, S.; Brownlee, D. Concentration and variability of the AIB amino acid in polar micrometeorites: Implications for the exogenous delivery of amino acids to the primitive Earth. Meteorit. Planet. Sci. 2004, 39, 1849–1858. [Google Scholar] [CrossRef]
- Flynn, G.J.; Keller, L.P.; Feser, M.; Wirick, S.; Jacobsen, C. The origin of organic matter in the solar system: Evidence from the interplanetary dust particles. Geochim. Cosmochim. Acta 2003, 67, 4791–4806. [Google Scholar] [CrossRef]
- Brinton, K.L.F.; Engrand, C.; Glavin, D.P.; Bada, J.L.; Maurette, M. A search for extraterrestrial amino acids in carbonaceous Antarctic micrometeorites. Orig. Life Evol. Biosph. 1998, 28, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Matrajt, G.; Bada, J.L. Re-examination of amino acids in Antarctic micrometeorites. Adv. Space Res. 2004, 33, 106–113. [Google Scholar] [CrossRef]
- Parker, E.T.; Engrand, C.; Dworkin, J.P.; Glavin, D.P. Amino acids in Antarctic micrometeorites. In Proceedings of the American Geophyical Union Fall Meeting, Washington, DC, USA, 10–14 December 2018. [Google Scholar]
- Fox, S.W.; Harada, K.; Hare, P.E. Accumulated analyses of amino acid precursors in returned lunar samples. Proc. Fourth Lunar Sci. Conf. Geochim. Cosmochim. Acta 1973, 2 (Suppl. 4), 2241–2248. [Google Scholar]
- Gehrke, C.W.; Zumwalt, R.W.; Kuo, K.; Rash, J.J.; Aue, W.A.; Stalling, D.L.; Kvenvolden, K.A.; Ponnamperuma, C. Research for amino-acids in lunar samples. Space Life Sci. 1972, 3, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Hare, P.E.; Windsor, C.R.; Fox, S.W. Evidence for compounds hydrolyzable to amino acids in aqueous extracts of Apollo 11 and Apollo 12 lunar fines. Science 1971, 173, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Modzeleski, V.E.; Modzeleski, J.E.; Mohammed, M.A.; Nagy, L.A.; Nagy, B.; Mcewan, W.S.; Urey, H.C.; Hamilton, P.B. Carbon-compounds in pyrolysates and amino-acids in extracts of Apollo-14 lunar samples. Nat. Phys. Sci. 1973, 242, 50–52. [Google Scholar] [CrossRef]
- Nagy, B.; Drew, C.M.; Hamilton, P.B.; Modzeles, V.E.; Murphy, M.E.; Scott, W.M.; Urey, H.C.; Young, M. Organic compounds in lunar samples. Pyrolysis products, hydrocarbons, amino acids. Science 1970, 167, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Modzeleski, J.E.; Modzeleski, V.E.; Mohammad, M.A.J.; Nagy, L.A.; Scott, W.M.; Drew, C.M.; Thomas, J.E.; Ward, R.; Hamilton, P.B.; et al. Carbon compounds in Apollo 12 lunar samples. Nature 1971, 232, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.E.; Harada, K.; Fox, S.W. Analyses for amino acids in lunar fines. Proc. Apollo 11 Lunar Sci. Conf. Geochim. Et Cosmochim. Acta 1970, 2 (Suppl. 4), 1799–1803. [Google Scholar]
- Lauretta, D.S.; Balram-Knutson, S.S.; Beshore, E.; Boynton, W.V.; d’Aubigny, C.D.; DellaGiustina, D.N.; Enos, H.L.; Gholish, D.R.; Hergenrother, C.W.; Howell, E.S.; et al. OSIRIS-REx: Sample Return from Asteroid (101955) Bennu. Space Sci. Rev. 2017, 212, 925–984. [Google Scholar] [CrossRef]
- Naraoka, H.; Aoki, D.; Fukushima, K.; Uesugi, M.; Ito, M.; Kitajima, F.; Mita, H.; Yabuta, H.; Takano, Y.; Yada, T.; et al. ToF-SIMS analysis of carbonaceous particles in the sample catcher of the Hayabusa spacecraft. Earth Planets Space 2015, 67, 67. [Google Scholar] [CrossRef]
- Naraoka, H.; Mita, H.; Hamase, K.; Mita, M.; Yabuta, H.; Saito, K.; Fukushima, K.; Kitajima, F.; Sandford, S.A.; Nakamura, T.; et al. Preliminary organic compound analysis of microparticles returned from Asteroid 25143 Itokawa by the Hayabusa mission. Geochem. J. 2012, 46, 61–72. [Google Scholar] [CrossRef]
- Sandford, S.A.; Aleon, J.; Alexander, C.M.O.D.; Araki, T.; Bajt, S.; Baratta, G.A.; Borg, J.; Bradley, J.P.; Brownlee, D.E.; Brucato, J.R.; et al. Organics Captured from Comet 81P/Wild 2 by the Stardust Spacecraft. Science 2006, 314, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Dworkin, J.P.; Sandford, S.A. Detection of cometary amines in samples returned by Stardust. Meteorit. Planet. Sci. 2008, 43, 399–413. [Google Scholar] [CrossRef]
- Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Cometary glycine detected in samples returned by Stardust. Meteorit. Planet. Sci. 2009, 44, 1323–1330. [Google Scholar] [CrossRef]
- Creamer, J.S.; Mora, M.F.; Willis, P.A. Enhanced resolution of chiral amino acids with capillary electrophoresis for biosignature detection in extraterrestrial samples. Anal. Chem. 2016, 89, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Burk, M.J.; Martinez, J.E.; Feaster, J.E.; Cosford, N. Catalytic asymmetric reductive amination of ketones via highly enantioselective hydrogenation of the C-N double bond. Tetrahedron 1994, 50, 4399–4428. [Google Scholar] [CrossRef]
- Corey, E.J.; Gilman, N.W.; Ganem, B.E. New methods for the oxidation of aldehydes to carboxylic acids and esters. J. Am. Chem. Soc. 1968, 90, 5616–5617. [Google Scholar] [CrossRef]
- Peltzer, E.T.; Bada, J.L.; Schlesinger, G.; Miller, S.L. The chemical conditions on the parent body of the Murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Adv. Space Res. 1984, 4, 69–74. [Google Scholar] [CrossRef]
- Huber, C.; Wächtershäuser, G. Primordial reductive amination revisited. Tetrahedron Lett. 2003, 44, 1695–1697. [Google Scholar] [CrossRef]
- Cooper, G.W.; Cronin, J.R. Linear and cyclic aliphatic carboxamides of the Murchison meteorite: Hydrolyzable derivatives of amino acids and other carboxylic acids. Geochim. Cosmochim. Acta 1995, 59, 1003–1015. [Google Scholar] [CrossRef]
- Bada, J.L.; Glavin, D.P.; McDonald, G.D.; Becker, L. A search for endogenous amino acids in martian meteorite ALH84001. Science 1998, 279, 362–365. [Google Scholar] [CrossRef]
- Glavin, D.P.; Bada, J.L.; Brinton, K.L.F.; McDonald, G.D. Amino acids in the Martian meteorite Nakhla. Proc. Natl. Acad. Sci. USA 1999, 96, 8835–8838. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Dworkin, J.P.; Aubrey, A.; Botta, O.; Doty, J.H., III; Martins, Z.; Bada, J.L. Amino acid analyses of Antarctic CM2 meteorites using liquid chromatography-time of flight-mass spectrometry. Meteorit. Planet. Sci. 2006, 41, 889–902. [Google Scholar] [CrossRef]
- Burton, A.S.; Glavin, D.P.; Callahan, M.P.; Dworkin, J.P.; Jenniskens, P.; Shaddad, M.H. Heterogeneous distributions of amino acids provide evidence of multiple sources within the Almahata Sitta parent body, asteroid 2008 TC3. Meteorit. Planet. Sci. 2011, 46, 1703–1712. [Google Scholar] [CrossRef]
- Burton, A.S.; Glavin, D.P.; Elsila, J.E.; Dworkin, J.P.; Jenniskens, P.; Yin, Q.Z. The amino acid composition of the Sutter’s Mill CM2 carbonaceous chondrite. Meteorit. Planet. Sci. 2014, 49, 2074–2086. [Google Scholar] [CrossRef]
- Mcdonald, G.D.; Bada, J.L. A Search for Endogenous Amino-Acids in the Martian Meteorite Eeta79001. Geochim. Cosmochim. Acta 1995, 59, 1179–1184. [Google Scholar] [CrossRef]
- Herd, C.D.K.; Hilts, R.W.; Skelhorne, A.W.; Simkus, D.N. Cold curation of pristine astromaterials: Insights from the Tagish Lake meteorite. Meteorit. Planet. Sci. 2016, 51, 499–519. [Google Scholar] [CrossRef]
- Calaway, M.J.; Allen, C.C.; Allton, J.H. Organic Contamination Baseline Study in NASA Johnson Space Center Astromaterials Curation Laboratories; NASA Center for AeroSpace Information: Hanover, MD, USA, 2014.
- Dworkin, J.P.; Adelman, L.A.; Ajluni, T.; Andronikov, A.V.; Aponte, J.C.; Bartels, A.E.; Beshore, E.; Bierhaus, E.B.; Brucato, J.R.; Bryan, B.H.; et al. OSIRIS-REx Contamination Control Strategy and Implementation. Space Sci. Rev. 2017, 214, 19. [Google Scholar] [CrossRef] [PubMed]
- Elsila, J.E.; Callahan, M.P.; Glavin, D.P.; Dworkin, J.P.; Brueckner, H. Distribution and stable isotopic composition of amino acids from fungal peptaibiotics: Assessing the potential for meteoritic contamination. Astrobiology 2011, 11, 123–133. [Google Scholar] [CrossRef]
- Penzias, A.A. Nuclear processing and isotopes in the galaxy. Science 1980, 208, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Robert, F.; Epstein, S. The concentration and isotopic composition of hydrogen, carbon, and nitrogen in carbonaceous meteorites. Geochim. Cosmochim. Acta 1982, 46, 81–95. [Google Scholar] [CrossRef]
- Sandford, S.A.; Bernstein, M.P.; Dworkin, J.P. Assessment of the interstellar processes leading to deuterium enrichment in meteoritic organics. Meteorit. Planet. Sci. 2001, 36, 1117–1133. [Google Scholar] [CrossRef]
- Yuen, G.U.; Kvenvolden, K.A. Monocarboxylic acids in Murray and Murchison carbonaceous meteorites. Nature 1973, 246, 301–302. [Google Scholar] [CrossRef]
- Lawless, J.G.; Yuen, G.U. Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 1979, 282, 396–398. [Google Scholar] [CrossRef]
- Shimoyama, A.; Naraoka, H.; Yamamoto, H.; Harada, K. Carboxylic acids in the Yamato-791198 carbonaceous chondrites from Antarctica. Chem. Lett. 1986, 15, 1561–1564. [Google Scholar] [CrossRef]
- Naraoka, H.; Shimoyama, A.; Harada, K. Molecular distribution of monocarboxylic acids in Asuka carbonaceous chondrites from Antarctica. Orig. Life Evol. Biosph. 1999, 29, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Aponte, J.C.; Tarozo, R.; Alexandre, M.R.; Alexander, C.M.O.D.; Charnley, S.B.; Hallmann, C.; Summons, R.E.; Huang, Y. Chirality of meteoritic free and IOM-derived monocarboxylic acids and implications for prebiotic organic synthesis. Geochim. Cosmochim. Acta 2014, 131, 1–12. [Google Scholar] [CrossRef]
- Aponte, J.C.; Whitaker, D.; Powner, M.W.; Elsila, J.E.; Dworkin, J.P. Analyses of aliphatic aldehydes and ketones in carbonaceous chondrites. Acs Earth Space Chem. 2019, 3, 463–472. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Aliphatic hydrocarbons of the Murchison meteorite. Geochim. Cosmochim. Acta 1990, 54, 2859–2868. [Google Scholar] [CrossRef]
- Krishnamurthy, R.V.; Epstein, S.; Cronin, J.R.; Pizzarello, S.; Yuen, G.U. Isotopic and molecular analyses of hydrocarbons and monocarboxylic acids of the Murchison meteorite. Geochim. Cosmochim. Acta 1992, 56, 4045–4058. [Google Scholar] [CrossRef]
- Callahan, M.P.; Smith, K.E.; Cleaves, H.J.; Ruzicka, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc. Natl. Acad. Sci. USA 2011, 108, 13995–13998. [Google Scholar] [CrossRef]
- Hilts, R.W.; Herd, C.D.K.; Simkus, D.N.; Slater, G.F. Soluble organic compounds in the Tagish Lake meteorite. Meteorit. Planet. Sci. 2014, 49, 526–549. [Google Scholar] [CrossRef]
- Martins, Z.; Botta, O.; Fogel, M.L.; Sephton, M.A.; Glavin, D.P.; Watson, J.S.; Dworkin, J.P.; Schwartz, A.W.; Ehrenfreund, P. Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet. Sci. Lett. 2008, 270, 130–136. [Google Scholar] [CrossRef]
- Martins, Z.; Modica, P.; Zanda, B.; d’Hendecourt, L.L.S. The amino acid and hydrocarbon contents of the Paris meteorite: Insights into the most primitive CM chondrite. Meteorit. Planet. Sci. 2015, 50, 926–943. [Google Scholar] [CrossRef]
- Huang, Y.; Aponte, J.C.; Zhao, J.; Tarozo, R.; Hallmann, C. Hydrogen and carbon isotopic ratios of polycyclic aromatic compounds in two CM2 carbonaceous chondrites and implications for prebiotic organic synthesis. Earth Planet. Sci. Lett. 2015, 426, 101–108. [Google Scholar] [CrossRef]
- Pizzarello, S.; Schrader, D.L.; Monroe, A.A.; Lauretta, D.S. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 11949–11954. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Wang, Y.; Alexandre, M.R.; Lee, T.; Rose-Petruck, C.; Fuller, M.; Pizzarello, S. Molecular and compound-specific isotopic characterization of monocarboxylic acids in carbonaceous meteorites. Geochim. Cosmochim. Acta 2005, 69, 1073–1084. [Google Scholar] [CrossRef]
- Pizzarello, S.; Huang, Y.; Alexandre, M.R. Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proc. Natl. Acad. Sci. USA 2008, 105, 3700–3704. [Google Scholar] [CrossRef] [PubMed]
- Herd, C.D.K.; Blinova, A.; Simkus, D.N.; Huang, Y.S.; Tarozo, R.; Alexander, C.M.O.; Gyngard, F.; Nittler, L.R.; Cody, G.D.; Fogel, M.L.; et al. Origin and evolution of prebiotic organic matter as inferred from the Tagish Lake meteorite. Science 2011, 332, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Aponte, J.C.; Alexandre, M.R.; Wang, Y.; Brearley, A.J.; Alexander, C.M.O.D.; Huang, Y. Effects of secondary alteration on the composition of free and IOM-derived monocarboxylic acids in carbonaceous chondrites. Geochim. Cosmochim. Acta 2011, 75, 2309–2323. [Google Scholar] [CrossRef]
- Aponte, J.C.; Woodward, H.K.; Abreu, N.M.; Elsila, J.E.; Dworkin, J.P. Molecular distribution, 13C-isotope, and enantiomeric compositions of carbonaceous chondrite monocarboxylic acids. Meteorit. Planet. Sci. 2019, 54, 415–430. [Google Scholar] [CrossRef]
- Dillon, J.T.; Tarozo, R.; Yin, Q.; Huang, Y. Analysis of carboxylic acid compounds in the Sutter’s Mill meteorite. In Proceedings of the 44th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 18–22 March 2013. [Google Scholar]
- Cronin, J.R. Acid-labile amino acid precursors in the Murchison meteorite I: Chromatographic fractionation. Orig. Life 1976, 7, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Aponte, J.C.; Dworkin, J.P.; Elsila, J.E. Assessing the origins of aliphatic amines in the Murchison meteorite from their compound-specific carbon isotopic ratios and enantiomeric composition. Geochim. Cosmochim. Acta 2014, 141, 331–345. [Google Scholar] [CrossRef]
- Glavin, D.P.; Elsila, J.E.; Burton, A.S.; Callahan, M.P.; Dworkin, J.P.; Hilts, R.W.; Herd, C.D.K. Unusual nonterrestrial L-proteinogenic amino acid excesses in the Tagish Lake meteorite. Meteorit. Planet. Sci. 2012, 47, 1347–1364. [Google Scholar] [CrossRef]
- Burton, A.S.; Elsila, J.E.; Hein, J.E.; Glavin, D.P.; Dworkin, J.P. Extraterrestrial amino acids identified in metal-rich CH and CB carbonaceous chondrites from Antarctica. Meteorit. Planet. Sci. 2013, 48, 390–402. [Google Scholar] [CrossRef]
- Cronin, J.R.; Moore, C.B. Amino acid analyses of the Murchison, Murray, and Allende carbonaceous chondrites. Science 1971, 172, 1327–1329. [Google Scholar] [CrossRef] [PubMed]
- Pollock, G.E.; Cheng, C.N.; Cronin, S.E.; Kvenvolden, K.A. Stereoisomers of isovaline in the Murchison meteorite. Geochim. Cosmochim. Acta 1975, 39, 1571–1573. [Google Scholar] [CrossRef]
- Peltzer, E.T.; Bada, J.L. Alpha-hydroxycarboxylic acids in the Murchison meteorite. Nature 1978, 272, 443–444. [Google Scholar] [CrossRef]
- Glavin, D.P.; Bada, J. Isolation of amino acids from natural samples using sublimation. Anal. Chem. 1998, 70, 3119–3122. [Google Scholar] [CrossRef]
- Nakaparksin, S.; Gilav, E.; Oro, J. Study of the racemization of some neutral a-amino acids in acid solution using gas chromatographic techniques. Anal. Biochem. 1970, 33, 374–382. [Google Scholar] [CrossRef]
- Jungbauer, A.; Hahn, R. Ion-Exchange Chromatography. In Methods in Enzymology Guide to Protein Purification, 2nd ed.; Burgess, R.R., Deutscher, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 463, pp. 349–371. [Google Scholar]
- Burton, A.S.; Stern, J.C.; Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 2012, 41, 5459–5472. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cronin, J.R. Non-racemic amino acids in the Murray and Murchison meteorites. Geochim. Cosmochim. Acta 2000, 64, 329–338. [Google Scholar] [CrossRef]
- Amelung, W.; Zhang, X. Determination of amino acid enantiomers in soils. Soil Biol. Biochem. 2001, 33, 553–562. [Google Scholar] [CrossRef]
- Meierhenrich, U.J.; Muñoz Caro, G.M.; Bredehöft, J.H.; Jessberger, E.K.; Thiemann, W.H.-P. Identification of diamino acids in the Murchison meteorite. Proc. Natl. Acad. Sci. USA 2004, 101, 9182–9186. [Google Scholar] [CrossRef] [PubMed]
- Myrgorodska, I.; Meinert, C.; Martins, Z.; d’Hendecourt, L.L.; Meierhenrich, U.J. Quantitative enantioseparation of amino acids by comprehensive two-dimensional gas chromatography applied to non-terrestrial samples. J. Chromatogr. A 2016, 1433, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.H.; Nagy, B. Distribution and enantiomeric composition of amino acids in the Murchison meteorite. Nature 1982, 296, 837–840. [Google Scholar] [CrossRef]
- Engel, M.H.; Macko, S.A.; Silfer, J.A. Carbon isotope composition of individual amino acids in the Murchison meteorite. Nature 1990, 348, 47–49. [Google Scholar] [CrossRef]
- Engel, M.H.; Macko, S.A. Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature 1997, 389, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Chiesl, T.N.; Chu, W.K.; Stockton, A.M.; Amashukeli, X.; Grunthaner, F.; Mathies, R.A. Enhanced Amine and Amino Acid Analysis Using Pacific Blue and the Mars Organic Analyzer Microchip Capillary Electrophoresis System. Anal. Chem. 2009, 81, 2537–2544. [Google Scholar] [CrossRef]
- Myrgorodska, I.; Javelle, T.; Meinert, C.; Meierhenrich, U.J. Enantioselective gas chromatography in search of the origin of biomolecular asymmetry in outer space. Isr. J. Chem. 2016, 56, 1016–1026. [Google Scholar] [CrossRef]
- Pizzarello, S. Identifying chiral molecules and their enantiomeric excesses in extraterrestrial samples: An experimental journey. Isr. J. Chem. 2016, 56, 1027–1035. [Google Scholar] [CrossRef]
- Burton, A.S.; Berger, E.L. Insights into abiotically-generated amino acid enantiomeric excesses found in meteorites. Life 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Sephton, M.A.; Gilmour, I. Compound-specific isotope analysis of the organic constituents in carbonaceous chondrites. Mass Spectrom. Rev. 2001, 20, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Eiler, J.M.; Cesar, J.; Chimiak, L.; Dallas, B.; Grice, K.; Griep-Raming, J.; Juchelka, D.; Kitchen, N.; Lloyd, M.; Makarov, A.; et al. Analysis of molecular isotopic structures at high precision and accuracy by Orbitrap mass spectrometry. Int. J. Mass Spectrom. 2017, 422, 126–142. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Amino acids in meteorites. Adv. Space Res. 1983, 3, 5–18. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Amino-Acids of the Murchison Meteorite. III. Seven Carbon Acyclic Primary Alpha-Amino Alkanoic Acids. Geochim. Cosmochim. Acta 1986, 50, 2419–2427. [Google Scholar] [CrossRef]
- Lamkin, W.M.; Gehrke, C.W. Quantitative gas chromatography of amino acids—Preparation of n-butyl N-trifluoroacetyl esters. Anal. Chem. 1965, 37, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Pereira, W.E.; Summons, R.E.; Rindfleisch, T.C.; Duffield, A.M.; Zeitman, B.; Lawless, J.G. Stable isotope mass fragmentography: Identification and hydrogen-deuterium exchange studies of eight Murchison meteorite amino acids. Geochim. Cosmochim. Acta 1975, 39, 163–172. [Google Scholar] [CrossRef]
- Mengerink, Y.; Kutlan, D.; Toth, F.; Csampai, A.; Molnar-Perl, I. Advances in the evaluation of the stability and characteristics of the amino acid and amine derivatives obtained with the o-phthaldialdehyde/3-mercaptopropionic acid and o-phthaldialdehyde/N-acetyl-l-cysteine reagents—High-performance liquid chromatography-mass spectrometry study. J. Chromatogr. A 2002, 949, 99–124. [Google Scholar] [CrossRef]
- Nuevo, M.; Auger, G.; Blanot, D.; d’Hendecourt, L. A detailed study of the amino acids produced from the vacuum UV irradiation of interstellar ice analogs. Orig. Life Evol. Biosph. 2008, 38, 37–56. [Google Scholar] [CrossRef]
- Salazar, C.; Armenta, J.M.; Cortes, D.F.; Shulaev, V. Combination of an AccQ·Tag-Ultra Performance Liquid Chromatographic Method with Tandem Mass Spectrometry for the Analysis of Amino Acids. In Amino Acid Analysis—Methods and Protocols; Alterman, M., Hunziker, P., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 828. [Google Scholar]
- Boogers, I.; Plugge, W.; Stokkermans, Y.Q.; Duchateau, A.L.L. Ultra-performance liquid chromatographic analysis of amino acids in protein hydrolysates using an automated pre-column derivatisation method. J. Chromatogr. A 2008, 1189, 406–409. [Google Scholar] [CrossRef]
- Pizzarello, S.; Feng, X.; Epstein, S.; Cronin, J.R. Isotopic analyses of nitrogenous compounds from the Murchison meteorite: Ammonia, amines, amino acids, and polar hydrocarbons. Geochim. Cosmochim. Acta 1994, 58, 5579–5587. [Google Scholar] [CrossRef]
- Pizzarello, S.; Holmes, W. Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim. Cosmochim. Acta 2009, 73, 2150–2162. [Google Scholar] [CrossRef]
- Monroe, A.A.; Pizzarello, S. The soluble organic compounds of the Bells meteorite: Not a unique or unusual composition. Geochim. Cosmochim. Acta 2011, 75, 7585–7595. [Google Scholar] [CrossRef]
- Pizzarello, S.; Yarnes, C.T. Enantiomeric excesses of chiral amines in ammonia-rich carbonaceous meteorites. Earth Planet. Sci. Lett. 2016, 443, 176–184. [Google Scholar] [CrossRef]
- Pizzarello, S.; Yarnes, C.T. The soluble organic compounds of the Mukundpura meteorite: A new CM chondrite fall. Planet Space Sci. 2018, 164, 127–131. [Google Scholar] [CrossRef]
- Yuen, G.; Blair, N.; Marais, D.J.D.; Chang, S. Carbon isotope composition of low molecular weight hydrocarbons and monocarboxylic acids from Murchison meteorite. Nature 1984, 307, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Simkus, D.N.; Aponte, J.C.; Elsila, J.E.; Hilts, R.W.; McLain, H.L.; Herd, C.D.K. New insights into the heterogeneity of the Tagish Lake meteorite: Soluble organic compositions of variously altered specimens. Meteorit. Planet. Sci. 2019. [Google Scholar] [CrossRef]
- Huang, Y.; Alexandre, M.R.; Wang, Y. Structure and isotopic ratios of aliphatic side chains in the insoluble organic matter of the Murchison carbonaceous chondrite. Earth Planet. Sci. Lett. 2007, 259, 517–525. [Google Scholar] [CrossRef]
- Simkus, D.N.; Aponte, J.C.; Hilts, R.W.; Elsila, J.E.; Herd, C.D.K. Compound-specific carbon isotope compositions of aldehydes and ketones in the Murchison meteorite. Meteorit. Planet. Sci. 2019, 54, 142–156. [Google Scholar] [CrossRef]
- Glavin, D.P.; Callahan, M.P.; Dworkin, J.P.; Elsila, J.E. The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 2011, 45, 1948–1972. [Google Scholar] [CrossRef]
- Seargent, D.A.J. The Murchison meteorite—Circumstances of its fall. Meteoritics 1990, 25, 341–342. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Glavin, D.P.; Bada, J. Extraterrestrial amino acids in the Murchison meteorite: Re-evaluation after thirty years. In Perspectives in Amino Acid and Protein Geochemistry; Goodfriend, G.A., Collins, M.J., Fogel, M.L., Macko, S.A., Wehmiller, J.F., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 7–14. [Google Scholar]
- Friedrich, J.M.; McLain, H.L.; Dworkin, J.P.; Glavin, D.P.; Towbin, W.H.; Hill, M.; Ebel, D.S. Effect of polychromatic X-ray microtomography imaging on the amino acid content of the Murchison CM chondrite. Meteorit. Planet. Sci. 2019, 54, 220–228. [Google Scholar] [CrossRef]
- Callahan, M.P.; Martin, M.G.; Burton, A.S.; Glavin, D.P.; Dworkin, J.P. Amino acid analysis in micrograms of meteorite sample by nanoliquid chromatography-high-resolution mass spectrometry. J. Chromatogr. A 2014, 1332, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.S.; Berger, E.L.; Locke, D.R.; Lewis, E.K. Correlated amino acid and mineralogical analyses of milligram and submilligram samples of carbonaceous chondrite Lonewolf Nunataks 94101. In Proceedings of the 49th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 19–23 March 2018. [Google Scholar]
- Simkus, D.N.; Goreva, Y.S.; McCoy, T.J.; Herd, C.D.K. TOF-SIMS analysis of prebiotic organic compounds in the Murchison meteorite. In Proceedings of the 46th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 16–20 March 2015. [Google Scholar]
- Hashiguchi, M.; Naraoka, H. High-mass resolution molecular imaging of organic compounds on the surface of Murchison meteorite. Meteorit. Planet. Sci. 2019, 54, 452–468. [Google Scholar] [CrossRef]
- Yesiltas, M.; Kebukawa, Y. Associations of organic matter with minerals in Tagish Lake meteorite via high spatial resolution synchrotron-based FTIR microspectroscopy. Meteorit. Planet. Sci. 2016, 51, 584–595. [Google Scholar] [CrossRef]
- Mita, H.; Shimoyama, A. Alpha-aminoisobutyric acid and isovaline in Tokyo Bay sediments. Geochim. Cosmochim. Acta 1998, 62, 47–50. [Google Scholar] [CrossRef]
- Glavin, D.P.; Brinkerhoff, W.; Dworkin, J.P.; Eigenbrode, J.; Franz, H.; Mahaffy, P.; Stern, J.; Allamandola, L.; Blake, D.; Sandford, S.A.; et al. Astrobiology Sample Analysis Program (ASAP) for advanced life detection instrumentation development and calibration. Astrobiology 2008, 8, 297. [Google Scholar]
- Peterson, E.; Horz, F.; Chang, S. Modification of amino acids at shock pressures of 3.5 to 32 GPa. Geochim. Cosmochim. Acta 1997, 61, 3937–3950. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Lawless, J.G.; Ponnamperuma, C. Nonprotein amino acids in Murchison meteorite. Proc. Natl. Acad. Sci. USA 1971, 68, 486–490. [Google Scholar] [CrossRef]
- Oró, J.; Gibert, J.; Lichtenstein, H.; Wikstrom, S.; Flory, D.A. Amino-acids, aliphatic and aromatic hydrocarbons in the Murchison meteorite. Nature 1971, 230, 105–106. [Google Scholar] [CrossRef]
- Oro, J.; Nakaparksin, S.; Lichtenstein, H.; Gilav, E. Configuration of amino-acids in carbonaceous chondrites and a Pre-Cambrian chert. Nature 1971, 230, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Lawless, J.G.; Kvenvolden, K.A.; Peterson, E.; Ponnamperuma, C.; Moore, C. Amino acids indigenous to Murray meteorite. Science 1971, 173, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Lawless, J.G.; Kvenvolden, K.A.; Peterson, E.; Ponnamperuma, C.; Jarosewich, E. Physical sciences: Evidence for amino-acids of extraterrestrial origin in the Orgueil meteorite. Nature 1972, 236, 66–67. [Google Scholar] [CrossRef]
- Lawless, J.G.; Peterson, E. Amino-acids in carbonaceous chondrites. Orig. Life Evol. Biosph. 1975, 6, 3–8. [Google Scholar] [CrossRef]
- Kotra, R.K.; Shimoyama, A.; Ponnamperuma, C.; Hare, P.E. Amino acids in a carbonaceous chondrite from Antarctica. J. Mol. Evol. 1979, 13, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, A.; Ponnamperuma, C.; Yanai, K. Amino acids in the Yamato-74662 meteorite, an Antarctic carbonaceous chondrite. Proc. Fourth Symp. Antarct. Meteor. 1979, 15, 196–205. [Google Scholar]
- Shimoyama, A.; Harada, K.; Yanai, K. Amino acids from the Yamato-791198 carbonaceous chondrite from Antarctica. Chem. Lett. 1985, 14, 1183–1186. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S.; Yuen, G.U. Amino-Acids of the Murchison Meteorite: II. Five Carbon Acyclic Primary Beta-Amino, Gamma-Amino and Delta-Amino Alkanoic Acids. Geochim. Cosmochim. Acta 1985, 49, 2259–2265. [Google Scholar] [CrossRef]
- Shimoyama, A.; Harada, K. Amino acid depleted carbonaceous chondrites (C2) from Antarctica. Geochem. J. 1984, 18, 281–286. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–955. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cooper, G.W. Molecular and chiral analyses of some protein amino acid derivatives in the Murchison and Murray meteorites. Meteorit. Planet. Sci. 2001, 36, 897–909. [Google Scholar] [CrossRef]
- Shimoyama, A.; Ogasawara, R. Dipeptides and diketopiperazines in the Yamato-791198 and Murchison carbonaceous chondrites. Orig. Life Evol. Biosph. 2002, 32, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Zolensky, M.; Turk, K.A. Nonracemic isovaline in the Murchison meteorite: Chiral distribution and mineral association. Geochim. Cosmochim. Acta 2003, 67, 1589–1595. [Google Scholar] [CrossRef]
- Pizzarello, S.; Huang, Y.S. The deuterium enrichment of individual amino acids in carbonaceous meteorites: A case for the presolar distribution of biomolecule precursors. Geochim. Cosmochim. Acta 2005, 69, 5163. [Google Scholar] [CrossRef]
- Chan, H.S.; Martins, Z.; Sephton, M.A. Amino acid analyses of type 3 chondrites Colony, Ornans, Chainpur, and Bishunpur. Meteorit. Planet. Sci. 2012, 47, 1502–1516. [Google Scholar] [CrossRef]
- Koga, T.; Naraoka, H. A new family of extraterrestrial amino acids in the Murchison meteorite. Sci. Rep. 2017, 7, 636. [Google Scholar] [CrossRef]
- Zenobi, R.; Philippoz, J.-M.; Zare, R.N.; Wing, M.R.; Bada, J.L.; Marti, K. Organic compounds in the Forest Vale, H4 ordinary chondrite. Geochim. Cosmochim. Acta 1992, 56, 2899–2905. [Google Scholar] [CrossRef]
- Bruckner, H.; Haasmann, S.; Langer, M.; Westhauser, T.; Wittner, R.; Godel, H. Liquid chromatographic determination of d- and l-amino acids by derivatization with o-phthaldialdehyde and chiral thiols. J. Chromatogr. A 1994, 666, 259–273. [Google Scholar] [CrossRef]
- Glavin, D.P.; Aubrey, A.D.; Callahan, M.P.; Dworkin, J.P.; Elsila, J.E.; Parker, E.T.; Bada, J.L.; Jenniskens, P.; Shaddad, M.H. Extraterrestrial amino acids in the Almahata Sitta meteorite. Meteorit. Planet. Sci. 2010, 45, 1695–1709. [Google Scholar] [CrossRef]
- Hutt, L.D.; Glavin, D.P.; Bada, J.L.; Mathies, R.A. Microfabricated capillary electrophoresis amino acid chirality analyzer for extraterrestrial exploration. Anal. Chem. 1999, 71, 4000–4006. [Google Scholar] [CrossRef]
- Glavin, D.P.; Bada, J.L. Survival of amino acids in micrometeorites during atmospheric entry. Astrobiology 2001, 1, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Botta, O.; Glavin, D.P.; Kminek, G.; Bada, J.L. Relative amino acid concentrations as a signature for parent body processes of carbonaceous chondrites. Orig. Life Evol. Biosph. 2002, 32, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Botta, O.; Martins, Z.; Ehrenfreund, P. Amino acids in Antarctic CM1 meteorites and their relationship to other carbonaceous chondrites. Meteorit. Planet. Sci. 2007, 42, 81–92. [Google Scholar] [CrossRef]
- Botta, O.; Martins, Z.; Emmenegger, C.; Dworkin, J.P.; Glavin, D.P.; Harvey, R.P.; Zenobi, R.; Bada, J.; Ehrenfreund, P. Polycyclic aromatic hydrocarbons and amino acids in meteorites and ice samples from LaPaz icefield, Antarctica. Meteorit. Planet. Sci. 2008, 43, 1465–1480. [Google Scholar] [CrossRef]
- Kminek, G.; Botta, O.; Glavin, D.P.; Bada, J.L. Amino acids in the Tagish Lake meteorite. Meteorit. Planet. Sci. 2002, 37, 697–701. [Google Scholar] [CrossRef]
- Martins, Z.; Hofmann, B.A.; Greenwood, R.C.; Verchovsky, A.B.; Franchi, I.A.; Jull, A.J.T.; Botta, O.; Glavin, D.P.; Dworkin, J.P.; Ehrenfreund, P. Amino acid composition, petrology, geochemistry, 14C terrestrial age, and oxygen isotopes of the Shisr 033 CR chondrite. Meteorit. Planet. Sci. 2007, 42, 1581–1595. [Google Scholar] [CrossRef]
- Martins, Z.; Alexander, C.M.O.D.; Orzechowska, G.E.; Fogel, M.L.; Ehrenfreund, P. Indigenous amino acids in primitive CR meteorites. Meteorit. Planet. Sci. 2007, 42, 2125–2136. [Google Scholar] [CrossRef]
- Glavin, D.P.; Dworkin, J.P. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. USA 2009, 106, 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.S.; Elsila, J.E.; Callahan, M.P.; Martin, M.G.; Glavin, D.P.; Johnson, N.M.; Dworkin, J.P. A propensity for n-ω-amino acids in thermally altered Antarctic meteorites. Meteorit. Planet. Sci. 2012, 47, 374–386. [Google Scholar] [CrossRef]
- Burton, A.S.; Grunsfeld, S.; Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. The effects of parent-body hydrothermal heating on amino acid abundances in CI-like chondrites. Polar Sci. 2014, 8, 255–263. [Google Scholar] [CrossRef]
- Burton, A.S.; McLain, H.; Glavin, D.P.; Elsila, J.E.; Davidson, J.; Miller, K.E.; Andronikov, A.V.; Lauretta, D.; Dworkin, J.P. Amino acid analyses of R and CK chondrites. Meteorit. Planet. Sci. 2015, 50, 470–482. [Google Scholar] [CrossRef]
- Callahan, M.P.; Burton, A.S.; Elsila, J.E.; Baker, E.M.; Smith, K.E.; Glavin, D.P.; Dworkin, J.P. A search for amino acids and nucleobases in the Martian meteorite Roberts Massif 04262 using liquid chromatography-mass spectrometry. Meteorit. Planet. Sci. 2013, 48, 786–795. [Google Scholar] [CrossRef]
- Chan, Q.H.S.; Zolensky, M.E.; Kebukawa, Y.; Fries, M.; Ito, M.; Steele, A.; Rahman, Z.; Nakato, A.; Kilcoyne, A.L.D.; Suga, H.; et al. Organic matter in extraterrestrial water-bearing salt crystals. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.H.S.; Nakato, A.; Kebukawa, Y.; Zolensky, M.E.; Nakamura, T.; Maisano, J.A.; Colbert, M.W.; Martinez, J.E.; Kilcoyne, A.L.D.; Suga, H.; et al. Heating experiments of the Tagish Lake meteorite: Investigation of the effects of short-term heating on chondritic organics. Meteorit. Planet. Sci. 2019, 54, 104–125. [Google Scholar] [CrossRef]
- Cronin, J.R.; Moore, C.B. Amino acids of the Nogoya and Mokoia carbonaceous chondrites. Geochim. Cosmochim. Acta 1976, 40, 853–857. [Google Scholar] [CrossRef]
- Cronin, J.R. Acid-labile amino acid precursors in the Murchison meteorite II: A search for peptides and amino acyl amides. Orig. Life 1976, 7, 343–348. [Google Scholar] [CrossRef]
- Epstein, S.; Krishnamurthy, R.V.; Cronin, J.R.; Pizzarello, S.; Yuen, G.U. Unusual stable isotope ratios in amino acids and carboxylic acid extracts from the Murchison meteorite. Nature 1987, 326, 477–479. [Google Scholar] [CrossRef]
- Pizzarello, S.; Krishnamurthy, R.V.; Epstein, S.; Cronin, J.R. Isotopic analyses of amino acids from the Murchison meteorite. Geochim. Cosmochim. Acta 1991, 55, 905–910. [Google Scholar] [CrossRef]
- Lerner, N.R.; Cooper, G.W. Iminodicarboxylic acids in the Murchison meteorite: Evidence of Strecker reactions. Geochim. Cosmochim. Acta 2005, 69, 2901–2906. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S.; Moore, C.B. Amino-acids in an Antarctic carbonaceous chondrite. Science 1979, 206, 335–337. [Google Scholar] [CrossRef]
- Aponte, J.C.; Dworkin, J.P.; Elsila, J.E. Indigenous aliphatic amines in the aqueously altered Orgueil meteorite. Meteorit. Planet. Sci. 2015, 50, 1733–1749. [Google Scholar] [CrossRef]
- Aponte, J.C.; McLain, H.L.; Dworkin, J.P.; Elsila, J.E. Aliphatic amines in Antarctic CR2, CM2, and CM1/2 carbonaceous chondrites. Geochim. Cosmochim. Acta 2016, 189, 296–311. [Google Scholar] [CrossRef]
- Aponte, J.C.; Abreu, N.M.; Glavin, D.P.; Dworkin, J.P.; Elsila, J.E. Distribution of aliphatic amines in CO, CV, and CK carbonaceous chondrites and relation to mineralogy and processing history. Meteorit. Planet. Sci. 2017, 52, 2632–2646. [Google Scholar] [CrossRef]
- Aponte, J.C.; Abreu, N.M.; Keller, L.P.; Elsila, J.E.; Dworkin, J.P. Soluble amines in anomalous CR chondrite Miller Range (MIL) 090001. In Proceedings of the 49th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 19–23 March 2018. [Google Scholar]
- Shimoyama, A.; Naraoka, H.; Komiya, M.; Harada, K. Analyses of carboxylic acids and hydrocarbons in Antarctic carbonaceous chondrites, Yamato-74662 and Yamato-793321. Geochem. J. 1989, 23, 181–193. [Google Scholar] [CrossRef]
- Briscoe, J.F.; Moore, C.B. Determination of formic acid and acetic acid in chondritic meteorites. Meteoritics 1993, 28, 330. [Google Scholar]

| Analyzed as | Extraction and Analytical Methodology | Author (Year Published) | Meteorites Analyzed |
|---|---|---|---|
| TFA alkyl (methyl, n-butyl, sec-butyl, (S)-sec-butyl, sec-pentyl, or isopropyl) ester derivatives | Meteorite powder heated or refluxed in water for 8–24 h at 110–110 °C. Aqueous portion removed, dried, optionally acid-hydrolyzed, desalted using cation exchange resin, and derivatized for GC, GC-MS and/or GC-IRMS analysis. | Kvenvolden et al. (1970, 1971) [13,134], Oró et al. (1971) [135,136], Lawless et al. (1971, 1972) [137,138], Lawless and Peterson (1975) [139], Pereira et al. (1975) [108], Kotra et al. (1979) [140], Shimoyama et al. (1979, 1985) [141,142], Cronin et al. (1981, 1985) [16,143], Peltzer et al. (1984) [45], Shimoyama and Harada (1984) [144], Cronin and Pizzarello (1986, 1997) [106,145], a Engel et al. (1990) [97]; a Engel and Macko (1997) [98]; Pizzarello and Cronin (2000) [92], Pizzarello and Cooper (2001) [146], Shimoyama and Ogasawara (2002) [147], Pizzarello et al. (2003, 2004, 2008, 2012) [18,74,76,148], Pizzarello and Huang (2005) [149], Pizzarello and Holmes (2009) [114], Monroe and Pizzarello (2011) [115], Chan et al. (2012) [150], Martins et al. (2015) [72], Koga and Naraoka (2017) [151], Pizzarello and Yarnes (2018) [117] | CI1: Orgueil [136,138]; CM2: ALH 77306 [140], Mukundpura [117], Murchison [13,16,18,45,92,97,98,106,108,134,135,139,143,145,146,147,148,149,151], Murray [92,136,137,146,148,149], Paris [72], Yamato 74662 [141], 791198 [142,147], 793321 [144]; CR1: GRO 95577 [74]; CR2: GRA 95229 [76,114], LAP 02342 [114], MIL 07525 [74], GRO 03116 [74], EET 92042 [74], GRA 06100 [74], PCA 91082 [74]; CR3: QUE 99177 [74], MET 00426 [74]; C2ung: Bells [115], Belgica 7904 [144]; CO3: Colony [150], Ornans [150]; CV3: Mokoia [136]; LL3: Chainpur [150], Bishunpur [150] |
| OPA/NAC, OPA/IBLC and OPA/IBDC-derivatives | Meteorite powder extracted in water for 6–24 h at 100–110 °C. Aqueous portion removed, dried, optionally acid-hydrolyzed, desalted using cation exchange resin, and derivatized for HPLC-FD and/or TOF-MS analysis. | Zenobi et al. (1992) [152], Brückner et al. (1994) [153], McDonald and Bada (1995) [53], Bada et al. (1998) [48], Glavin et al. (1999, 2006, 2010, 2011, 2012) [49,50,83,122,154], b Hutt et al. (1999) [155], Ehrenfreund et al. (2001) [17], Glavin and Bada (2001) [156], Botta et al. (2002, 2007, 2008) [157,158,159], Kminek et al. (2002) [160], Martins et al. (2007) [161,162], Glavin and Dworkin (2009) [163], Elsila et al. (2011) [57], Herd et al. (2011) [77], Burton et al. (2011, 2012, 2013, 2014, 2015) [51,52,84,164,165,166], Callahan et al. (2013, 2014) [126,167], Aponte et al. (2014) [82], Chan et al. (2018, 2019) [168,169], Friedrich et al. (2019) [125]; Simkus et al. (2019) [119] | CI: Ivuna [17,157,165], Orgueil [17,122,157,158,161,163], Y-86029 [165], Y-980115 [165]; CM1: ALH 88045 [158], MET 01070 [122,158], LAP 02277 [158], SCO 06043 [122]; CM1/2: ALH 83100 [50]; CM2: Murchison [17,50,52,57,82,122,125,126,153,155,156,157,158,161,163], Murray [17], Nogoya [157], Mighei [157], LEW 90500 [50,122,163], LON 94102 [122,163], Sutter’s Mill [52]; CR: Shişr 033 [161]; CR1: GRO 95577 [122,162]; CR2: Renazzo [157], EET 92042 [122,162,163], GRA 95229 [162]; CR3: QUE 99177 [122,163]; C2ung: Essebi [157], Tagish Lake [157,160,169], Tagish Lake 5b, 11h, 11i [77,83]; Tagish Lake 1, 4, 10a [119]; CO3: ALH 77307 [164], MIL 05013 [164], DOM 08006 [164]; CH3: ALH 85085 [84], PCA 91467 [84], PAT 91546 [84]; CBb: MAC 02675 [84]; CB: MIL 05082 [84], MIL 07411 [84]; CV3: Allende [157], ALH 84028 [164], EET 96026 [164], LAP 02206 [164], LAR 06317 [164], GRA 06101 [164]; CK3: NWA 5956 [166]; CK4: LAR 04318 [166], ALH 85002 [166]; CK4/5: PCA 82500 [166]; CK5: LAP 03784 [159], EET 92002 [166]; CK6: LEW 87009 [166], LAR 06872 [166]; R3: LAP 03834 [166]; R4: LAP 031135 [166]; LL5: LAP 03624 [159], LAP 03573 [159], LAP 03637 [159]; L6: Shişr 031 [161], Shişr 035 [161]; H3-6: Zag halite crystals [168]; Ureilite: Almahata Sitta [51,154], ALH 77257 [164], EET 83309 [164], LEW 85328 [164], GRA 95205 [164], LAR 04315 [164]; H4: LAP 03553 [159], Forest Vale [152]; Lunar: MAC 88105 [48]; Martian: EET 79001 [53], ALH 84001 [48], Nakhla [49], RBT 04262 [167] |
| OPA/NAC derivatives | Meteorite powder extracted overnight at room temperature in 1 M or 6 M HCl. Supernatant separated, dried, optionally acid-hydrolyzed, desalted using cation exchanged resin, then derivatized for HPLC analysis. | Bada et al. (1998) [48], Glavin et al. (1999) [49] | Lunar: MAC 88105 [48]; Martian: ALH 84001 [48], Nakhla [49] |
| Ninhydrin derivatives | Meteorite powder refluxed in water for 20–24 h. Aqueous portion separated, dried, re-dissolved in water and split in half for acid-hydrolyzed and unhydrolyzed analyses using LC. | Cronin and Moore (1971,1976) [85,170], Cronin (1976) [81,171] | CM2: Murchison [81,85,171], Murray [85], Nogoya [170]; CV3: Allende [85], Mokoia [170] |
| Bulk underivatized amino acids | Meteorite powder refluxed in water at 100–110 °C for 24 h. Aqueous portion removed, dried, acid hydrolyzed, and then desalted using cation exchange resin for IRMS. | Epstein et al. (1987) [172], Pizzarello et al. (1991, 1994) [113,173] | CM2: Murchison |
| PFP alkyl ((S)-sec-butyl) ester derivatives | Meteorite powder refluxed in water at 100 °C for 8 h. Aqueous portion separated by filtration, dried in a rotary evaporator, hydrolyzed, dried and desalted using cation exchange resin for GC-MS or GC-IRMS analyses. | Engel and Nagy (1982) [96] | CM2: Murchison |
| PFP ester derivatives | Meteorite powder refluxed in water at 100 °C for 6 h. Aqueous portion separated, dried, acid hydrolyzed, and derivatized for GC-MS analysis. | Hilts et al. (2014) [70] | C2ung: Tagish Lake 5b, 11h, 11i |
| MTBSTFA derivatives | Meteorite powder stirred in water for 46 h with intermittent sonication. Aqueous portion concentrated by rotary evaporation, treated with ethanol for removal of salts, then acidified and desalted using cation exchange resin for GC-MS analysis. | Cooper and Cronin (1995) [47], Lerner and Cooper (2005) [174] | CM2: Murchison |
| ECEE or ECHFBE derivatives | Meteorite powder extracted in water at 100 °C for 20 h. Aqueous portion was separated by centrifugation, split for acid-hydrolyzed and unhydrolyzed analyses, dried for derivatization and multidimensional (GC x GC) analysis. | Meierhenrich et al. (2004) [94], Myrgorodska et al. (2016) [95] | CM2: Murchison |
| PBSE derivatives | Meteorite powder extracted via subcritical water extraction at 200 °C and 3000 MPa with 5 min equilibration time. Portion of the extract was diluted, derivatized and analyzed using micellar electrokinetic chromatography. | Chiesl et al. (2009) [99] | CM2: Murchison |
| OPA derivatives | Meteorite powder in water at 110 °C or at room temperature for 24 h. Aqueous portion separated by filtration, dried in a rotary evaporator, acid hydrolyzed, dried, and re-dissolved in aqueous sodium citrate for liquid chromatography. | Cronin et al. (1979) [175], Matrajt et al. (2004) [24] | CM2: ALH 77306 [175]; Murchison [24]; CO3: ALH 77307 [175]; South Pole Water Well micrometeorites [24] |
| Analyzed as | Extraction and Analytical Methodology | Author (Year Published) | Meteorites Analyzed |
|---|---|---|---|
| Underivatized amines, DNP derivatives, and OPA derivatives | Meteorite powders refluxed for 24 h. Aqueous portion removed after centrifugation, then dried and acid-hydrolyzed. Separate set of meteorite powders extracted in water at 120 °C for four days with no subsequent hydrolysis step. Analyzed by GC-MS and ion exchange chromatography | Jungclaus et al. (1976) [15] | CM2: Murchison |
| PFP and OPA derivatives | Meteorite powders extracted in triple-distilled water at 110 °C for 24 h. Aqueous portion removed after acidification and cryogenic transfer. Residue taken to pH > 12.5 using NaOH, frozen and acidified for cryogenic transfer of volatile amines for GC-MS and ion exchange chromatography | Pizzarello et al. (1994) [113] | CM2: Murchison |
| TFA derivatives (amides) | Meteorite powder put in degassed glass vial with triple-distilled water at 100 °C and intermittent sonication for 20 h. Aqueous extract was made acidic and concentrated, then it was made basic and cryogenically transferred, acidified, dried in rotary evaporator, and derivatized for GC-MS analysis | Pizzarello et al. (2008) [76] | CR2: GRA 95229 |
| TFA derivatives (amides) | Meteorite powder put in degassed glass vial with triple-distilled water at 100 °C and intermittent sonication for 20 h. Aqueous portion separated by centrifugation, dried completely, acid hydrolyzed, re-dried and re-dissolved in water, made basic, cryogenically transferred, acidified, dried in rotary evaporator, and derivatized for GC-MS analysis | Pizzarello and Holmes (2009) [114] | CR2: LAP 02342 |
| TFA derivatives (amides) | Meteorite powders extracted in water at 25 °C for 24 h, followed by a 100 °C 24 h extraction. Aqueous portions separated after each extraction period were made basic, cryogenically transferred, acidified, dried in rotary evaporator, and derivatized for GC-MS analysis | Monroe and Pizzarello (2011) [115] | C2ung: Bells; CI1: Ivuna |
| TFA derivatives (amides) | Meteorite powder put in degassed glass vial with triple-distilled water at 100 °C for 20 h. Aqueous portion separated by decantation, concentrated, basified, cryogenically transferred, acidified, dried in rotary evaporator, and derivatized for GC-MS analysis | Pizzarello et al. (2012) [74], Pizzarello and Yarnes (2016, 2018) [116,117] | CM2: Mukundpura [117]; CR1: GRO 95577 [74]; CR2: GRA 95229 [116]; LAP 02342 [116]; MIL 07525 [74,116], PCA 91082 [74,116], EET 92042 [74,116]; CR3: QUE 99177 [74,116], MET 00426 [74,116] |
| TPC derivatives (amides) | Meteorite powders extracted in water at 100 °C for 24 h. Aqueous extracts separated after centrifugation were acidified and taken to dryness. Dry residues were re-dissolved in water and treated with NaOH, then centrifuged and the aqueous fractions separated from precipitate were re-acidified and dried completely. The residues were dissolved in diluted aqueous NaOH, extracted using DCM and derivatized for GC-MS and GC-IRMS analyses. | Aponte et al. (2014, 2015, 2016, 2017) [82,176,177,178,179] | CI1: Orgueil [176]; CM1/2: ALH 83100 [177]; CM2: Murchison [82], LEW 90500 [177], LON 94101 [177]; CR2: MIL 090001 [179], LAP 02342 [177], GRA 95229 [177]; CO3: DOM 08006 [178], MIL 05013 [178]; CV3: Allende [178], LAP 02206 [178], GRA 06101 [178]; CK4: ALH 85002 [178]; CK5: EET 92002 [178] |
| Analyzed as | Extraction and Analytical Methodology | Author (Year Published) | Meteorites Analyzed |
|---|---|---|---|
| Methyl esters | Meteorite powder refluxed in glass flask or in degassed glass tube using 1%, 5% or 10% KOH/MeOH for 3 h. Water added to extract for DCM or benzene partition. Aqueous fraction taken to dryness, re-dissolved in water, acidified, and DCM-extracted for GC-MS analysis | Yuen and Kvenvolden (1973) [61], Shimoyama et al. (1986, 1989) [63,180], Naraoka et al. (1999) [64] | CM2: Murchison [61], Murray [61], and Asuka 881280 [64], 881334 [64], 881458 [64]; C2: Yamato 791198 [63], 74662 [180] and 793321 [180] |
| Methyl esters | Meteorite powder refluxed in 6% KOH/MeOH for 3 h. Aqueous portion separated by decantation, solution taken to dryness, acidification, filtration, and diethyl ether-extraction for GC and GC-IRMS analyses | Lawless and Yuen (1979) [62] | CM2: Murchison |
| Underivatized MCAs | Meteorite chips put in degassed flask with deionized water, disaggregation of chips by freeze-thaw cycles and sonication. Aqueous portion separated, neutralized, taken to dryness by rotary evaporation, re-dissolved in water, and vacuum distilled for suppression chromatography and GC-IRMS analyses | Yuen et al. (1984) [118] | CM2: Murchison |
| Underivatized MCAs | No details provided about extraction protocol. Analysis of MCAs performed using ion exclusion chromatography | Briscoe and Moore (1993) [181] | CM2: Murchison; CV3: Allende; LL3: Parnallee; L6: Leedey; E4: Abee |
| Underivatized MCAs | Meteorite chips or powder extracted in degassed glass tube or round-bottom flask with ultrapure deionized or double-distilled water at 100 or 110 °C for 6 or 24 h. Aqueous extract taken to pH > 10, concentrated by rotary evaporation, acidified for SPME, GC-FID, GC-MS and GC-IRMS analyses | Huang et al. (2005) [75], Aponte et al. (2011) [78], Herd et al. (2011) [77], Dillon et al. (2013) [80], Hilts et al. (2014) [70]; Simkus et al. (2019) [119] | CM2: Murchison [75,78], ALH 84033 [78], Sutter’s Mill [80], WIS 91600 [78]; C2ung: Tagish Lake 5b, 11h, 11i and 11v [70,77]; Tagish Lake 1, 4, 10a [119]; CM1: ALH 84034 [78]; CR2: EET 87770 [78]; CV3: MET 00430 [78] |
| Underivatized MCAs | Meteorite powder put in degassed glass vial with triple-distilled water at 100 °C and intermittent sonication for 20 h. Aqueous extract taken to pH = 11.5, concentrated by rotary evaporation, acidified, cryogenically transferred and DCM-extracted for GC-MS analysis | Pizzarello et al. (2008) [76] | CR2: GRA 95229 |
| Underivatized MCAs | Meteorite powder put in degassed glass vial with distilled water at 100 °C for 20 h or at 25 °C for 24 h, followed by a 100 °C 24 h extraction. Aqueous portions separated after each extraction period were made acid, cryogenically transferred, acidified, and DCM-extracted for GC-MS analysis | Monroe and Pizzarello (2011) [115] | CI1: Ivuna; C2ung: Bells |
| Underivatized MCAs | Meteorite powder put in degassed glass vial with distilled water at 100 °C for 20 h. Aqueous portions separated after each extraction period were made acid, cryogenically transferred, acidified, and DCM-extracted for GC-MS analysis | Pizzarello et al. (2012) [74], Pizzarello and Yarnes (2018) [117] | CM2: Mukundpura [117]; CR1: GRO 95577 [74]; CR2: MIL 07525 [74], PCA 91082 [74]; CR3: QUE 99177 [74], MET 00426 [74] |
| Underivatized MCAs and as (S)-1-phenylethyl esters | Meteorite powder put in Teflon tube containing aqueous 1 N NaOH, 30 min sonication, stirring for 2 h at room temperature. Acidification of aqueous extract and DCM-partition for GC-FID and GCMS underivatized analyses, and esterification for GC-MRM and GC-IRMS analyses | Aponte et al. (2014) [65] | CM2: Murchison, LON 94101 |
| Derivatized as (S)-2-methylbutyl esters | Meteorite powder extracted in distilled water at 100 °C for 24 h. Aqueous portion separated, MgCl2 solution added, and solution taken to dryness by centrifugal evaporation. Acid-catalyzed esterification of residue for GC-MS and GC-IRMS analyses | Aponte et al. (2019) [79] | CM1/2: ALH 83100; CM2: Murchison, LEW 90500, LON 94101, EET 96029; CR2: MIL 090001, LAP 02342, GRA 95229, MIL 090657; CO3: DOM 08006, MIL 05013; CV3: Allende, LAP 02206, GRA 06101; CK4: ALH 85002, CK5: EET 92002 |
| Analyzed as | Extraction and Analytical Methodology | Author (Year Published) | Meteorites Analyzed |
|---|---|---|---|
| Underivatized carbonyls for GC-MS MBTH derivatives (azines) for colorimetric | Meteorite powder extracted in water at 120 °C for 4 days. Aqueous portion removed after centrifugation for head space GC-MS of volatile species, followed by derivatization for colorimetric analyses, and removal of derivatization tag for GC-MS re-analysis | Jungclaus et al. (1976) [14] | CM2: Murchison, Murray |
| PFBHA derivatives (oximes) | Meteorite powders extracted in water at 80 °C for 2 h, then at 80 °C for 20 h, then at 100 °C 24 h. Aqueous portions separated after each extraction period were derivatized (35 °C, 2 h), then acidified. PFBHA derivatives were extracted from solution in hexane for GC-MS analysis | Pizzarello and Holmes (2009) [114] | CM2: Murchison; CR2: GRA 95229, LAP 02342 |
| PFBHA derivatives (oximes) | Meteorite powders extracted in water at 25 °C for 24 h, followed by a 100 °C 24 h extraction. Aqueous portions separated after each extraction period were derivatized (35 °C, 2 h), acidified, and extracted using hexane for GC-MS and GC-IRMS analyses | Monroe and Pizzarello (2011) [115] | CI1: Ivuna; C2ung: Bells |
| PFBHA derivatives (oximes) | Meteorite powders extracted in water at 100 °C for 24 h. Aqueous portions were derivatized (35 °C, 2 h), acidified, and extracted using hexane for GC-MS analysis | Pizzarello et al. (2012) [74] | CR1: GRO 95577; CR2: MIL 07525, PCA 91082; CR3: QUE 99177, MET 00426 |
| PFBHA derivatives (oximes) | Meteorite powders extracted in water at 100 °C for 24 h. Aqueous portions were derivatized (room temperature, 24 h), acidified, and extracted using DCM for GC-MS and GC-IRMS analyses | Simkus et al. (2019) [119,121] | CM2: Murchison [121]; C2ung: Tagish Lake 1, 4, and 10a [119] |
| DMB derivatives (acetals) | Meteorite powders extracted in DCM at 100 °C for 24 h. DCM portions were derivatized for GC-MS and GC-IRMS analyses | Aponte et al. (2019) [66] | CI1: Orgueil; CM1/2: ALH 83100; CM2: Murchison, LEW 90500, LON 94101, EET 96029; CR2: MIL 090001, LAP 02342, GRA 95229, MIL 090657; CV3: Allende |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simkus, D.N.; Aponte, J.C.; Elsila, J.E.; Parker, E.T.; Glavin, D.P.; Dworkin, J.P. Methodologies for Analyzing Soluble Organic Compounds in Extraterrestrial Samples: Amino Acids, Amines, Monocarboxylic Acids, Aldehydes, and Ketones. Life 2019, 9, 47. https://doi.org/10.3390/life9020047
Simkus DN, Aponte JC, Elsila JE, Parker ET, Glavin DP, Dworkin JP. Methodologies for Analyzing Soluble Organic Compounds in Extraterrestrial Samples: Amino Acids, Amines, Monocarboxylic Acids, Aldehydes, and Ketones. Life. 2019; 9(2):47. https://doi.org/10.3390/life9020047
Chicago/Turabian StyleSimkus, Danielle N., José C. Aponte, Jamie E. Elsila, Eric T. Parker, Daniel P. Glavin, and Jason P. Dworkin. 2019. "Methodologies for Analyzing Soluble Organic Compounds in Extraterrestrial Samples: Amino Acids, Amines, Monocarboxylic Acids, Aldehydes, and Ketones" Life 9, no. 2: 47. https://doi.org/10.3390/life9020047
APA StyleSimkus, D. N., Aponte, J. C., Elsila, J. E., Parker, E. T., Glavin, D. P., & Dworkin, J. P. (2019). Methodologies for Analyzing Soluble Organic Compounds in Extraterrestrial Samples: Amino Acids, Amines, Monocarboxylic Acids, Aldehydes, and Ketones. Life, 9(2), 47. https://doi.org/10.3390/life9020047







