Toxic Cyanobacterial Bloom Triggers in Missisquoi Bay, Lake Champlain, as Determined by Next-Generation Sequencing and Quantitative PCR
Abstract
:1. Introduction
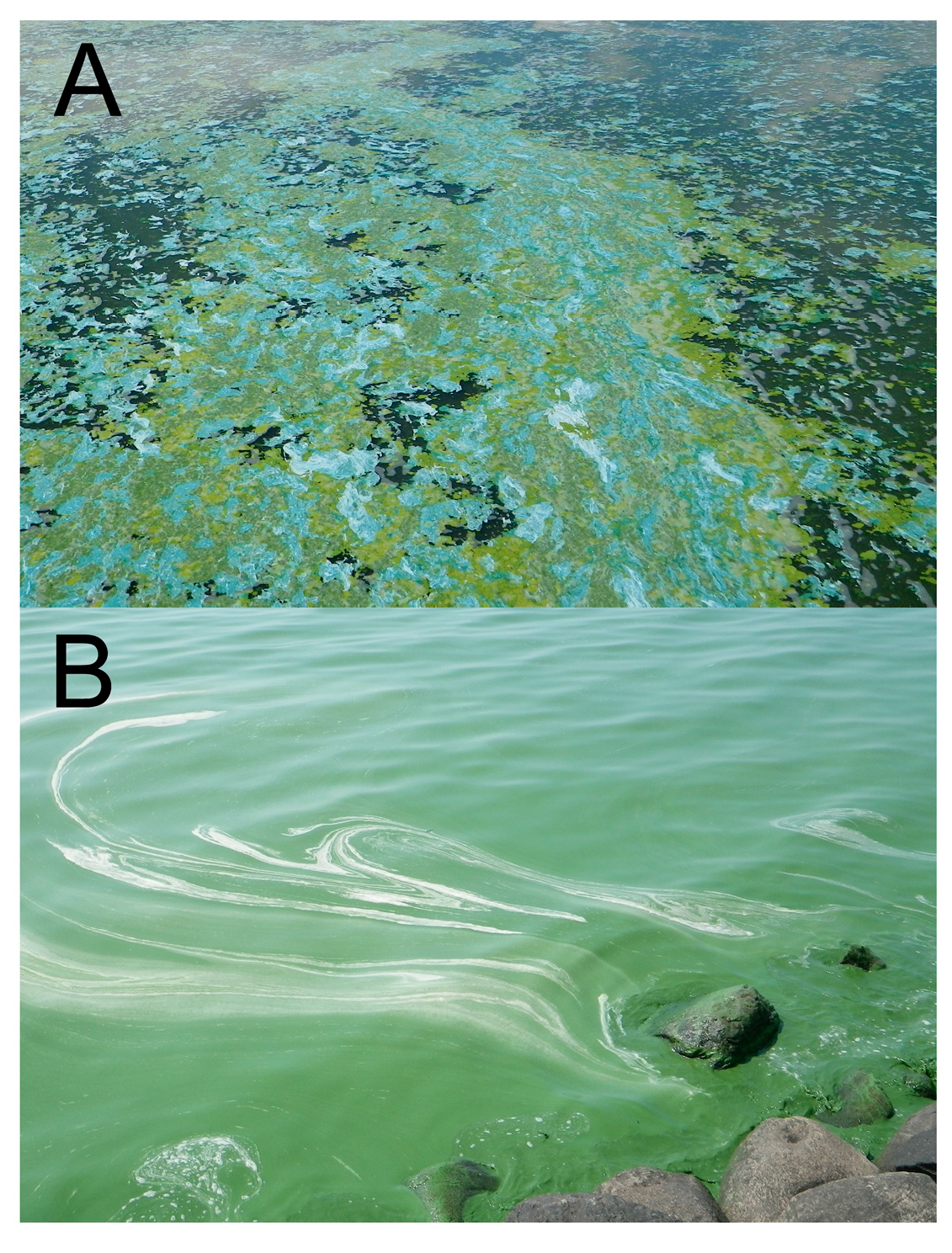
2. Experimental Section
2.1. Sampling Sites
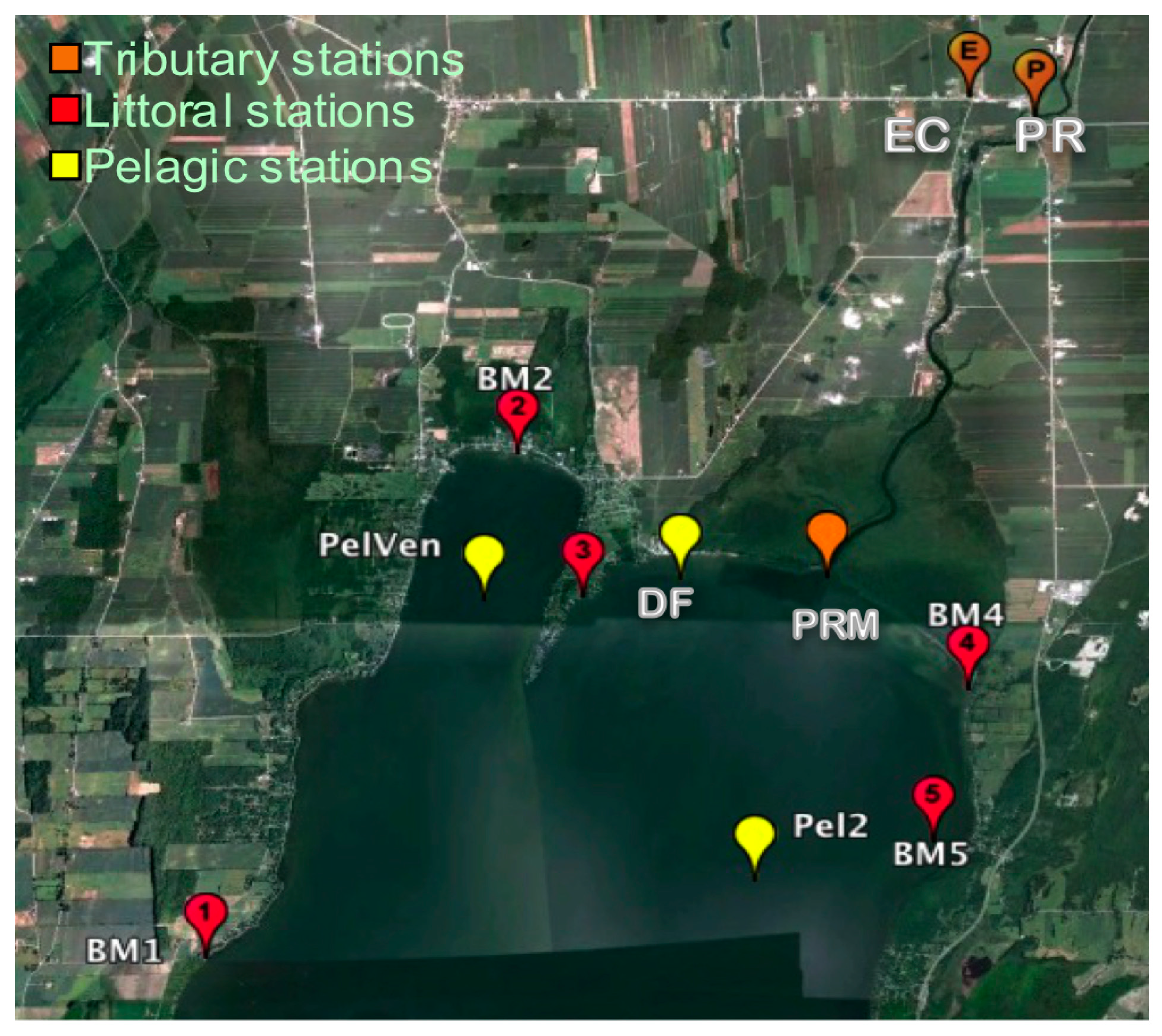
2.2. Sample Collection
2.3. Cyanobacterial Abundance, E. coli Counts and Physico-Chemical Parameters
2.4. Microcystin Concentration Analyses
2.5. Data and Statistical Analyses
2.6. DNA Sample Preparation, Extraction and Quantification
2.7. Quantitative PCR of Microcystis 16S rRNA and mcyD Genes
2.8. Cyanobacterial and Microcystis 16S rRNA Gene Amplification for High-Throughput Sequencing
2.9. Bioinformatic Analyses
3. Results
3.1. Physico-Chemical Parameters in Missisquoi Bay and Its Tributaries

| Parameter | Pelagic | Littoral | Tributaries |
|---|---|---|---|
| DP (μg/L) | 23.12 a | 30.24 a | 61.00 b |
| TP (μg/L) | 69.21 a | 70.84 a,b | 157.73 b |
| DN (mg/L) | 0.39 a | 0.50 b | 2.40 c |
| TN (mg/L) | 0.53 a | 0.74 b | 2.50 c |
3.2. Spatial Analysis of Phosphorus and Nitrogen Concentrations and Their Relationship with Rainfall
| Parameter | Cochran–Mantel–Haenszel (CSMH Correlation/p) | Spearman Rank Correlations (Rho Estimates/n/p) |
|---|---|---|
| DP | 18.72 (p < 0.0001) | 0.40/126 (p < 0.01) |
| TP | 4.37 (p = 0.0366) | 0.21/121 (p < 0.05) |
| DN | 48.46 (p < 0.0001) | 0.64/119 (p < 0.01) |
| TN | 55.63 (p < 0.0001) | 0.72/111 (p < 0.01) |
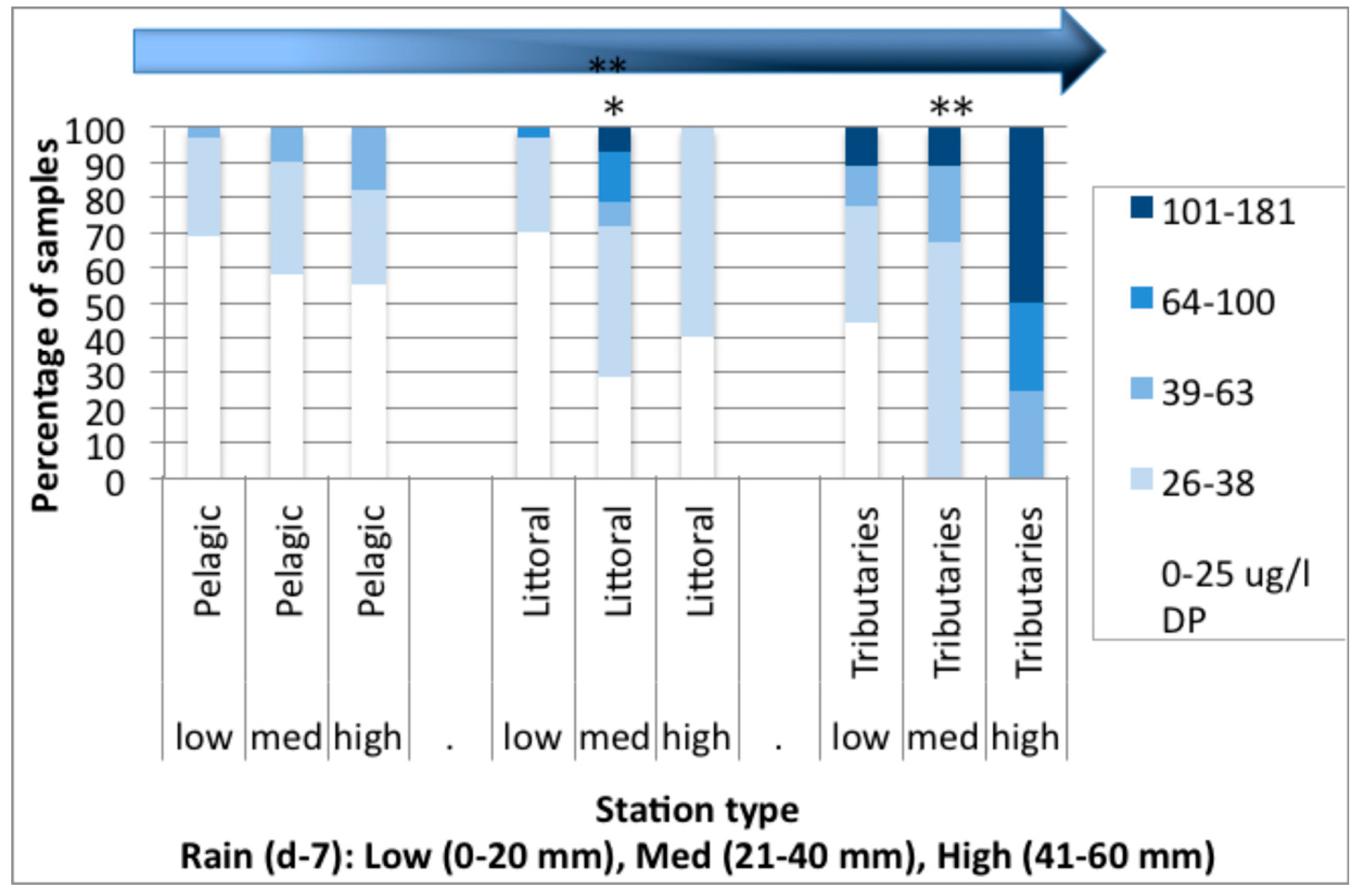
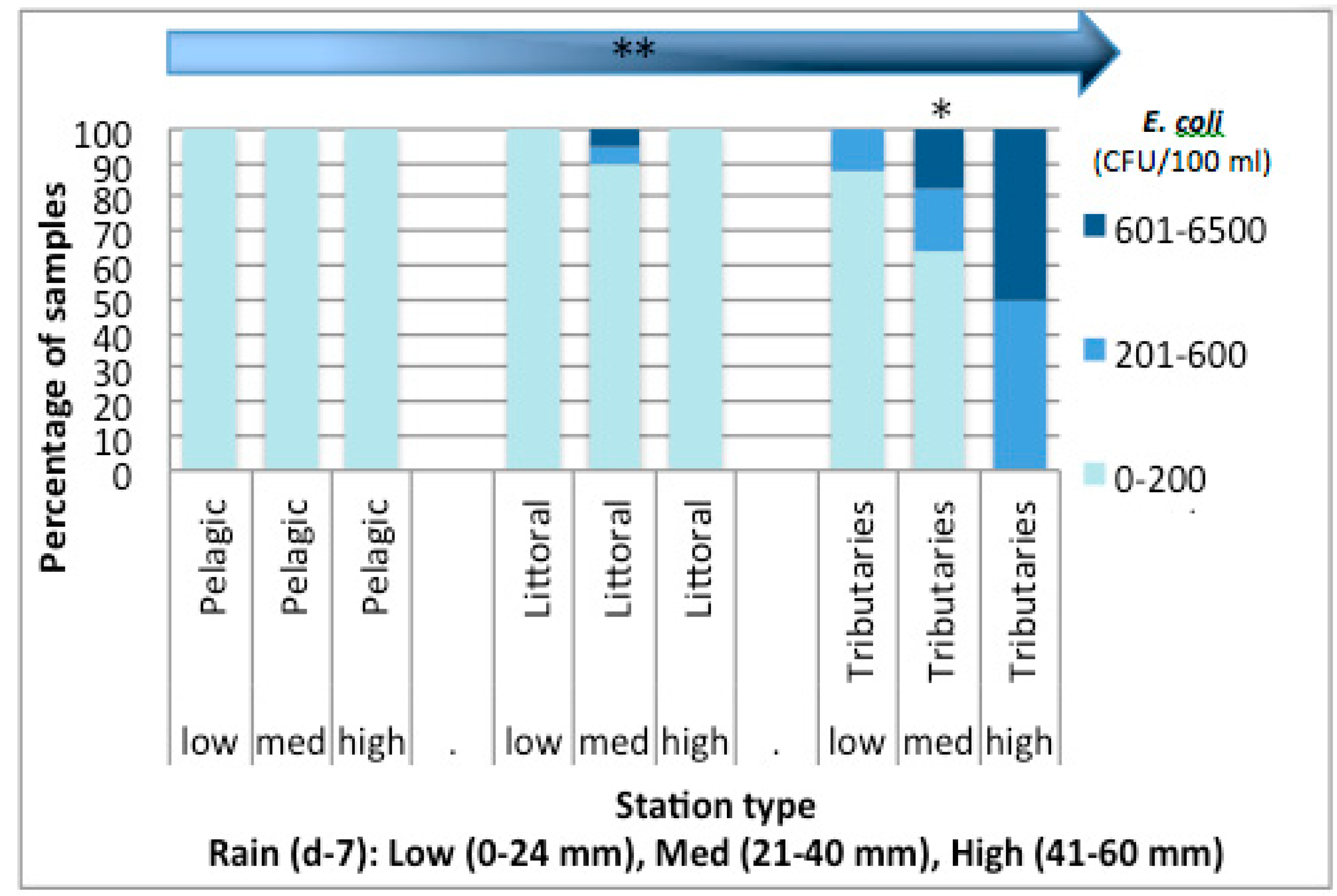
3.3. Nutrient Input in Relation to E. coli Abundance and Cyanobacterial Blooms in Missisquoi Bay and Pike River
| DATE | E. coli; Coliform Counts at Station: | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lit BM1 | Lit BM2 | Lit BM3 | Lit BM4 | Lit BM5 | Pel Ven | Pel 2 | EC | PR | PRM | DF | |
| 19 April 2009 | 0;0 | 25;85 | 70;260 | 25;85 | |||||||
| 26 April 2009 | 0;0 | 20;60 | 70;620 | ||||||||
| 5 May 2009 | 0;92 | 20;160 | 10;455 | ||||||||
| 8 May 2009 | 0;278 | 4;78 | |||||||||
| 20 May 2009 | 8;998 | 2;70 | 170;2020 | 185;2125 | 42;752 | 0;6 | |||||
| 6 June 2009 | 24;928 | 540;5120 | 60;920 | ||||||||
| 17 June 2009 | 0;64 | 0;TNC | 820;TNC | 470;TNC | 655;TNC | 12;736 | |||||
| 30 June 2009 | 0;74 | ||||||||||
| 19 July 2009 | 425;2725 | 2410;TNC | 90;3490 | 10;TNC | 5;1005 | 0;TNC | NSW | ||||
| 25 July 2009 | 5;TNC | 50;TNC | 5;TNC | 0;0 | 0;TNC | 0;545 | 0;1750 | 85;TNC | 10;TNC | 0;2400 | |
| 1 August 2009 | 35;TNC | 125;1850 | 0;90 | 10;TNC | 0;665 | NSW | NSW | ||||
| 8 August 2009 | 0;950 | 35;1385 | 0;20 | 0;815 | 5;515 | 0;230 | 0;0 | 55;2595 | 0;205 | ||
| 16 August 2009 | 0;2400 | 120;1120 | 0;2750 | 0;1900 | 0;1000 | 0;110 | 0;155 | ||||
| 22 August 2009 | 0;140 | 0;210 | 15;415 | 5;1555 | 0;210 | ||||||
| 31 August 2009 | 10;550 | TNC;TNC | 1420;TNC | ||||||||
| 23 September 2009 | 5;650 | 25;530 | 240;810 | 10;1395 | |||||||
| 10 November 2009 | 0;500 | 0;140 | 0;305 | 5;950 | 0;25 | 5;60 | |||||
| 6 December 2009 | 150;TNC | 330;TNC | |||||||||
| TP | DN | TN | E. coli | |
|---|---|---|---|---|
| DP | 0.48/122 (p < 0.01) | 0.50/117 (p < 0.01) | 0.40/111 (p < 0.01) | 0.51/61 (p < 0.01) |
| TP | 0.23/109 (p < 0.01) | 0.44/106 (p < 0.01) | 0.36/56 (p < 0.01) | |
| DN | 0.82/114 (p < 0.01) | 0.65/52 (p < 0.01) | ||
| TN | 0.63/46 (p < 0.01) |
3.4. Spatio-Temporal Characterization of Major Cyanobacterial Taxa and Their Association with Environmental Parameters in Missisquoi Bay/Pike River during 2009


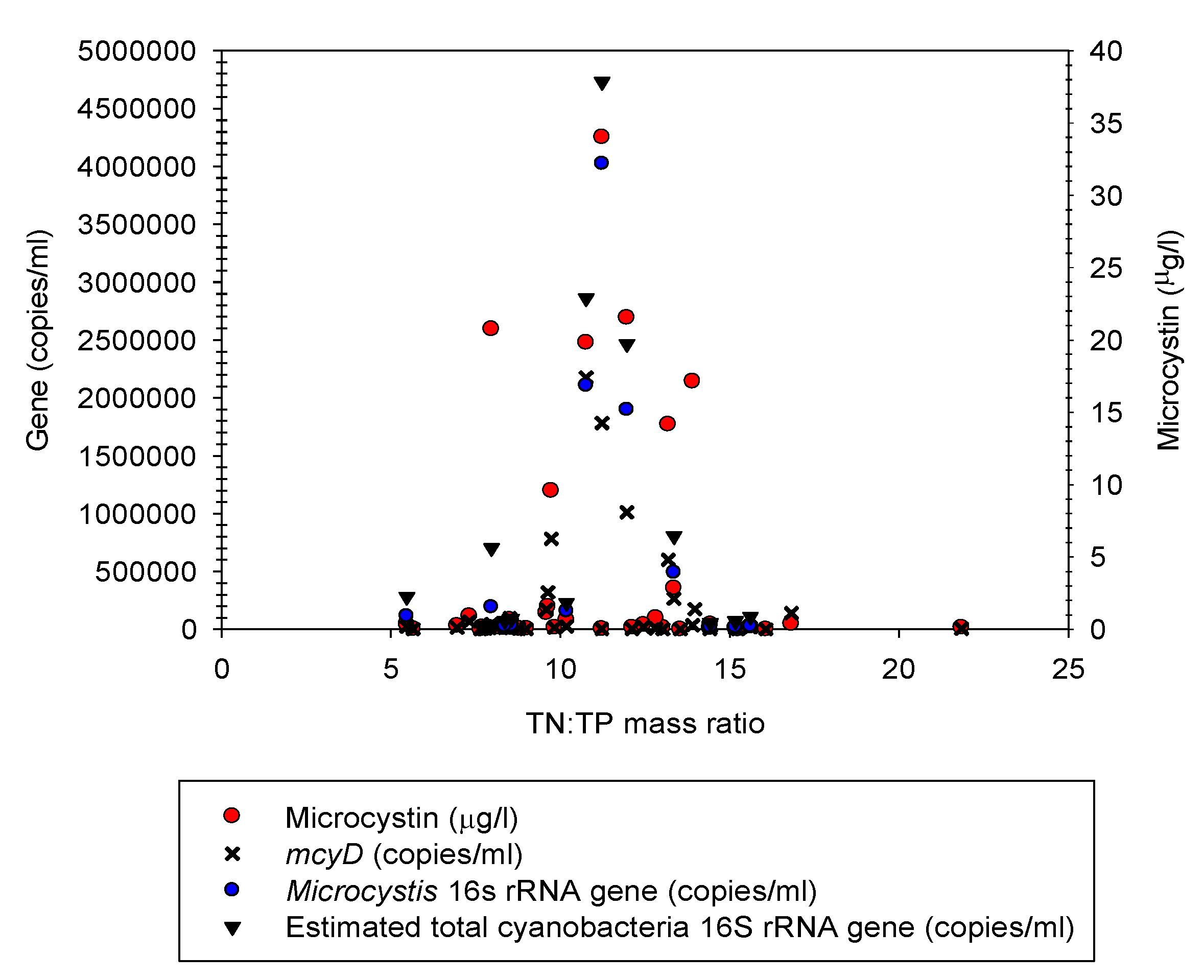
3.5. Microcystin Analyses
3.6. Q-PCR Analyses of Microcystis 16S rRNA and mcyD Genes
4. Discussion
4.1. Nutrient Input in Relation to Rainfall, E. coli and Cyanobacterial Blooms
4.2. Absolute Nutrient Concentrations, Nutrient Ratios, and the Dynamics of Microcystis
4.3. Major Cyanobacterial Taxa and Their Distribution Dynamics
4.4. Toxin and Q-PCR Analyses of 16S rRNA and mcyD Genes
4.5. The Impact of Climate Change on Cyanobacterial Blooms
4.6. Transboundary Challenges
5. Conclusions
Acknowledgments
Author Contributions
Appendix
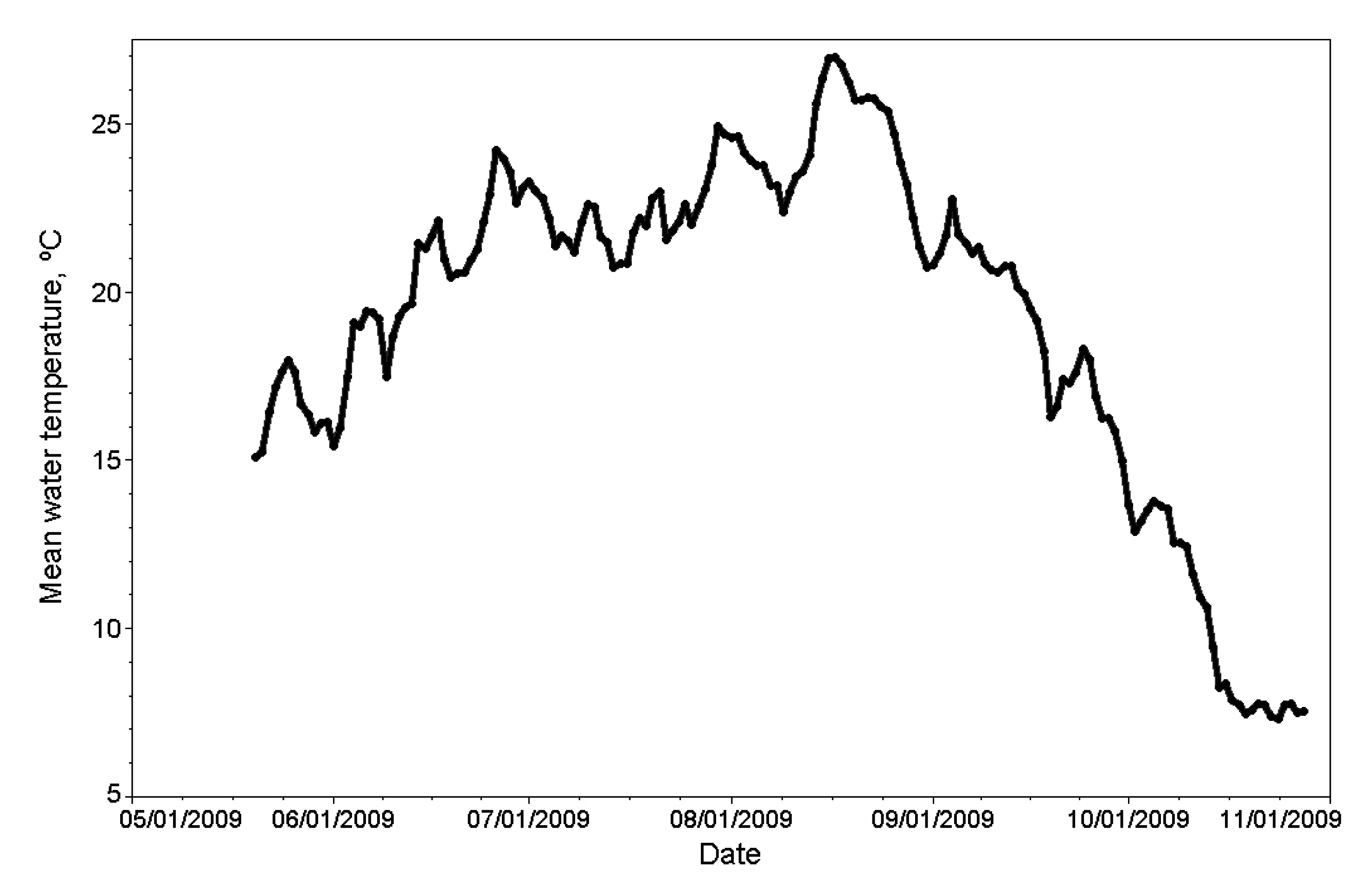

Conflicts of Interest
References
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, V. Eutrophication, toxic cyanobacteria and cyanotoxins: When ecosystems cry for help. Limnetica 2006, 25, 425–432. [Google Scholar]
- Davis, C. Evidence for the eutrophication of Lake Erie from phytoplankton records. Limnol. Oceanogr. 1964, 9, 275–283. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef]
- Lévesque, B.; Gervais, M.-C.; Chevalier, P.; Gauvin, D.; Anassour-Laouan-Sidi, E.; Gingras, S.; Fortin, N.; Brisson, G.; Greer, C.; Bird, D. Prospective study of acute health effects in relation to exposure to cyanobacteria. Sci. Tot. Environ. 2014, 466–467, 397–403. [Google Scholar] [CrossRef]
- Agence française de sécurité sanitaire des aliments et Agence française de sécurité sanitaire de l’environnement et du travail (AFSSA-AFSSET). Risques sanitaires liés à la présence de cyanobactéries dans l’eau; AFSSA-AFSSET: Paris, France, 2006. [Google Scholar]
- Stewart, I.; Webb, P.M.; Schluter, P.J.; Fleming, L.E.; Burns, J.W., Jr.; Gantar, M.; Baker, L.C.; Shaw, G.R. Epidemiology of recreational exposure to freshwater cyanobacteria-an international prospective cohort study. BMC Pub. Health 2006, 6, 93–103. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Azevedo, S.M.F.O.; An, J.S.; Molica, R.J.R.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R. An overview of problems caused by toxic blue-green algae (cyanobacteria) in drinking and recreational water. Environ. Toxicol. 1999, 14, 5–12. [Google Scholar] [CrossRef]
- Pilotto, L.S.; Douglas, R.M.; Burch, M.D.; Cameron, S.; Beers, M.; Rouch, G.J.; Robinson, P.; Kirk, M.; Cowie, C.T.; Hardiman, S.; Moore, C.; Attewell, R.G. Health effects of exposure to cyanobacteria (blue-green algae) during recreational water-related activities. Aust. N. Z. J. Public Health 1997, 21, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.R.; Runnegar, M.T.C.; Jackson, A.R.B.; Falconer, I.R. Severe hepatotoxicity caused by tropical cyanobacterium (blue-green algae) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Appl. Environ. Microbiol. 1985, 50, 1292–1295. [Google Scholar] [PubMed]
- Zamyadi, A.; MacLeod, S.L.; Fan, Y.; McQuaid, N.; Dorner, S.; Sauvé, S.; Prévost, M. Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: A monitoring and treatment challenge. Water Res. 2012, 46, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; McQuaid, N.; Prévost, M.; Dorner, S. Monitoring of potentially toxic cyanbacteria using an online multi-probe in drinking water sources. J. Environ. Monit. 2012, 14, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, E.; Fortin, N.; Greer, C.W.; Vo Duy, S.; Fayad, P.; Sauvé, S.; Prévost, M.; Dorner, S. Biodegradation of multiple microcystins and cylindrospermopsin in water and clarifier sludge: Effect of particulate matter. Ecotoxicol. Environ. Saf. 2014. submitted for publication. [Google Scholar]
- Zamyadi, A.; Dorner, S.; Sauvé, S.; Ellis, D.; Bolduc, A.; Bastien, C.; Prévost, M. Species-dependence of cyanobacteria removal efficiency by different drinking water treatment processes. Water Res. 2013, 47, 2689–2700. [Google Scholar] [CrossRef] [PubMed]
- Ghernaout, B.; Ghernaout, D.; Saiba, A. Algae and cyanotoxins removal by coagulation/flocculation: A review. Desalin. Water Treat. 2010, 20, 133–143. [Google Scholar] [CrossRef]
- Anderson, D.M.; Hoagland, P.; Kaoru, Y.; White, A.W. Estimated Annual Economic Impacts from Harmful Algal Blooms (HABs) in the United States; Technical Report WHOI-2000-11; Woods Hole Oceanographic Institution: Woods Hole, MA, USA, 2011; pp. 3–8. [Google Scholar]
- Fortin, N.; Aranda-Rodriguez, R.; Jing, H.; Pick, F.; Bird, D.; Greer, C.W. Detection of microcystin-producing cyanobacteria in Missisquoi Bay, Quebec, Canada, using quantitative PCR. Appl. Environ. Microbiol. 2010, 76, 5105–5112. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, S.; Briand, J.-F.; Leboulanger, C.; Avois-Jacquet, C.; Oberhaus, L.; Tassin, B.; Vinçon-Leite, B.; Paolini, G.; Druart, J.-C.; Anneville, O.; Humbert, J.-F. The proliferation of the toxic cyanobacterium Planktothrix rubescens following restoration of the largest natural French lake (Lac du Bourget). Harm. Alg. 2005, 4, 651–672. [Google Scholar] [CrossRef]
- Geider, R.J. Light and temperature dependence of the carbon to chlorophyll a ratio in microalgae and cyanobacteria: Implications for physiology and growth of phytoplankton. New Phytol. 1987, 106, 1–34. [Google Scholar] [CrossRef]
- Davis, T.W.; Harke, M.J.; Marcoval, M.A.; Goleski, J.; Orano-Dawson, C.; Berry, D.L.; Gobler, C.J. Effects of nitrogenous compounds and phosphorus on the growth of toxic and non-toxic strains of Microcystis during cyanobacterial blooms. Aqua. Microbiol. Ecol. 2010, 61, 149–162. [Google Scholar] [CrossRef]
- Gobler, C.J.; Davis, T.W.; Coyne, K.J.; Boyer, G.L. Interactive influences of nutrient loading, zooplankton grazing, and microcystin synthetase gene expression on cyanobacterial bloom dynamics in a eutrophic New York lake. Harmful Algae 2007, 6, 119–133. [Google Scholar] [CrossRef]
- Huisman, J.; Sharples, J.; Stroom, J.M.; Visser, P.M.; Kardinaal, W.E.A.; Verspagen, J.M.H.; Sommeijer, B. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 2004, 85, 2960–2970. [Google Scholar] [CrossRef]
- Soares, M.C.S.; Marinho, M.M.; Azevedo, S.M.O.F.C.; Branco, W.C.; Huszar, V.L.M. Eutrophication and retention time affecting spatial heterogeneity in a tropical reservoir. Limnol. Ecol. Manag. Inland Waters 2012, 42, 197–203. [Google Scholar] [CrossRef]
- Paerl, H.W. Nuisance phytoplankton blooms in coastal, estuarine, and inland waters. Limnol. Oceanogr. 1988, 33, 823–847. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.M.; Konopko, E.A.; DeBruyn, J.M.; Bourbonniere, R.A.; Boyer, G.L.; Wilhelm, S.W. Lake Erie Microcystis: Relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae 2009, 8, 665–673. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Chen, Y. The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu, China: An 11-year investigation. Harmful Algae 2011, 10, 337–343. [Google Scholar] [CrossRef]
- Te, S.H.; Gin, K.Y.-H. The dynamics of cyanobacteria and microcystin production in a tropical reservoir of Singapore. Harmful Algae 2011, 10, 319–329. [Google Scholar] [CrossRef]
- Al-Tebrineh, J.; Merrick, C.; Ryan, D.; Humpage, A.; Bowling, L.; Neilan, B.A. Community composition, toxigenicity, and environmental conditions during a cyanobacterial bloom occurring along 1100 Kilometers of the Murray River. Appl. Environ. Microbiol. 2012, 78, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sitoki, L.; Kurmayer, R.; Rott, E. Spatial variation of phytoplankton composition, biovolume, and resulting microcystin concentrations in the Nyanza Gulf (Lake Victoria, Kenya). Hydrobiologia 2012, 691, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Werner, V.R.; Laughinghouse, H.D.; Fiore, M.F.; Sant’Anna, C.L.; Hoff, C.; de Souza Santos, K.R.; Neuhaus, E.B.; Molica, R.J.R.; Honda, R.Y.; Echenique, R.O. Morphological and molecular studies of Sphaerospermopsis torques-reginae (Cyanobacteria, Nostocales) from South American water blooms. Phycologia 2012, 51, 228–238. [Google Scholar] [CrossRef]
- Owens, O.V.H.; Esaias, W.E. Physiological responses of phytoplankton to major environmental factors. Annu. Rev. Plant Physiol. 1976, 27, 461–483. [Google Scholar] [CrossRef]
- Hecky, R.E.; Kilham, P. Nutrient limitation of phytoplankton in freshwater and marine environments: A review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 1988, 33, 796–822. [Google Scholar] [CrossRef]
- Schindler, D.W. The dilemma of controlling cultural eutrophication of lakes. Proc. R. Soc. B Biolog. Sci. 2012, 279, 4322–4333. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Nat. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed]
- Kotak, B.G.; Lam, A.K.Y.; Prepas, E.E.; Hrudey, S.E. Role of chemical and physical variables in regulating microcystin-LR concentrations in phytoplankton of eutrophic lakes. Can. J. Fish. Aquat. Sci. 2000, 57, 1584–1593. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T. Throwing fuel on the fire: Synergistic effects of excessive nitrogen inputs and global warming on harmful algal blooms. Environ. Sci. Technol. 2010, 44, 7756–7758. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic change. Sci. Tot. Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef]
- Lewis, W.M., Jr.; Wurtsbaugh, W.A.; Paerl, H.W. Rationale for control of anthropogenic nitrogen and phosphorus to reduce eutrophication of inland waters. Environ. Sci. Technol. 2011, 45, 10300–10305. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Gao, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Elser, J.J.; Marzolf, E.R.; Goldman, C.R. Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: A review and critique of experimental enrichments. Can. J. Fish. Aquat. Sci. 1990, 47, 1468–1477. [Google Scholar] [CrossRef]
- Sakamoto, M. Primary production by phytoplankton community in some Japanese lakes and its dependence on lake depth. Arch. Hydrobiol. 1966, 62, 1–28. [Google Scholar]
- Smith, V.H. The nitrogen and phosphorus dependance of algal biomass in lakes: An empirical and theoretical analysis. Limnol. Oceanogr. 1982, 27, 1101–1112. [Google Scholar] [CrossRef]
- Smith, V.H. Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science 1983, 221, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H.; Bierman, V.J.; Jones, B.L.; Havens, K.E. Historical trends in the Lake Okeechobee ecosystem IV. Nitrogen:phosphorus ratios, cyanobacterial dominance, and nitrogen fixation potential. Arch. Hydrobiol. 1995, 107, 71–88. [Google Scholar]
- Reynolds, C. What factors influence the species composition of phytoplankton in lakes of different trophic status? Hydrobiologia 1998, 369–370, 11–26. [Google Scholar] [CrossRef]
- Deposition of Air Pollutants to the Great Waters; Second Report to Congress, Report No. EPA-453/R-97-011; Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency: Research Triangle Park, NC, USA, 1997.
- Levine, S.N.; Lini, A.; Ostrofsky, M.L.; Bunting, L.; Burgess, H.; Leavitt, P.R.; Reuter, D.; Lami, A.; Guilizzoni, P.; Gilles, E. The eutrophication of Lake Champlain’s northeastern arm: Insights from paleolimnological analyses. J. Great Lakes Res. 2012, 38, 35–48. [Google Scholar] [CrossRef]
- Smeltzer, E.; Shambaugh, A.D.; Stangel, P. Environmental change in Lake Champlain revealed by long-term monitoring. J. Great Lakes Res. 2012, 38, 6–18. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Algae and cyanobacteria in fresh water. In Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2003; Volume 1, Coastal and fresh waters; pp. 136–158. [Google Scholar]
- Beck, E.; van Bochove, E.; Smeltzer, E.; Leblanc, D. International Missisquoi Bay Study Board. Missisquoi Bay Critical Source Area Study, Final report to the International Joint Commission. 15 February 2012. Available online: http://www.ijc.org/files/publications/HH5.pdf (accessed on 14 January 2015).
- State of Vermont Natural Resources Board, Water Resources Panel. Vermont Water Quality Standards. Vt. Code R. 12 004 052. January 2008. Available online: http://www.nrb.state.vt.us/wrp/publications/wqs.pdf (accessed on 14 January 2015).
- Simoneau, M. État de l’écosystème aquatique du bassin versant de la baie Missisquoi. Available online: http://www.mddelcc.gouv.qc.ca/Eau/bassinversant/bassins/missisquoi/faits.htm (accessed on 4 May 2015).
- Troy, A. Updating the Lake Champlain basin land use data to improve prediction of phosphorus loading. In Technical Report No.34; Wang, D., Ed.; Lake Champlain Basin Program: Grand Isle, VT, USA, 2007. [Google Scholar]
- Hegman, W.; Wang, D.; Borer, C. Estimation of Lake Champlain basin wide nonpoint source phosphorus export. In Technical Report No. 31; Lake Champlain Basin Program: Grand Isle, VT, USA, 1999. [Google Scholar]
- Madramootoo, C.A.; Michaud, A.R.; Whalen, J.; Poirier, S.-C. Mesures précises et approches innovantes en modélisation de la dynamique des nutriments en bassin et en plan d’eau contribuant aux fleurs des cyanobactéries. Rapport finale de recherche; No.2009-CY-130646, Programme de recherche en partenariat sur les cyanobactéries; Fonds québécois de la recherche sur la nature et les technologies et Fonds de la recherche en santé du Québec: Quebec, QC, Canada, 2012. [Google Scholar]
- Gasser, M.; Bolinder, O.M.; Martel, S.; Poulin, D.; Beaudin, I.; Michaud, A.R.; Drouin, A. Impacts Agroenvironnementaux Associés à la Culture et au Prélèvement de Biomasses Végétales Agricoles Pour la Production de Bioproduits Industriels; Rapport Final; Institut de recherche et de développement en agroenvironnement (IRDA): Quebec, Canada, 2010; pp. 20–42. [Google Scholar]
- Michaud, A.; Laverdière, M.R. Cropping, soil type and manure application effects on phosphorus export and bioavailability. Can. J. Soil Sci. 2004, 84, 295–305. [Google Scholar] [CrossRef]
- Howery, L.D.; Pfister, J.A. Dietary and fecal concentrations of nitrogen and phosphorus in penned white-tailed deer does. J. Wild. Man. 1990, 54, 383–389. [Google Scholar] [CrossRef]
- Kirchmann, H.; Pettersson, S. Human urine—Chemical composition and fertilizer use efficiency. Nutr. Cycl. Agroecosyst. 1994, 40, 149–154. [Google Scholar]
- Sheldrick, W.F.; Syers, J.K.; Lingard, J. Contribution of livestock excreta to nutrient balances. Nutr. Cycl. Agrosyst. 2003, 66, 119–131. [Google Scholar]
- Iyengar, V.; Albaugh, G.P.; Lohani, A.; Nair, P.P. Human stools as a source of viable colonic epithelial cells. FASEB J. 1991, 5, 2856–2859. [Google Scholar] [PubMed]
- Valiere, N.; Taberlet, P. Urine collected in the field as a source of DNA for species and individual identification. Mol. Ecol. 2000, 9, 2150–2152. [Google Scholar] [CrossRef] [PubMed]
- Padia, R.; Karthikeyan, R.; Mukhtar, S.; Parker, I. Occurrence and fate of E. coli from various non-point sources in a subtropical watershed. J. Nat. Environ. Sci. 2012, 3, 9–18. [Google Scholar]
- Hardina, C.M.; Fujioka, R.S. Soil: The environmental source of Escherichia coli and enterococci in Hawaii’s streams. Environ. Toxicol. Water. Qual. 1991, 6, 185–195. [Google Scholar] [CrossRef]
- Byappanahalli, M. Indigenous soil bacteria and low moisture may limit but allow faecal bacteria to multiply and become a minor population in tropical soils. Water Sci. Technol. 2004, 50, 27–32. [Google Scholar] [PubMed]
- Ishii, S.; Ksoll, W.B.; Hicks, R.E.; Sadowsky, M.J. Presence and growth of naturalized Escherichia coli in temperate soils from Lake Superior watersheds. Appl. Environ. Microbiol. 2006, 72, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Whitman, R.L.; Nevers, M.B.; Korinek, G.C.; Byappanahalli, M.N. Solar and temporal effects on Escherichia coli concentration at a Lake Michigan swimming beach. Appl. Environ. Microbiol. 2004, 70, 4276–4285. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Hulot, F.D. Population dynamics of harmful cyanobacteria. In Harmful Cyanobacteria; Huisman, J., Matthijs, H.C.P., Visser, P.M., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 143–176. [Google Scholar]
- Google Earth. Available online: http://www.google.com/earth/ (accessed on 3 May 2015).
- Utermöhl, H. Zur Vervolkommnung der quantitativen phytoplankton-Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Centre d’expertise en analyses environnementales du Québec (CEAEQ). Recherche et Dénombrement Simultanés des Coliformes Totaux et d’Escherichia coli dans l’eau Potable avec le Milieu de Culture MI; Méthode par Filtration sur Membrane, MA.700-ECCtmi 1.0, Rév.1; Ministère du Développement durable, de l’Environnement et des Parcs du Québec: Québec, Canada, 2006. [Google Scholar]
- Environment Canada. Available online: http://climat.meteo.gc.ca/climateData/dailydata_f.html?timeframe=2&Prov=&StationID=5431&Year=2009 (accessed on 19 January 2015).
- Wetzel, R.G.; Likens, G.E. Inorganic nutrients: Nitrogen, phosphorus and other nutrients. In Limnological Analyses, 3rd ed.; Wetzel, R.G., Likens, G.E., Eds.; Springer: New York, NY, USA, 2000; pp. 85–111. [Google Scholar]
- Patton, C.J.; Kryskalla, J.R. Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory—Evaluation of alkaline persulfate digestion as an alternative to Kjeldahl digestion for determination of total and dissolved nitrogen and phosphorus in water. U.S. Geological Survey, Water-Resources Investigations 2003, Report 03-4174. Available online: http://nwql.usgs.gov/rpt.shtml?WRIR-03-4174 (accessed on 3 May 2015).
- Munoz-Ramos, V. Relationship between Toxic Cyanobacterial Blooms, Physico-Chemical Factors and Potential Multiple Source Excretra Contamination in an Affected Watershed. Master’s Thesis, McGill University, Montreal, QC, Canada, April 2013. [Google Scholar]
- NCBI Primer-BLAST program. Available online: http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome (accessed on 6 May 2015).
- Ashelford, K.E.; Weightman, A.J.; Fry, J.C. PRIMROSE: A computer program for generating and estimating the phylogenetic range of 16S rRNA oligonucleotide probes and primers in conjunction with theRDP-II database. Nucleic Acids Res. 2002, 109, 85–101. [Google Scholar]
- Yergeau, E.; Lawrence, J.R.; Sanschagrin, S.; Waiser, M.J.; Korber, D.R.; Greer, C.W. Next-generation sequencing of microbial communities in the Athabasca River and its tributaries in relation to oil sands mining activities. Appl. Environ. Microbiol. 2012, 78, 7626–7637. [Google Scholar] [CrossRef] [PubMed]
- Engene, N.; Gerwick, W.H. Intra–genomic 16S rRNA gene heterogeneity in cyanobacterial genomes. Fottea 2011, 1, 17–24. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Mitra, S.; Ruscheweyh, H.-J.; Weber, N.; Schuster, S.C. Integrative analysis of environmental sequences using MEGAN4. Gen. Res. 2011, 21, 1552–1560. [Google Scholar] [CrossRef]
- Wacklin, P.; Hoffmann, L.; Komárek, J. Nomenclatural validation of the genetically revised cyanobacterial genus Dolichospermum (Ralfs ex BoRnet et flahault) comb. nova. Fottea 2009, 9, 59–64. [Google Scholar] [CrossRef]
- Bornet, E.; Flahault, C. Révision des Nostocacées hétérocystées. Ann. Sci. Nat.-Bot. 1888, 7, 171–262. [Google Scholar]
- Klebahn, H. Anabaena spiroides Klebahn. Flor. Bot. Ztg. 1895, 268, 1895. [Google Scholar]
- Testa, J.M.; Kemp, W.M. Hypoxia-induced shifts in nitrogen and phosphorus cycling in Chesapeake Bay. Limnol. Oceanogr. 2012, 57, 835–850. [Google Scholar] [CrossRef]
- Enright, P.; Madramootoo, C.A. Phosphorus losses in in surface runoff and subsurface drainage waters on two agricultural fields in Quebec. In Proceedings of the 8th International Drainage Symposium—Drainage VIII, ASAE, St. Joseph, MI, Sacramento, CA, USA, 21–24 March 2004.
- Rasouli, S. Soluble and Particulate Nitrogen Losses from Tile Drained Agricultural Fields in Southern Quebec, Canada. Ph.D. Thesis, McGill University, Montreal, QC, Canada, August 2013. [Google Scholar]
- Johnston, W.R.; Ittihadieh, F.; Daum, R.M.; Pillsbury, A.F. Nitrogen and phosphorus in tile drainage effluent. Soil Sci. Soc. Am. J. 1965, 29, 287–289. [Google Scholar] [CrossRef]
- Simard, G. Nutrient Transport from Agricultural Fields. Master’s Thesis, McGill University, Montreal, QC, Canada, August 2005. [Google Scholar]
- Michaud, A.; Deslandes, J.; Beaudin, I. Modélisation de l’Hydrologie et des Dynamiques de Pollution Diffuse dans le Basin-Versant de la Rivière aux Brochets à l’aide du modèle SWAT; Final Report; Institut de Recherche et de Développement en Agroenvironnement (IRDA): Quebec, QC, Canada, 2006. [Google Scholar]
- Lewandoski, A. Manure Management-Soil Scientist, Bullletin 07401. Available online: http://www.extension.umn.edu/agriculture/tillage/soil-management/soil-management-series/manure-management/#soil-scientist (accessed on 14 January 2015).
- Meals, D.W.; Braun, D.C. Demonstration of Methods to Reduce Indicator Bacteria Levels in Agriculture Runoff in Vermont; Final Report SEI #021334-F; Lake Champlain Basin Program: Grand Isle, VT, USA, 2005. [Google Scholar]
- Ministére des affaires municipales et de l’occupation du territoire (MAMROT 2009). Evaluation de performance des ouvrages municipaux d’assainissement des eaux pour l’année 2009. Available online: http://www.mamrot.gouv.qc.ca/pub/infrastructures/suivi_ouvrages_assainissement_eaux/eval_perform_rapport_2009.pdf (accessed on 19 January 2015).
- Ginn, H.P.; Pearson, L.A.; Neilan, B.A. Ntc A from Microcystis Aeruginosa PCC7806 is autoregulatory and binds to the microcystin promoter. Appl. Environ. Microbiol. 2010, 76, 4362–4368. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.R.; Rizzo, D.M.; Watzin, M.C.; Druschel, G.K. Unraveling associations between cyanobacteria blooms and in-lake-environmental conditions in Missisquoi Bay, Lake Champlain, USA, using a modified self-organizing map. Environ. Sci. Technol. 2013, 47, 14267–14274. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, J.M.; Collier, D.C.; Welch, E.B.; Hardy, F.J.; Crayton, M. Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Can. J. Fish. Aquat. Sci. 2000, 57, 231–240. [Google Scholar] [CrossRef]
- Tilman, D.; Kilham, S.S.; Kilham, P. Phytoplankton community ecology: The role of limiting nutrients. Annu. Rev. Ecol. Evol. Syst. 1982, 13, 349–372. [Google Scholar] [CrossRef]
- Rhee, G.Y.; Gotham, I.J. Optimum N:P ratios and coexistence of planktonic algae. J. Phycol. 1980, 16, 486–489. [Google Scholar] [CrossRef]
- Oh, H.-M.; Rhee, G.Y. A comparative study of microalgae isolated from flooded rice paddies: Light-limited growth, C fixation, growth efficiency and relative N and P requirement. J. Appl. Phycol. 1991, 3, 211–220. [Google Scholar] [CrossRef]
- Bulgakov, N.G.; Levich, A.P. The nitrogen:phosphorus ratio as a factor regulating phytoplankton community structure. Arch. Hydrobiol. 1999, 146, 3–22. [Google Scholar]
- Li, Y.; Li, D. Competition between toxic Microcystis aeruginosa and nontoxic Microcystis wesenbergii with Anabaena PCC7120. J. Appl. Phycol. 2012, 24, 69–78. [Google Scholar] [CrossRef]
- Briand, E.; Bormans, M.; Quiblier, C.; Salençon, M.-J.; Humbert, J.-F. Evidence of the cost of the production of microcystins by Microcystis aeruginosa under differing light and nitrate environmental conditions. PLoS ONE 2012, 7, e29981. [Google Scholar] [CrossRef] [PubMed]
- Schatz, D.; Keren, Y.; Vardi, A.; Sukenik, A.; Carmeli, S.; Börner, T.; Dittmann, E.; Kaplan, A. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol. 2007, 9, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Kardinaal, W.E.A.; Tonk, L.; Janse, I.; Hol, S.; Slot, P.; Huisman, J.; Visser, P.M. Competition for light between toxic and nontoxic strains of the harmful cyanobacterium Microcystis. Appl. Environ. Microbiol. 2007, 73, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.S. Nutrient uptake. In Ecology of Phytoplankton; Usher, M., Saunders, D., Peet, R., Dobson, A., Eds.; Cambridge University Press: Cambridge, New York, NY, USA, 2006; pp. 145–177. [Google Scholar]
- Haselkorn, R. Heterocyst differentiation and nitrogen fixation in Anabaena. In Biological Nitrogen Fixation, Sustainable Agriculture and the Environment; Wang, Y.-P., Lin, M., Tian, Z.-X., Elmerich, C., Newton, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 41, pp. 65–68. [Google Scholar]
- Robarts, R.D.; Zohary, T. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. N. Z. J. Mar. Freshw. Res. 1987, 21, 391–399. [Google Scholar] [CrossRef]
- Chu, Z.; Jin, X.; Yang, B.; Zeng, Q. Buoyancy regulation of Microcystis flos-aquae during phosphorus-limited and nitrogen-limited growth. J. Plank. Res. 2007, 29, 739–745. [Google Scholar] [CrossRef]
- Brookes, J.D.; Ganf, G.G. Variations in the buoyancy response of Microcystis aeruginosa to nitrogen, phosphorus and light. J. Plank. Res. 2001, 23, 1399–1411. [Google Scholar] [CrossRef]
- Ngwa, F.; Madramootoo, C.; Jabaji, S. Monitoring toxigenic Microcystis strains in the Missisquoi Bay, Quebec, by PCR targeting multiple toxic gene loci. Microbiol. Open 2012. [Google Scholar] [CrossRef]
- Ngwa, F.; Madramootoo, C.; Jabaji, S. Development and application of a multiplex qPCR technique to detect multiple microcystin-producing cyanobacterial genera in a Canadian freshwater lake. J. Appl. Phycol. 2014, 26, 1675–1687. [Google Scholar] [CrossRef]
- Xie, L.; Xie, P.; Li, S.; Tang, H.; Liu, H. The low TN:TP ratio, a cause or a result of Microcystis blooms? Water Res. 2003, 37, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Orihel, D.M.; Bird, D.F.; Brylinsky, M.; Chen, H.; Donald, D.B.; Huang, D.Y.; Giani, A.; Kinniburgh, D.; Kling, H.; Kotak, B.G.; et al. High microcystin concentrations occur only at low nitrogen-to-phosphorus ratios in nutrient-rich Canadian lakes. Can. J. Fish. Aquat. Sci. 2012, 69, 1457–1462. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Davidson, T.A.; Trolle, D.; Søndergaard, M.; Lauridsen, T.L.; Beklioğlu, M.; Brucet, S.; Volta, P.; González-Bergonzoni, I.; et al. Climate change impacts on lakes: An integrated ecological perspective based on a multi-faceted approach, with special focus on shallow lakes. J. Limonol. 2014, 73, 88–111. [Google Scholar]
- Rigosi, A.; Carey, C.C.; Ibelings, B.W.; Brookes, J.D. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 2014, 59, 99–114. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Bécares, E.; Costa, L.S.; van Donk, E.; Hansson, L.-A.; Jeppesen, E.; Krug, C.; Lacerot, G.; Mazzeo, N.; et al. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Chang. Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- El-Shehawy, R.; Gorokhova, E.; Fernandez-Pinas, F.; del Campo, F.F. Global warming an hepatotoxin production by cyanobacteria: What can we learn from experiments? Wat. Res. 2012, 46, 1420–1429. [Google Scholar] [CrossRef]
- Reichwaldt, E.S.; Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: Between simplistic scenarios and complex dynamics. Water. Res. 2012, 46, 1372–1393. [Google Scholar] [CrossRef] [PubMed]
- Maske, S.; Sangolkar, L.; Chakrabarti, T. Temporal variation in density and diversity of cyanobacteria and cyanotoxins in lakes at Nagpur (Maharashtra State), India. Environ. Monit. Assess. 2010, 169, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Jöhnk, K.D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, P.M.; Strooms, J.M. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Chang. Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef]
- Takamura, N.; Iwakuma, T.; Yasuno, M. Photosynthesis and primary production of Microcystis aeruginosa Kütz. in Lake Kasumigaura. J. Plankton. Res. 1985, 7, 303–312. [Google Scholar] [CrossRef]
- Konopka, A.; Brock, T.D. Effect of temperature on blue-green algae (cyanobacteria) in Lake Mendota. Appl. Environ. Microbiol. 1978, 36, 572–576. [Google Scholar] [PubMed]
- Jensen, H.S.; Andersen, F.O. Importance of temperature, nitrate, and pH for phosphate release from aerobic sediments of four shallow, eutrophic lakes. Limnol. Oceanogr. 1992, 37, 577–589. [Google Scholar] [CrossRef]
- Shanley, J.B.; Denner, J.C. The hydrology of the Lake Champlain basin. In Lake Champlain in Transition: From Research toward Restoration; Manley, T.O., Manley, P.L., Eds.; Americal Geophysical Union: Washington, DC, USA, 1999; pp. 41–67. [Google Scholar]
- Castle, S.S.; Howe, E.A.; Bird, E.L.; Howland, W.G. Flood Resilience in the Lake Champlain basin and upper Richelieu River; Final Report; Lake Champlain Basin Program: Grand Isle, VT, USA, 2013. [Google Scholar]
- Osherenko, G. Understanding the failure to reduce phosphorus loading in Lake Champlain: Lessons for governance. Vt. J. Environ. Law 2014, 15, 323–360. [Google Scholar]
- Patoine, M.; D’Auteuil-Potvin, F. Tendances de la qualité de l’eau de 1999 à 2008 dans dix Bassins Versants Agricoles au Québec; Ministère du Développement durable, de l’Environnement, de la Faune et des Parcs, Direction du suivi de l’état de l’Environnement: Quebec, QC, Canada, 2013. [Google Scholar]
- Medalie, L.; Hirsch, R.M.; Archfield, S.A. Use of flow-normalization to evaluate nutrient concentration and flux changes in Lake Champlain tributaries, 1990–2009. J. Great Lakes Res. 2012, 38, 58–67. [Google Scholar] [CrossRef]
- USGS Inundation Map & USGS/NOAA Lake Champlain Gage. Available online: http://waterdata.usgs.gov/vt/nwis/uv?site_no=04294500 (accessed on 14 January 2015).
- Ministère des Affaires Municipales et de l’Occupation du Territoire. Evaluation de Performance des OMAE Pour l’année 2011. Available online: http://www.mamrot.gouv.qc.ca/pub/infrastructures/suivi_ouvrages_assainissement_eaux/annexe_bilan_partie_1.pdf (accessed on 19 January 2015).
- Beaulieu, M.; Pick, F.; Gregory-Eaves, I. Nutrients and water temperature are significant predictors of cyanobacterial biomass in a 1147 lakes data set. Limnol. Oceanogr. 2013, 58, 1736–1746. [Google Scholar] [CrossRef]
- Leavitt, P. Degradation and destabilization of lake ecosystems by anthropogenic nitrogen: A new paradigm for future lake eutrophication. In Proceedings of the 60e Conférence Canadienne de la Recherche sur les Pêches et Rencontre Annuelle de la Société Canadienne de Limnologie, Montréal, QC, Canada, 4–6 January 2007.
- Blomqvisti, P.; Pettersson, A.; Hyenstrand, P. Ammonium-nitrogen: A key regulatory factor causing dominance of non-nitrogen-fixing cyanobacteria in aquatic systems. Arch. Hydrobiol. 1994, 132, 141–164. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortin, N.; Munoz-Ramos, V.; Bird, D.; Lévesque, B.; Whyte, L.G.; Greer, C.W. Toxic Cyanobacterial Bloom Triggers in Missisquoi Bay, Lake Champlain, as Determined by Next-Generation Sequencing and Quantitative PCR. Life 2015, 5, 1346-1380. https://doi.org/10.3390/life5021346
Fortin N, Munoz-Ramos V, Bird D, Lévesque B, Whyte LG, Greer CW. Toxic Cyanobacterial Bloom Triggers in Missisquoi Bay, Lake Champlain, as Determined by Next-Generation Sequencing and Quantitative PCR. Life. 2015; 5(2):1346-1380. https://doi.org/10.3390/life5021346
Chicago/Turabian StyleFortin, Nathalie, Valentina Munoz-Ramos, David Bird, Benoît Lévesque, Lyle G. Whyte, and Charles W. Greer. 2015. "Toxic Cyanobacterial Bloom Triggers in Missisquoi Bay, Lake Champlain, as Determined by Next-Generation Sequencing and Quantitative PCR" Life 5, no. 2: 1346-1380. https://doi.org/10.3390/life5021346
APA StyleFortin, N., Munoz-Ramos, V., Bird, D., Lévesque, B., Whyte, L. G., & Greer, C. W. (2015). Toxic Cyanobacterial Bloom Triggers in Missisquoi Bay, Lake Champlain, as Determined by Next-Generation Sequencing and Quantitative PCR. Life, 5(2), 1346-1380. https://doi.org/10.3390/life5021346





