Hydrothermal Microflow Technology as a Research Tool for Origin-of-Life Studies in Extreme Earth Environments
Abstract
:1. Introduction
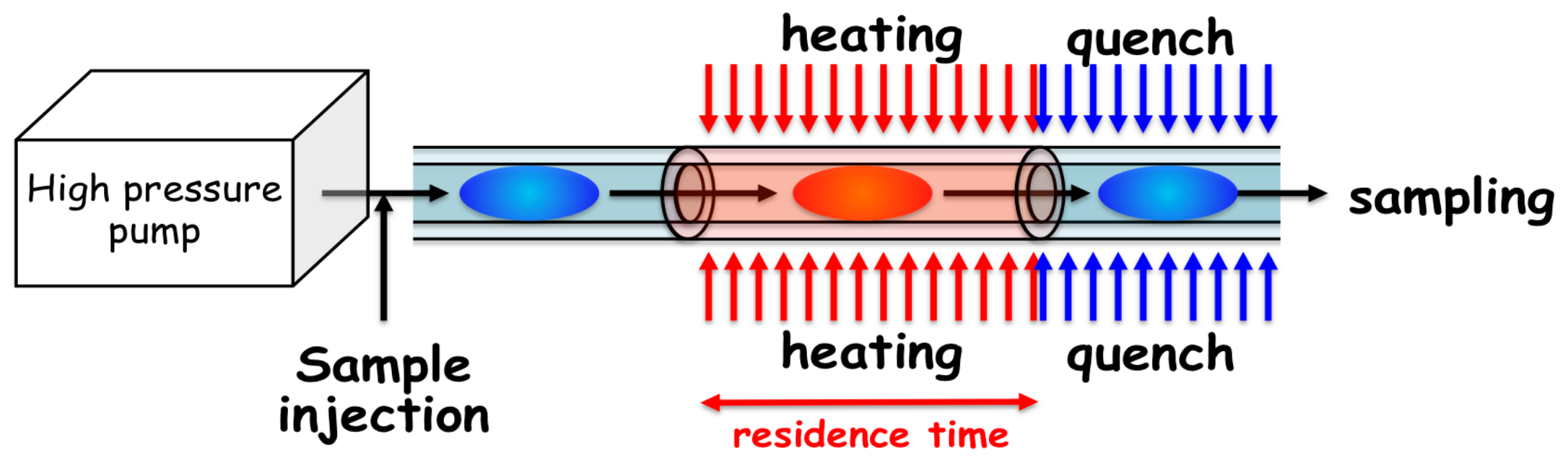
2. Development of Hydrothermal Microflow Reactor Systems
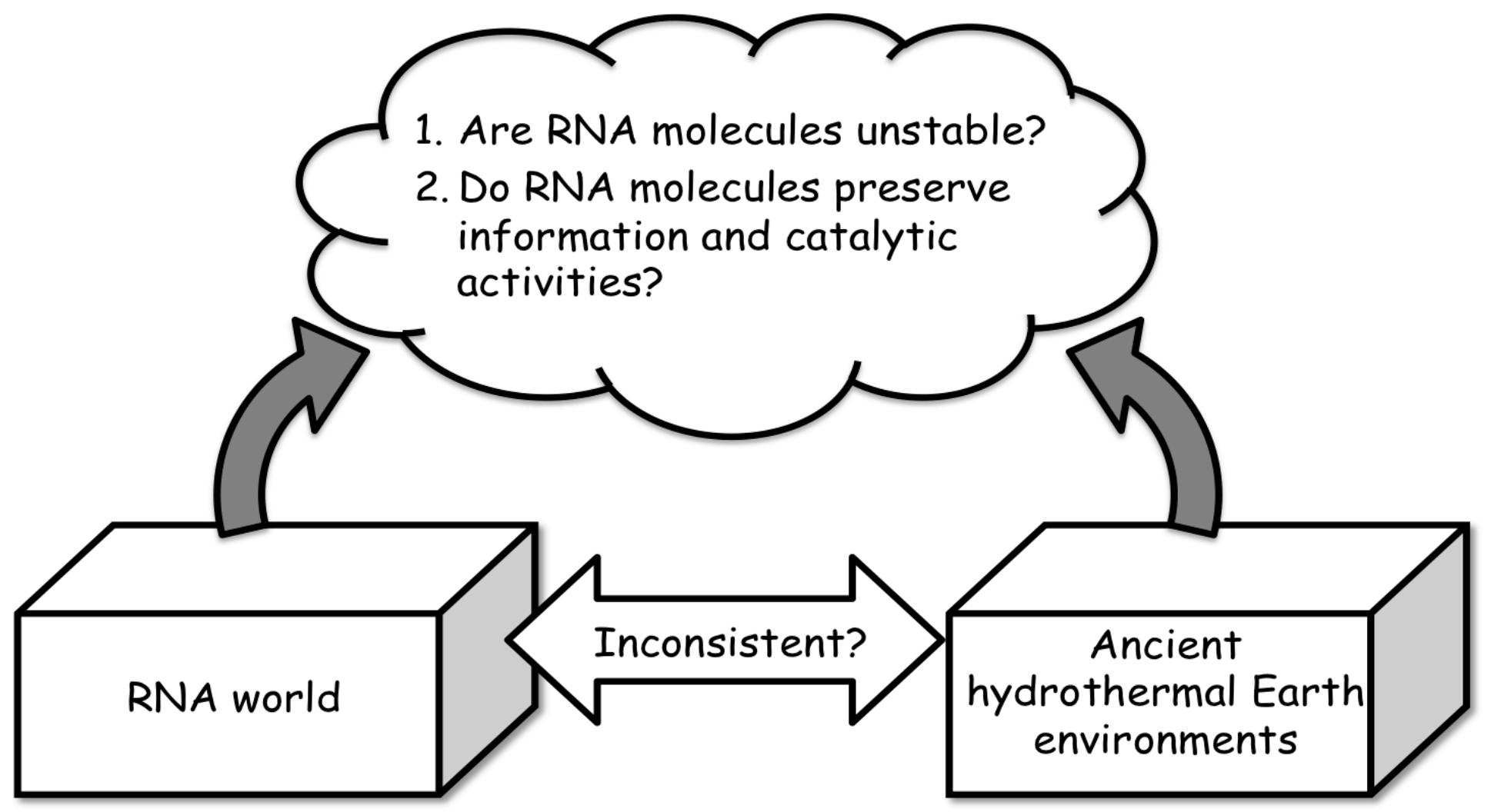
2.1. Importance of Hydrothermal Systems in Relation to the RNA World Hypothesis
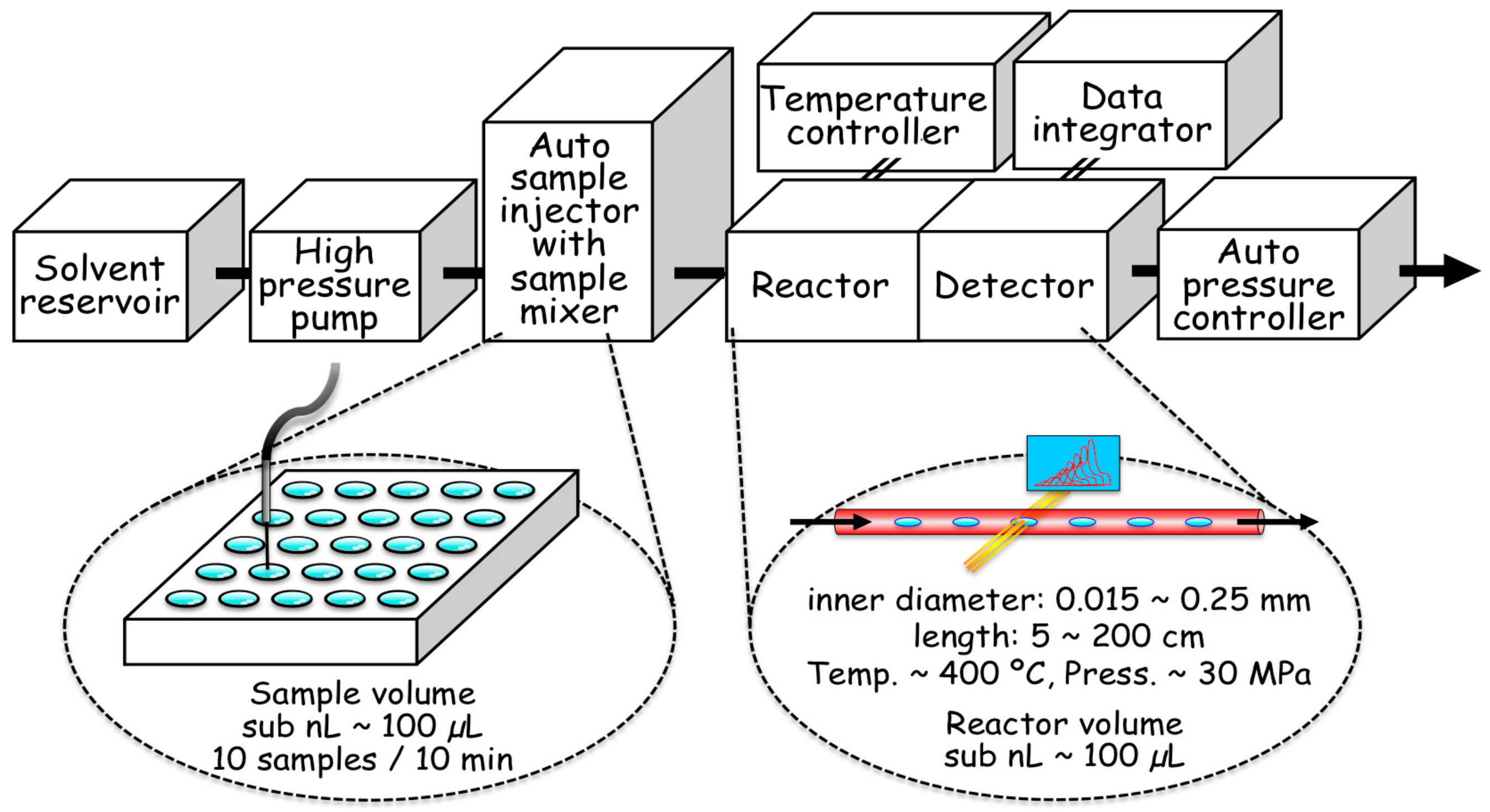
2.2. Hydrothermal Flow System
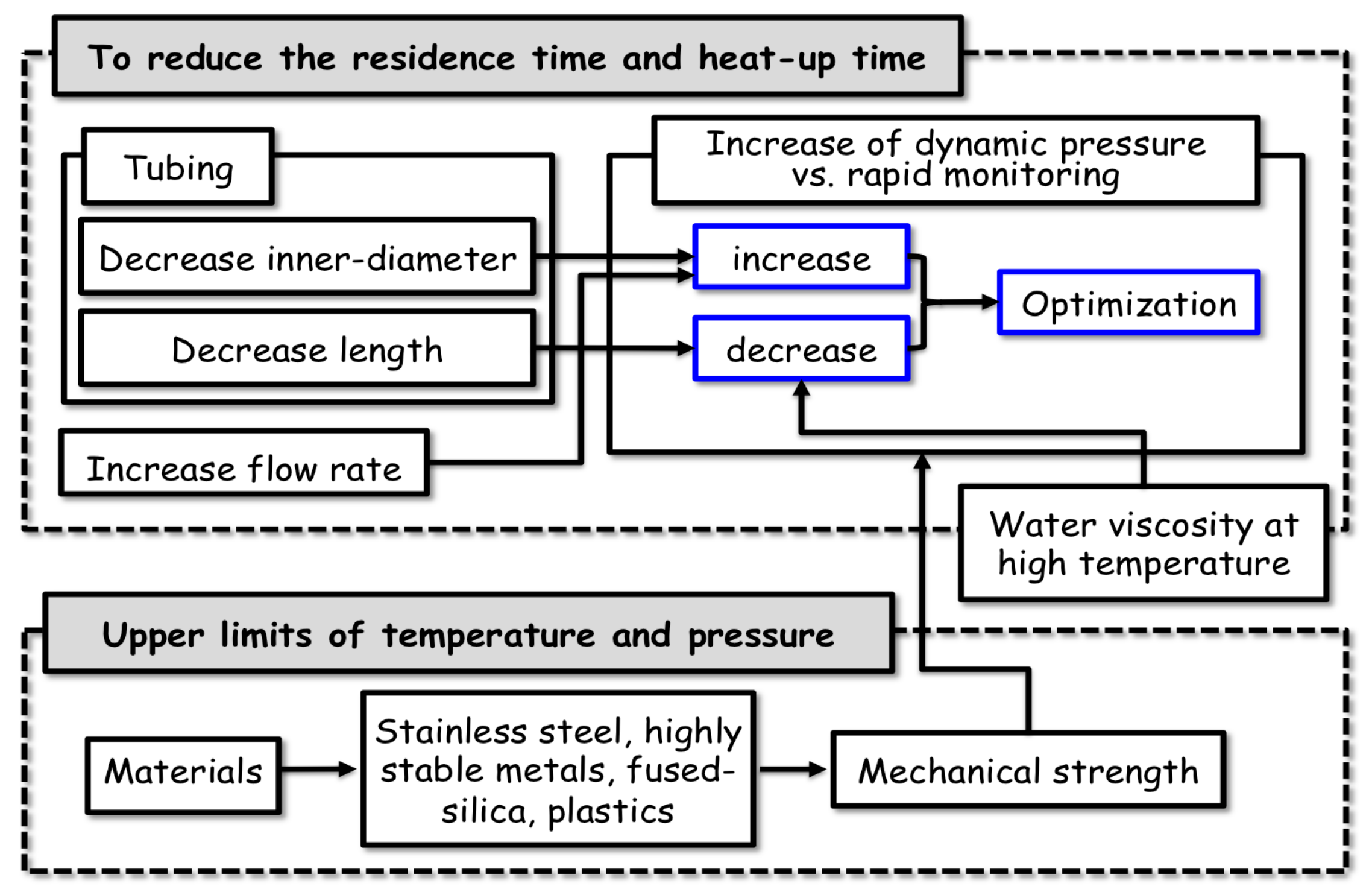
2.3. Details of the Mechanical Characteristics of the Flow System
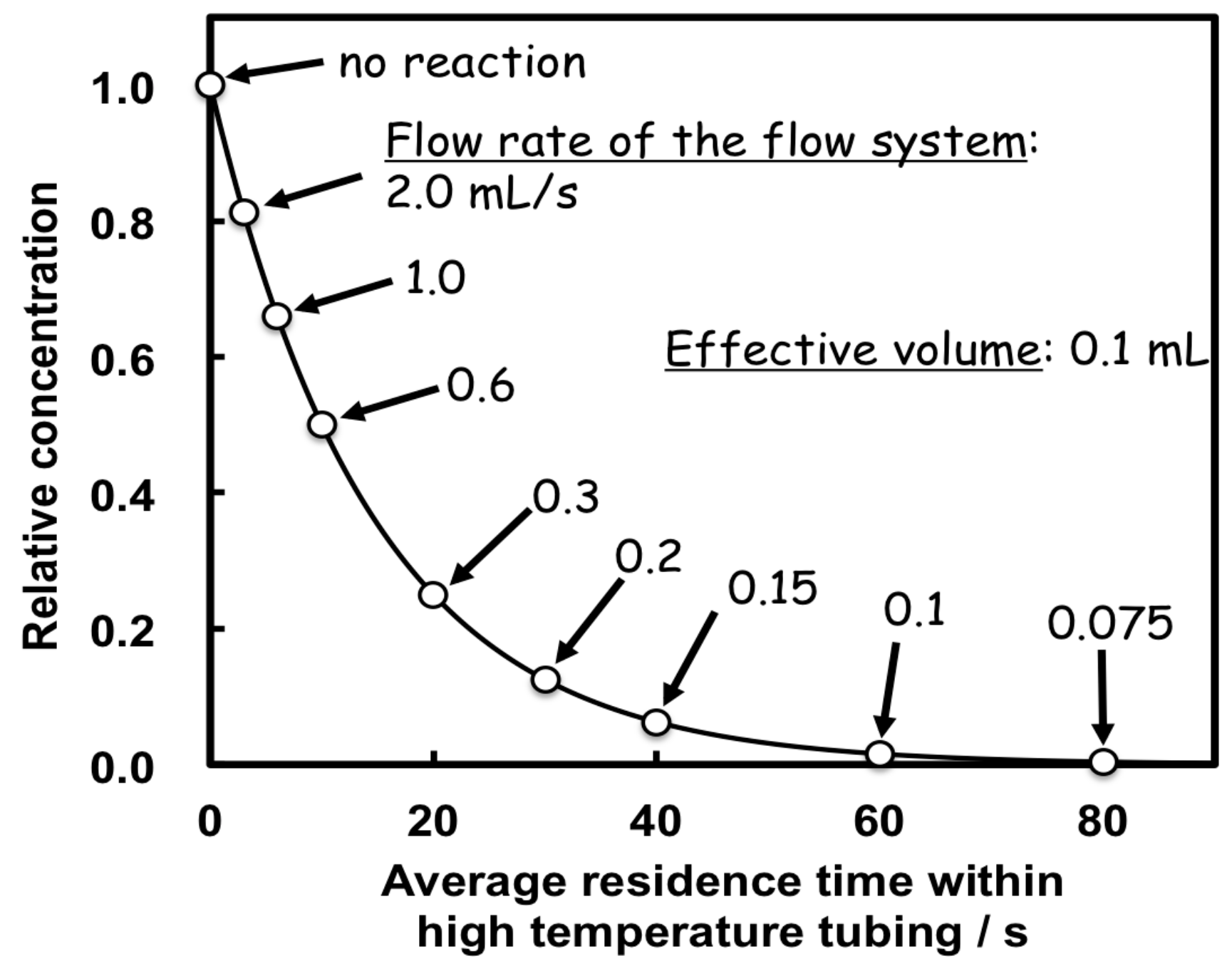
2.4. Kinetic Measurements by the Flow System
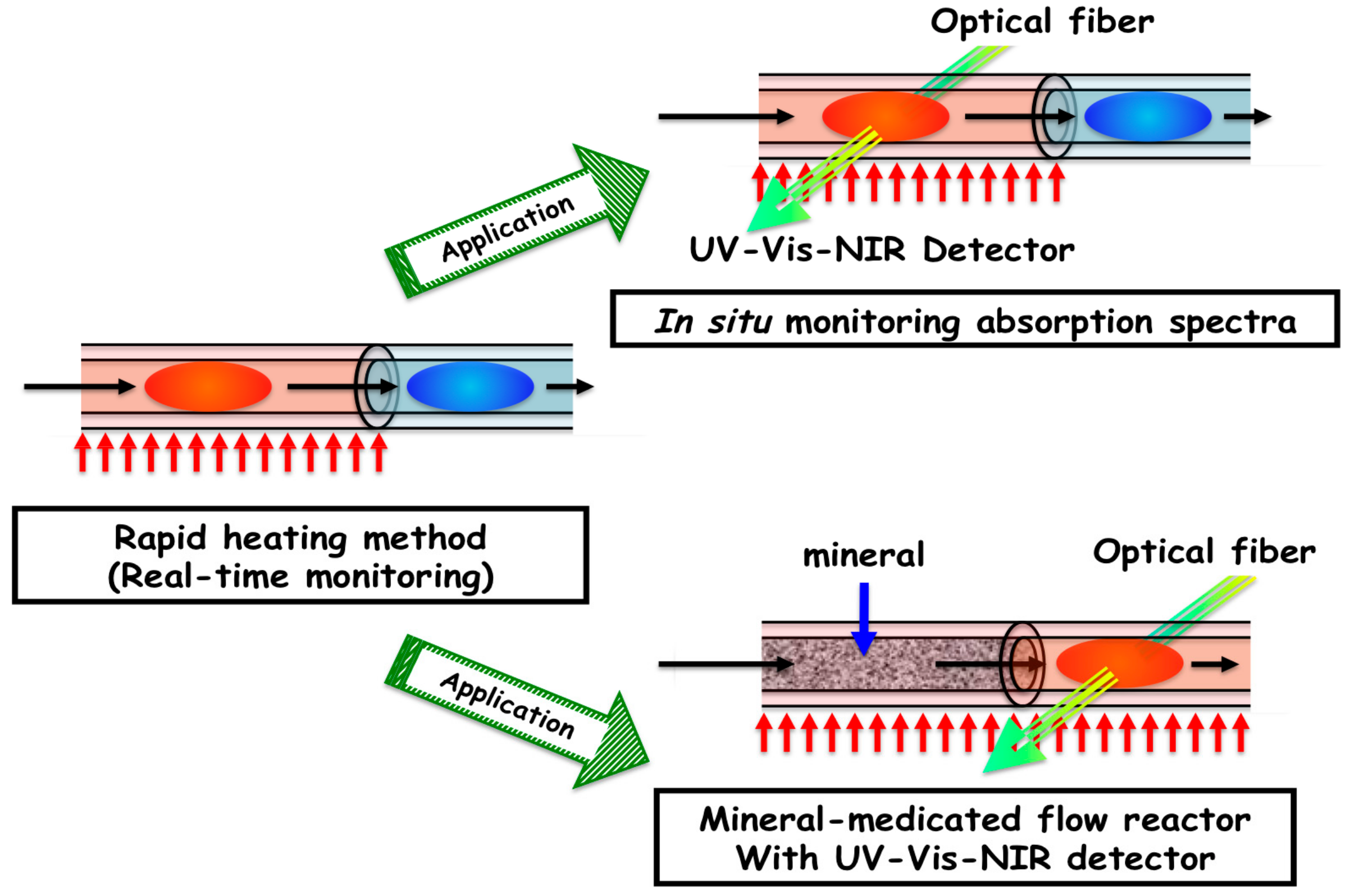
2.5. In situ Measurement of Absorption Spectra
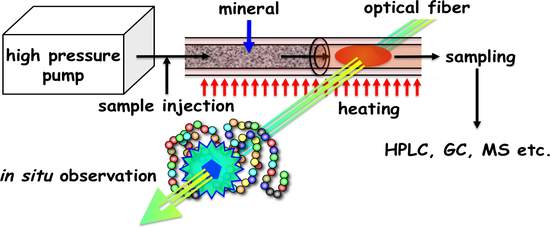
2.6. Mineral-Mediated Hydrothermal System
2.7. High-Throughput Modifications
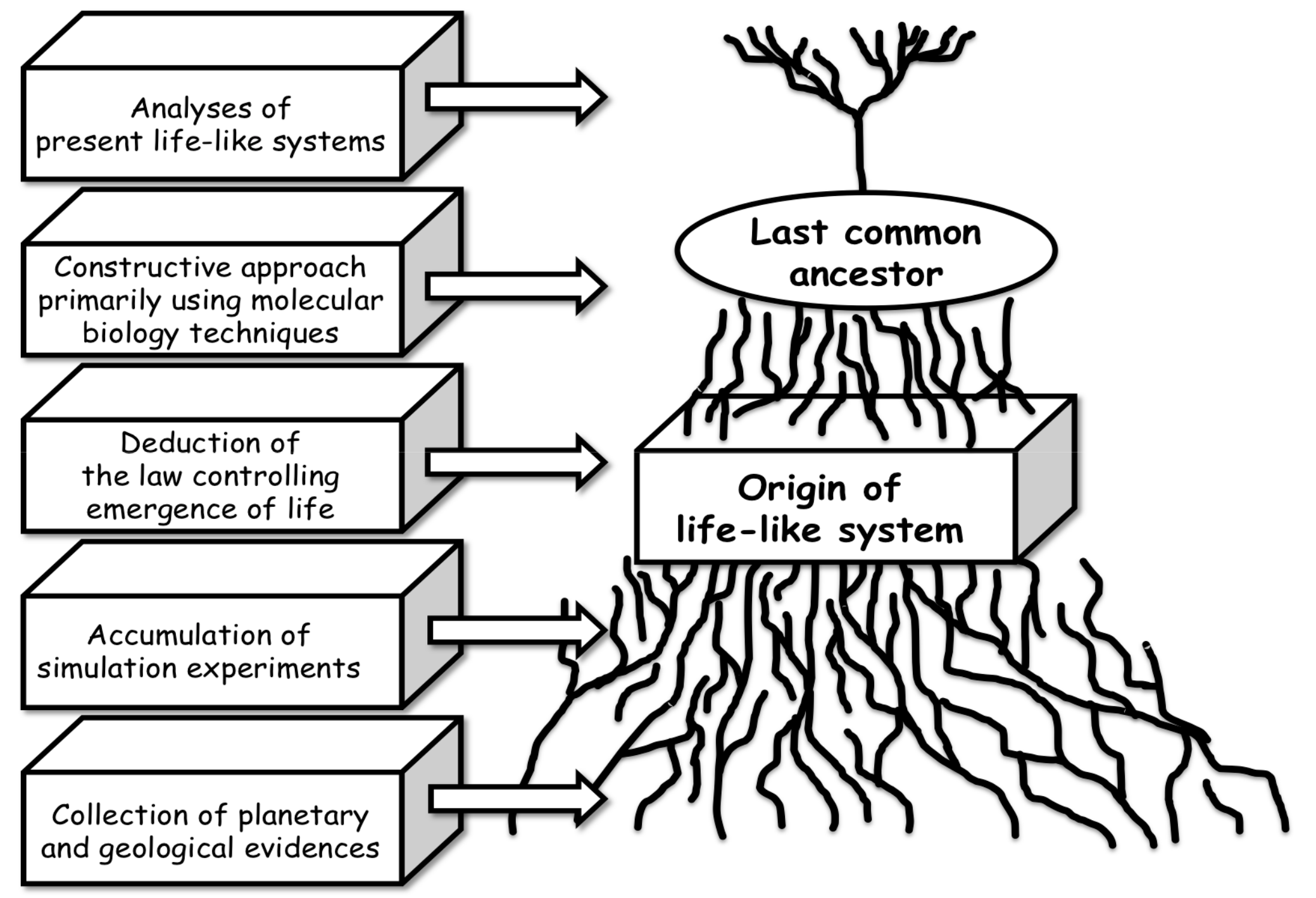
3. What Information Regarding the Origin of Life Can We Obtain from a Hydrothermal System?
3.1. Degradation and Formation of Biomolecules
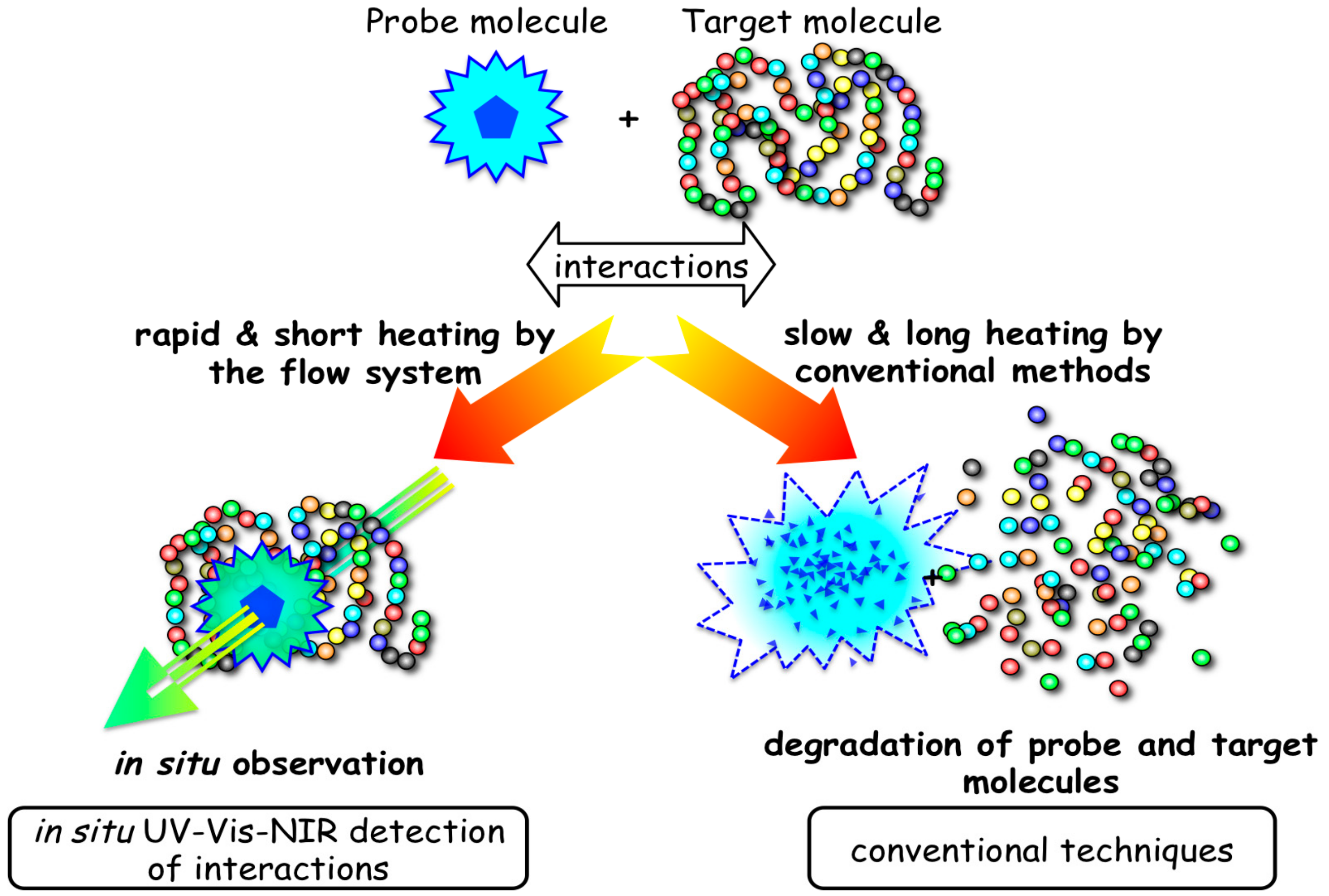
3.2. Interaction of Biomolecules under Hydrothermal Conditions
3.3. Chemical Evolution of Biomolecules under Hydrothermal Conditions
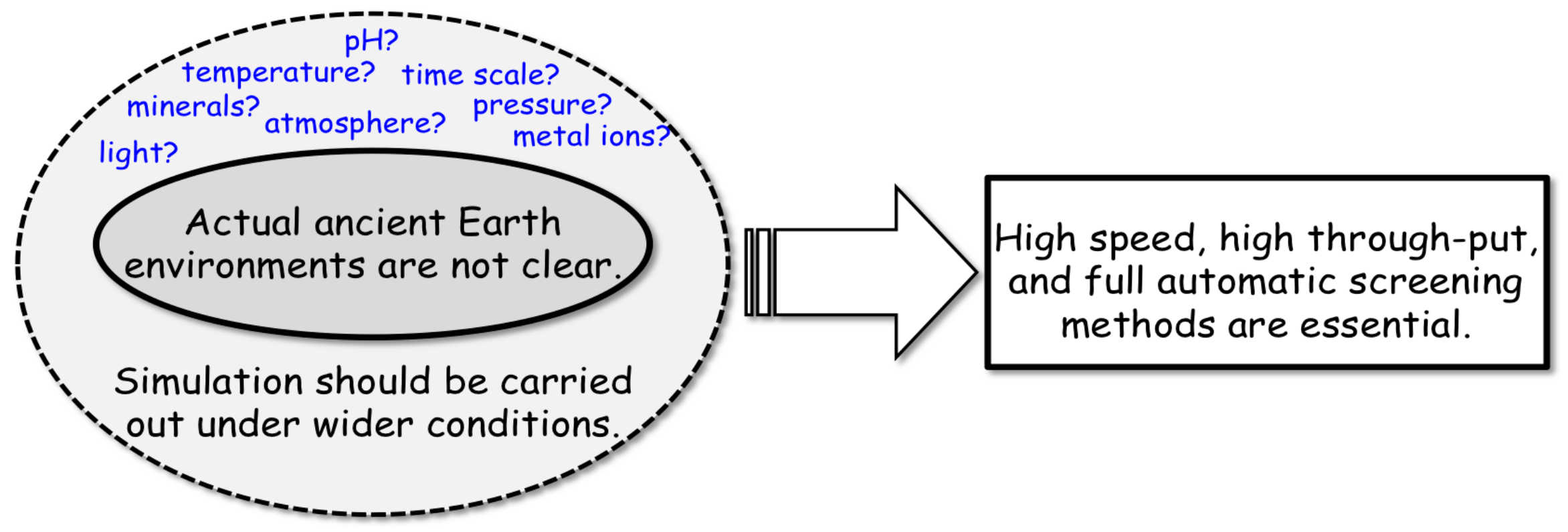
3.4. Strategy of Simulation Experiments for Chemical Evolution towards the Origin of Life
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Oró, J. Machanism of synthesis of adenine from hydrogen cyanide under possible primitive earth conditions. Nature 1961, 191, 1193–1194. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, R.; Orgel, L.E. Prebiotic activation processes. Nature 1973, 244, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Sawai, H. Catalysis of internucleotide bond formation by divalent metal ions. J. Am. Chem. Soc. 1976, 98, 7037–7039. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Joshi, P.G.; Edelson, E.H.; Lawless, J.G. HCN: A plausible source of purines, pyrimidines and amino acids on the primitive earth. J. Mol. Evol. 1977, 11, 293–311. [Google Scholar] [CrossRef]
- Sawai, H.; Shibata, T.; Ohno, M. Preparation of oligoadenylares with 2’-5’ linkage using Pb2+ ion catalyst. Tetrahedron 1981, 37, 481–485. [Google Scholar] [CrossRef]
- Inoue, T.; Orgel, L.E. Oligomerization of (guanosine 5’-phosphor)-2’-methylimidazolide on poly(C). An RNA polymerase model. J. Mol. Biol. 1982, 162, 201–217. [Google Scholar] [CrossRef]
- Inoue, T.; Orgel, L.E. A nonenzymatic RNA polymerase model. Science 1983, 219, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Ertem, G. Oligomerization of ribonucleotides on montmorillonite: Reaction of the 5’-phosphorimidazolide of adenosine. Science 1992, 257, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Ferris, J.P. Kinetics and mechanistic analysis of dinucleotide and oligonucleotide formation from the 5’-phosphorimidazolide of adenosine on Na+-montmorillonite. J. Am. Chem. Soc. 1994, 116, 7564–7572. [Google Scholar] [CrossRef]
- Ferris, J.P.; Hill, A.R.; Liu, J.R.; Orgel, L.E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 1996, 381, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Pino, S.; Ciciriello, F.; Di Mauro, E. Generation of long RNA chains in water. J. Biol. Chem. 2009, 284, 33206–33216. [Google Scholar] [CrossRef] [PubMed]
- El-Murr, N.; Maurel, M.-C.; Rihova, M.; Vergne, J.; Hervé, G.; Kato, M.; Kawamura, K. Behavior of a hammerhead ribozyme in aqueous solution at medium to high temperatures. Naturwissenschaften 2012, 99, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.; Maurel, M.-C.; Deamer, D. Salt-promoted synthesis of RNA-like molecules in simulated hydrothermal conditions. J. Mol. Evol. 2015, 80, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. A production of amino acids under porrible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Harada, K. Thermal copolymerization of amino acids to a product resembling protein. Science 1958, 128, 1214. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Harada, K. The thermal copolymerization of amino acids common to protein. J. Am. Chem. Soc. 1960, 82, 3745–3751. [Google Scholar] [CrossRef]
- Miller, S.L.; Urey, H.G. Organic compound synthesis on the primitive earth. Science 1959, 130, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.; Wächtershäuser, G. Peptides by activation of amino acids with CO on (Ni,Fe)S surfaces: implications for the origin of life. Science 1998, 281, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Imai, E.; Honda, H.; Hatori, K.; Brack, A.; Matsuno, K. Elongation of oligopeptides in a simulated submarine hydrothermal system. Science 1999, 283, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Suwannachot, Y.; Rode, B.M. Mutual amino acid catalysis in salt-induced peptide formation supports this mechanism’s role in prebiotic peptide evolution. Origins Life Evol. Biosph. 1999, 29, 463–471. [Google Scholar] [CrossRef]
- Maurel, M.C.; Orgel, L.E. Oligomerization of thioglutamic acid. Origins Life Evol. Biosphere 2000, 30, 423–430. [Google Scholar] [CrossRef]
- Kawamura, K.; Nishi, T.; Sakiyama, T. Consecutive elongation of alanine oligopeptides at the second time range under hydrothermal condition using a microflow reactor system. J. Am. Chem. Soc. 2005, 127, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Shimahashi, M. One-step formation of oligopeptide-like molecules from Glu and Asp in hydrothermal environments. Naturwissenschaften 2008, 95, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Takeya, H.; Kushibe, T.; Koizumi, Y. Mineral-enhanced hydrothermal oligopeptide formation at the second time scale. Astrobiology 2011, 11, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Drawbacks of the ancient RNA-based life-like system under primitive earth conditions. Biochimie 2012, 94, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. A Hypothesis: Life initiated from two genes, as deduced from the RNA world hypothesis and the characteristics of life-like systems. Life 2016, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, W. The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Szostak, J.W. In vitro selection of functional nucleic acids. Ann. Rev. Biochem. 1999, 68, 611–647. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.; Polisky, B.; Uhlenbeck, O.; Yarus, M. Diversify of oligonucleotide functions. Ann. Rev. Biochem. 1999, 68, 763–797. [Google Scholar]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Gesteland, R.F.; Cech, T.R.; Atkins, J.F. (Eds.) The RNA World, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2005. [Google Scholar]
- Kaddour, H.; Vergne, J.; Hervé, G.; Maurel, M.C. High-pressure analysis of a hammerhead ribozyme from Chrysanthemum chlorotic mottle viroid reveals two different populations of self-cleaving molecule. FEBS J. 2011, 278, 3739–3747. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sato, K.; Wakabayashi, M.; Nakaishi, T.; Ko-Mitamura, E.P.; Shima, Y.; Urabe, I.; Yomo, T. Synthesis of functional protein in liposome. J. Biosci. Bioeng. 2001, 92, 590–593. [Google Scholar] [CrossRef]
- Mansy, S.S.; Schrum, J.P.; Krishnamurthy, M.; Tobe, S.; Treco, D.A.; Szostak, J.W. Template-directed synthesis of a genetic polymer in a model protocell. Nature 2008, 454, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, N.; Miyamoto-Sato, E.; Husimi, Y.; Yanagawa, H. In vitro virus: Bonding of mRNA bearing puromycin at the 3’-terminal end to the C-terminal end of its encoded protein on the ribosome in vitro. FEBS Lett. 1997, 414, 405–408. [Google Scholar] [PubMed]
- Ichihashi, N.; Usui, K.; Kazuta, Y.; Sunami, T.; Matsuura, T.; Yomo, T. Darwinian evolution in a translation-coupled RNA replication system within a cell-like compartment. Nature Comm. 2013, 2494. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Ikoma, M.; Genda, H.; Hirose, K.; Yokoyama, T.; Santosh, M. The naked planet earth: Most essential pre-requisite for the origin and evolution of life. Geosci. Front. 2013, 4, 141–165. [Google Scholar] [CrossRef]
- Peck, W.H.; Valley, J.W.; Wilde, S.A.; Graham, C.M. Oxygen isotope ratios and rare earth elements in 3.3 to 4.4 Ga zircons: Ion microprobe evidence for high δ18O continental crust and oceans in the early. Archean. Geochim. Cosmochim. Acta 2001, 65, 4215–4229. [Google Scholar] [CrossRef]
- Mojzsis, S.J.; Harrison, T.M.; Pidgeon, R.T. Oxygen-isotope evidence from ancient zircons for liquid water at the Earth's surface 4,300 Myr ago. Nature 2001, 409, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Valley, J.W.; Cavosie, A.J.; Ushikubo, T.; Reinhard, D.A.; Lawrence, D.F.; Larson, D.J.; Clifton, P.H.; Kelly, T.F.; Wilde, S.A.; Moser, D.E.; Spicuzza, M.J. Hadean age for a post-magma-ocean zircon confirmed by atom-probe tomography. Nature Geosci. 2014, 7, 219–223. [Google Scholar] [CrossRef]
- Kawamura, K.; Maurel, M.-C. Walking over 4 Gya: Chemical evolution from photochemistry to mineral and organic chemistries leading to an RNA world, online first. Orig. Life Evol. Biosphe. 2017, 47, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Corliss, J.B.; Baross, J.A.; Hoffman, S.E. An hypothesis concerning the relationship between submarine hot springs and the origin of life on Earth. Oceanol. Acta (Suppl.) 1981, 4, 59–69. [Google Scholar]
- Pace, N.P. Origin of life—facing up to the physical setting. Cell 1991, 65, 531–533. [Google Scholar] [CrossRef]
- Forterre, P. A hot topic: The origin of hyperthermopiles. Cell 1996, 85, 789–792. [Google Scholar] [CrossRef]
- Galtier, N.; Tourasse, N.; Gouy, M. A nonhyperthermophilic common ancestor to extant life forms. Science 1999, 283, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Akanuma, S.; Nakajima, Y.; Yokoboria, S.; Kimura, M.; Nemoto, N.; Mase, T.; Miyazono, K.; Tanokura, M.; Yamagishia, A. Experimental evidence for the thermophilicity of ancestral life. Proc. Natl. Acad. Sci. USA 2013, 110, 11067–11072. [Google Scholar] [CrossRef] [PubMed]
- Akanuma, S. Characterization of reconstructed ancestral proteins suggests a change in temperature of the ancient biosphere. Life 2017, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Eigen, M. Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften 58, 465–523. [CrossRef]
- Ikehara, K. Possible steps to emergence of life: The [GADV]-protein world hypothesis. Chem. Rec. 2005, 5, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Eigen, M.; Gardiner, W.; Schuster, P.; Winkler-Oswatitsch, R. The origin of genetic information. Sci. Am. 1981, 64, 541–565. [Google Scholar] [CrossRef]
- Kauffman, S.A. Autocatalytic sets of proteins. J. Theor. Biol. 1986, 119, 1–24. [Google Scholar] [CrossRef]
- Mojzsis, S.J.; Arrhenius, G.; McKeegan, K.D.; Harrison, T.M.; Nutman, A.P.; Friend, C.R.L. Evidence for life on Earth before 3,800 million years ago. Nature 1996, 384, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.C.G. Carbon-dioxide on the early earth. Orig. Life Evol. Biosph. 1985, 16, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Kasting, J.F.; Pollack, J.B. Effects of high CO2 levels on surface temperature and atmospheric oxidation state of the early earth. J. Atom. Chem. 1984, 1, 403–428. [Google Scholar] [CrossRef]
- Kasting, J.F.; Ackerman, T.P. Climatic consequences of very high-carbon dioxide levels in the earths early atmosphere. Science 1986, 234, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Kasting, J.F. Earth’s early atmosphere. Science 1993, 259, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Schaechter, M.; Maaløe, O.; Kjeldgaard, N.O. Dependency on medium and temperature of cell size and chemical composition during balanced growth of salmonella typhimurium. Microbiology 1958, 19, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. Molecular Biology of the Gene, 2nd ed.; Saunders: Philadelphia, PA, USA, 1972. [Google Scholar]
- Orgel, L.E.; Crick, F.H.C. Anticipating an RNA world some past speculations on the origin of life: Where are they today? FASEB J. 1993, 7, 238–239. [Google Scholar] [PubMed]
- Kawamura, K. Behavior of RNA under hydrothermal conditions and the origins of life. Inter. J. Astrobiol. 2004, 3, 301–309. [Google Scholar] [CrossRef]
- Kawamura, K. Monitoring hydrothermal reactions on the millisecond time scale using a micro-tube flow reactor and kinetics of ATP hydrolysis for the RNA world hypothesis. Bull. Chem. Soc. Jpn. 2000, 73, 1805–1811. [Google Scholar] [CrossRef]
- Kawamura, K. Kinetics and activation parameter analyses of hydrolysis and interconversion of 2’,5’- and 3’,5’-linked dinucleoside monophosphate at extremely high temperatures. Biochim. Biophys. Acta 2003, 1620, 199–210. [Google Scholar] [CrossRef]
- Kawamura, K. Kinetic analysis of cleavage of ribose phosphodiester bond within guanine and cytosine rich oligonucleotides and dinucleotides at 65–200 °C and its implications on the chemical evolution of RNA. Bull. Chem. Soc. Jpn. 2003, 76, 153–162. [Google Scholar] [CrossRef]
- Baross, J.A.; Hoffman, S.E. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life Evol. Biosph. 1985, 15, 327–345. [Google Scholar] [CrossRef]
- Nisbet, E.G. RNA and hot-water springs. Nature 1986, 322, 206. [Google Scholar] [CrossRef]
- White, R.H. Hydrolytic stability of biomolecules at high temperatures and its implication for life at 250 °C. Nature 1984, 310, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Westall, F.; Campbell, K.A.; Bréhéret, J.G.; Foucher, F.; Gautret, P.; Hubert, A.; Sorieul, S.; Grassineau, N.; Guido, D.M. Archean (3.33 Ga) microbe-sediment systems were diverse and flourished in a hydrothermal context. Geology 2015, 43, 615–618. [Google Scholar] [CrossRef] [Green Version]
- Larralde, R.; Robertson, M.P.; Miller, S.L. Rates of decomposition of ribose and other sugars: Implications for chemical evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 8158–8160. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Yosida, A.; Matumoto, O. Kinetic investigations for the hydrolysis of adenosine 5’-triphosphate at elevated temperatures: prospects for the chemical evolution of RNA. Viva Origino 1997, 25, 177–190. [Google Scholar]
- Levy, M.; Miller, S.L. The stability of the RNA bases: Implications for the origin of life. Proc. Natl. Acad. Sci. USA 1998, 95, 7933–7938. [Google Scholar]
- Cowan, D.A. The upper temperature for life–where do we draw the line? Trends Microbiol. 2003, 12, 58–60. [Google Scholar] [CrossRef]
- Kargel, J.S.; Kaye, J.Z.; Head, J.W., III; Marion, G.M.; Sassen, R.; Crowley, J.K.; Ballesteros, O.P.; Grant, S.A.; Hogenboom, D.L. Europa’s crust and ocean: origin, composition, and the prospects for life. Icarus 2000, 148, 226–265. [Google Scholar] [CrossRef]
- Hsu, H.W.; Postberg, F.; Sekine, Y.; Shibuya, T.; Kempf, S.; Horányi, M.; Juhász, A.; Altobelli, N.; Suzuki, K.; Masaki, Y.; Kuwatani, T.; Tachibana, S.; Sirono, S.; Georg Moragas-Klostermeyer, G.; Srama, R. Ongoing hydrothermal activities within Enceladus. Nature 2015, 519, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.G. (Ed.) Special issue-marine hydrothermal systems and the origin of life. Orig. Life Evol. Biosphe. 1992, 22, 5–242, and therein. [Google Scholar]
- Hennet, R.J.C.; Holm, N.G.; Engel, M.H. Abiotic synthesis of amino-acids under hydrothermal conditions and the origin of life—a perpetual phenomenon. Naturwissenschaften 1992, 79, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.G.; Ertem, G.; Ferris, J.P. The bonding and reactions of nucleotids and polynucleotides on iron oxide hydroxide polymorphs. Orig. Life Evol Biosphere 1993, 23, 195–215. [Google Scholar] [CrossRef]
- Holm, N.G.; Andersson, E. Hydrothermal simulation experiments as a tool for studies of the origin of life on earth and other terrestrial planets: A review. Astrobiology 2005, 5, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W.; Georgiou, C.D. Hydrothermal conditions and the origin of cellular life. Astrobiology 2015, 15, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Yukioka, M. Kinetics of the racemization of amino acids at 225–275 °C using a real-time monitoring method of hydrothermal reactions. Thermochim. Acta 2001, 375, 9–16. [Google Scholar] [CrossRef]
- Kawamura, K.; Kameyama, N.; Matumoto, O. Kinetics of hydrolysis of ribonucleotide polymers in aqueous solution at elevated temperatures: implications of chemical evolution of RNA and primitive ribonuclease. Viva Origino 1999, 27, 107–118. [Google Scholar]
- Kawamura, K. Kinetic analysis of hydrothermal reactions by flow tube reactor—Hydrolysis of adenosine 5'-triphosphate at 398–573 K—. Nippon Kagaku Kaishi 1998, 255–262. [Google Scholar] [CrossRef]
- Kawamura, K. Monitoring of hydrothermal reactions in 3 ms using fused-silica capillary tubing. Chem. Lett. 1999, 28, 125–126. [Google Scholar] [CrossRef]
- Masten, D.A.; Foy, B.R.; Harradine, D.M.; Dyer, R.B. In situ Raman spectroscopy of reactions in supercritical water. J. Phys. Chem. 1993, 97, 8557–8559. [Google Scholar] [CrossRef]
- Bennett, G.E.; Johnston, K.P. UV-visible absorbance spectroscopy of organic probes in supercritical water. J. Phys. Chem. 1994, 98, 441–447. [Google Scholar] [CrossRef]
- Kieke, M.L.; Schopperlrei, J.W.; Brill, T.B. Spectroscopy of hydrothermal reactions. 1. The CO2−H2O system and kinetics of urea decomposition in an FTIR spectroscopy flow reactor cell operable to 725 K and 335 bar. J. Phys. Chem. 1996, 100, 7455–7462. [Google Scholar] [CrossRef]
- Islam, M.N.; Kaneko, T.; Kobayashi, K. Reaction of amino acids in a Supercritical water-flow reactor simulating submarine hydrothermal systems. Bull. Chem. Soc. Jpn. 2003, 76, 1171–1178. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Aubrey, A.D.; Bada, J.L. An evaluation of the critical parameters for abiotic peptide synthesis in submarine hydrothermal systems. Orig. Life Evol. Biosphe. 2009, 39, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. In situ UV-VIS detection of hydrothermal reactions using fused-silica capillary tubing within 0.08–3.2 s at high temperatures. Anal. Sci. 2002, 18, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Development of micro-flow hydrothermal monitoring systems and their applications to the origin of life study on earth. Anal. Sci. 2011, 27, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. In situ UV-VIS detection of the association of water-soluble anionic porphyrin and aromatic bases in aqueous solution at high temperatures using a capillary flow hydrothermal reactor system. Anal. Sci. 2003, 19, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. A new probe for the indirect measurement of the conformation and interaction of biopolymers at extremely high temperatures using a capillary flow hydrothermal reactor system for UV-visible spectrophotometry. Anal. Chim. Acta. 2005, 543, 236–241. [Google Scholar] [CrossRef]
- Kawamura, K.; Nagayoshi, H.; Yao, T. In situ analysis of proteins at high temperatures mediated by capillary-flow hydrothermal UV-Vis spectrophotometer with a water-soluble chromogenic reagent. Anal. Chim. Acta 2010, 667, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Ikoma, K.; Igarashi, S.; Hisamoto, H.; Yao, T. Flow injection analysis combined with a hydrothermal flow reactor: application to kinetic determination of trace amounts of iridium using a water-soluble porphyrin. Talanta 2011, 84, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Nakai, T.; Ikoma, K.; Hisamoto, H. High-throughput Ru(III) analysis using the hydrothermal flow reactor-mediated FIA by the extreme acceleration of Ru(III) complexation with 1,10-phenanthroline. Talanta 2012, 99, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Yasuda, T.; Hatanaka, T.; Hamahiga, K.; Matsuda, N.; Ueshima, M.; Nakai, K. Oxidation of aliphatic alcohols and benzyl alcohol by H2O2 under the hydrothermal conditions in the presence of solid-state catalysts using batch and flow reactors. Chem. Eng. J. 2016, 285, 49–56. [Google Scholar] [CrossRef]
- Kawamura, K.; Yasuda, T.; Hatanaka, T.; Hamahiga, K.; Matsuda, N.; Ueshima, M.; Nakai, K. In situ UV-VIS spectrophotometry within the second time scale as a research tool for solid-state catalyst and liquid-phase reactions at high temperatures: Its application to the formation of HMF from glucose and cellulose. Chem. Eng. J. 2017, 307, 1066–1075. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Boyce, A.J.; Fallick, A.E. On hydrothermal convection systems and the emergence of life. Eco. Geo. 2005, 100, 419–438. [Google Scholar]
- Qian, Y.; Engel, M.H.; Macko, S.A.; Carpenter, S.; Deming, J.W. Kinetics of peptide hydrolysis and amino acid decomposition at high temperature. Geochim. Cosmochim. Acta 1993, 57, 3281–3293. [Google Scholar] [CrossRef]
- Kawamura, K. Reality of the emergence of life-like systems from simple prebiotic polymers on primitive earth. In Genesis–in the Beginning: Precursors of Life, Chemical Models and Early Biological Evolution; Seckbach, J., Gordon, R., Eds.; Springer: New York, NY, USA, 2012; pp. 123–144. [Google Scholar]
- Kawamura, K.; Nagayoshi, H. Behavior of DNA under hydrothermal conditions with MgCl2 additive using an in situ UV-visible spectrophotometer. Thermochim. Acta 2007, 466, 63–68. [Google Scholar] [CrossRef]
- Kawamura, K. Temperature limit for the emergence of life-like system deduced from the prebiotic chemical kinetics under the hydrothermal conditions. In Proceedings of the Twelfth International Conference on the Simulation and Synthesis of Living Systems, Odense, Denmark; Fellermann, H., Dörr, M., Hanczyc, M.M., Lauren, L.L., Maurer, S., Merkle, D., Monnard, P.A., Støy, K., Rasmussen, S., Eds.; 2010; pp. 37–44. [Google Scholar]
- Kawamura, K.; Da Silva, L.; Ogawa, M.; Konagaya, N.; Maurel, M.C. Verification of chemical evolution of RNA under hydrothermal environments on the primitive Earth. BIO Web Conf. 2015, 4, 00011. [Google Scholar] [CrossRef]
- McMullen, J.P.; Jensen, K.F. Integrated microreactors for reaction automation: New approaches to reaction development. Ann. Rev. Anal. Chem. 2010, 3, 19–42. [Google Scholar] [CrossRef] [PubMed]
| Tubing Size | Heat-Up Time (ms) | |
|---|---|---|
| Length (cm) | Inner Diameter (mm) | |
| 200 | 0.25 | 3000 |
| 50 | 0.10 | 300 |
| 20 | 0.05 | 40 |
| 10 | 0.025 | 4 |
| 5 | 0.015 | 2 |
| Type of Flow Reactor | Improvement | References |
|---|---|---|
| Real-time monitoring of hydrothermal reactions using narrow tubing | Monitoring at 0.002–200 s, at 400 °C, at 30 MPa. | [63,83,84] |
| In situ monitoring of hydrothermal reactions with UV-visible absorption spectrophotometer | In situ measurement of UV-visible-NIR absorption spectra at 200–600 nm, at 0.3–60 s, at 400 °C, at 30 MPa | [90,91,92,93,94] |
| Mineral-mediated hydrothermal flow reactor | High-temperature reactor column packed with mineral particles at 0.3–60 s, at 300 °C, 30 MPa. | [24] |
| Flow-injection analysis for high temperatures with hydrothermal flow reactor | Reactions are accelerated with high-temperature reactor at temperatures up to 400 °C | [95,96] |
| In situ monitoring in the presence of solid-state catalysts with UV-visible-NIR spectrophotometer | High-temperature reactor column packed with solid-state catalysts and in situ analysis at UV-visible-NIR region. | [97,98] |
| Molecules | Half-Life (s) | ||
|---|---|---|---|
| 100 °C | 200 °C | 300 °C | |
| oligoRNA | 2400~4500 | 2.0~3.4 | 0.02~0.04 |
| C3’pG | 13000 | 29 | 0.54 |
| dCpdG | 570000 | 46 | 0.098 |
| Half-Life (C3’pG)/Half-Life (dCpdG) | 0.023 | 0.630 | 5.5 |
| 5’-ATP | 1300 | 0.37 | 0.0019 |
| Alanine | 16000000 | 3400 | 14 |
| Half-Life (ATP)/Half-Life (Alanine) | 8.1 × 10−5 | 1.1 × 10−4 | 1.4 × 10−4 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamura, K. Hydrothermal Microflow Technology as a Research Tool for Origin-of-Life Studies in Extreme Earth Environments. Life 2017, 7, 37. https://doi.org/10.3390/life7040037
Kawamura K. Hydrothermal Microflow Technology as a Research Tool for Origin-of-Life Studies in Extreme Earth Environments. Life. 2017; 7(4):37. https://doi.org/10.3390/life7040037
Chicago/Turabian StyleKawamura, Kunio. 2017. "Hydrothermal Microflow Technology as a Research Tool for Origin-of-Life Studies in Extreme Earth Environments" Life 7, no. 4: 37. https://doi.org/10.3390/life7040037
APA StyleKawamura, K. (2017). Hydrothermal Microflow Technology as a Research Tool for Origin-of-Life Studies in Extreme Earth Environments. Life, 7(4), 37. https://doi.org/10.3390/life7040037