Photosynthesis in Hydrogen-Dominated Atmospheres
Abstract
:1. Introduction
1.1. Role and Evolution of Photosynthesis
1.2. Photosynthesis beyond Earth
1.3. Hydrogen-Rich Rocky Exoplanets
2. Approach, Methods and Data Sources
2.1. Overall Approach
2.2. Energy Calculations
3. Results
3.1. Carbon-Containing Species in an H2-Dominated Atmosphere
- (1)
- Minimal methane: atmospheric carbon present almost entirely as CO or CO2, because there is no life to generate methane, and only a small amount of methane is outgassed.
- (2)
- Methane and carbon dioxide: atmospheric carbon present as methane and carbon dioxide in a ratio of between 1:10 and 10:1, because of higher rates of outgassing of methane than on Earth and/or some limited life.
- (3)
- High methane: atmospheric carbon present almost entirely as methane, because carbon is outgassed almost entirely as methane (an unlikely scenario, but possible) and/or because life is abundant.
| Element | Dominant Environmental Form | |
|---|---|---|
| Oxidized Environment | Reduced Environment | |
| C | CO2 | CH4 |
| S | SO42− | H2S |
| N | N2 | N2 |
| P | PO42− | PO42− |
| O | H2O | H2O |
3.2. Overall Reaction for Photosynthesis in a CH4/H2 Atmosphere
3.3. Energy Requirements for Biomass Building in a Reduced Environment
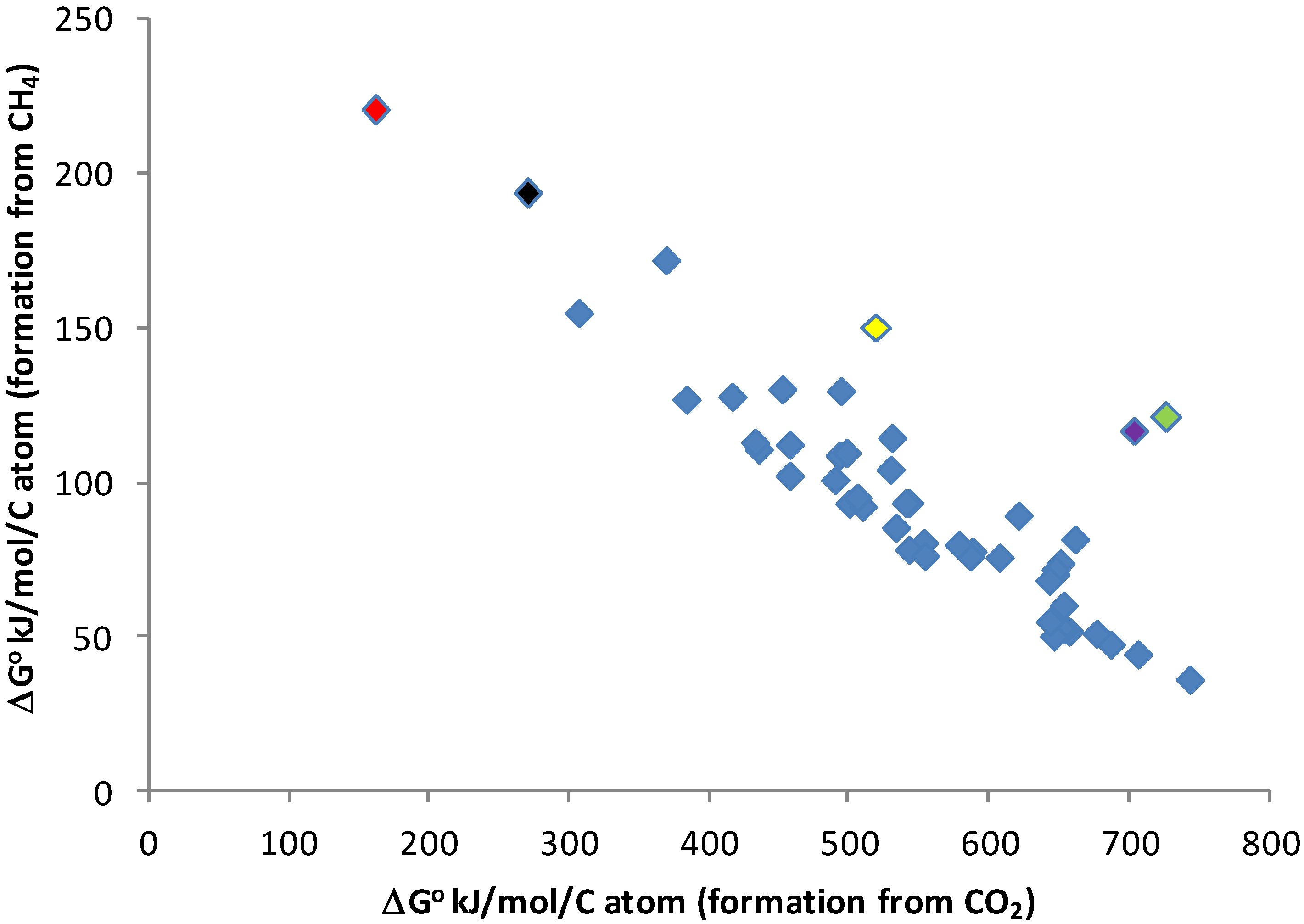

3.4. Electron Acceptors Other Than Hydrogen
| Element | Reaction | Free Energy Change (kJ/mol) | Ref for Free Energy Data |
|---|---|---|---|
| Nitrogen | ½ N2 + 1½ H2 → NH3 | −62.61 | [65] |
| ½ N2 + ½ H2 + H2O → NH2OH | +183.8 | [69] | |
| Phosphorus | H2 + HPO42− → HPO32− + H2O | +27.2 | [70] |
| H2 + HPO32− + H+ → H2PO2− + H2O | +84.3 | [70,71] | |
| ½H2 + H2PO2− + H+ → P(s) + 2H2O | +52.8 | [70,71] | |
| P(s) + 1½H2 → PH3 | +5.4 | [70,72] | |
| Overall 4 H2 + HPO42− + 2H+ → PH3 + 4H2O | +169.8 | ||
| Sulfur | SO42− + H2 → SO32− + H2O | +12.45 | [65] |
| SO32− + 2H2 +2H+ → S(s) + 3H2O | −248.29 | [65] | |
| S(s) + H2 → H2S | −44.81 | [65] | |
| Overall SO42− + 2H+ + 4H2 → H2S + 4H2O | −280.8 | ||
| Iron | ½ H2 + Fe3+ + OH− → Fe2+ + H2O | −125.8 | [65] |
| H2 + Fe2+ + 2OH− → Fe(s) + 2H2O | −6.1 | [65] | |
| Manganese | Mn3+ + ½ H2 + OH− > Mn2+ + H2O | −273.3 | [65] |
| H2 + Mn2+ + 2OH− → Mn(s) + 2H2O | −24.9 | [65] | |
| Silicon | 2H2 + H4SiO4 (s) → Si(s) + 4H2O | +384.5 | [71] |
| 2H2(g) + SiO2(s) > Si(s) + 2H2O | +382.1 | [71] | |
| Si(s) + 2H2(g) → SiH4(g) | +56.9 | [71] | |
| Aluminium | 3H2 (g) + Al2O3 (s) → 2Al(s) +3 H2O | +871.0 | [71] |
| Copper | Cu2+ + ½H2 → Cu+ + H+ | −19.4 | [65] |
| Cu+ + ½ H2 → Cu(s) + H+ | −57.8 | [65] | |
| Vanadium | H2VO4− +2H+ + ½H2 → HVO2+ + 2H2O | −113.8 | [65] |
| HVO2+ + ½H2 → VO+ + H2O | −243.5 | [65] | |
| VO+ + ½ H2 → VOH+ | 17.5 | [65] | |
| VOH+ + H2 → V(s) + H+ + H2O | 122.7 | [65] |
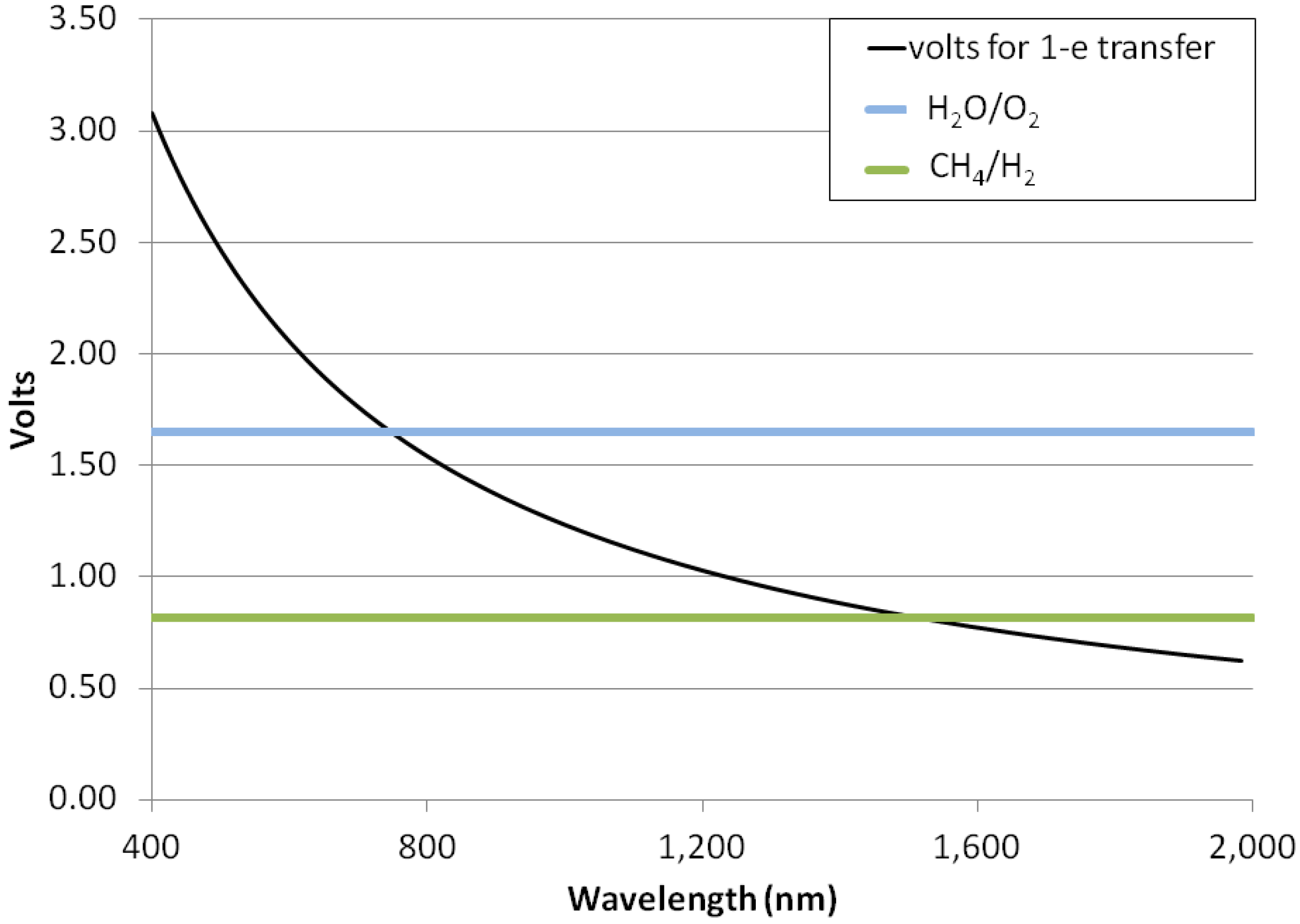
3.5. Photon Energies for H2-Dominated Photosynthesis
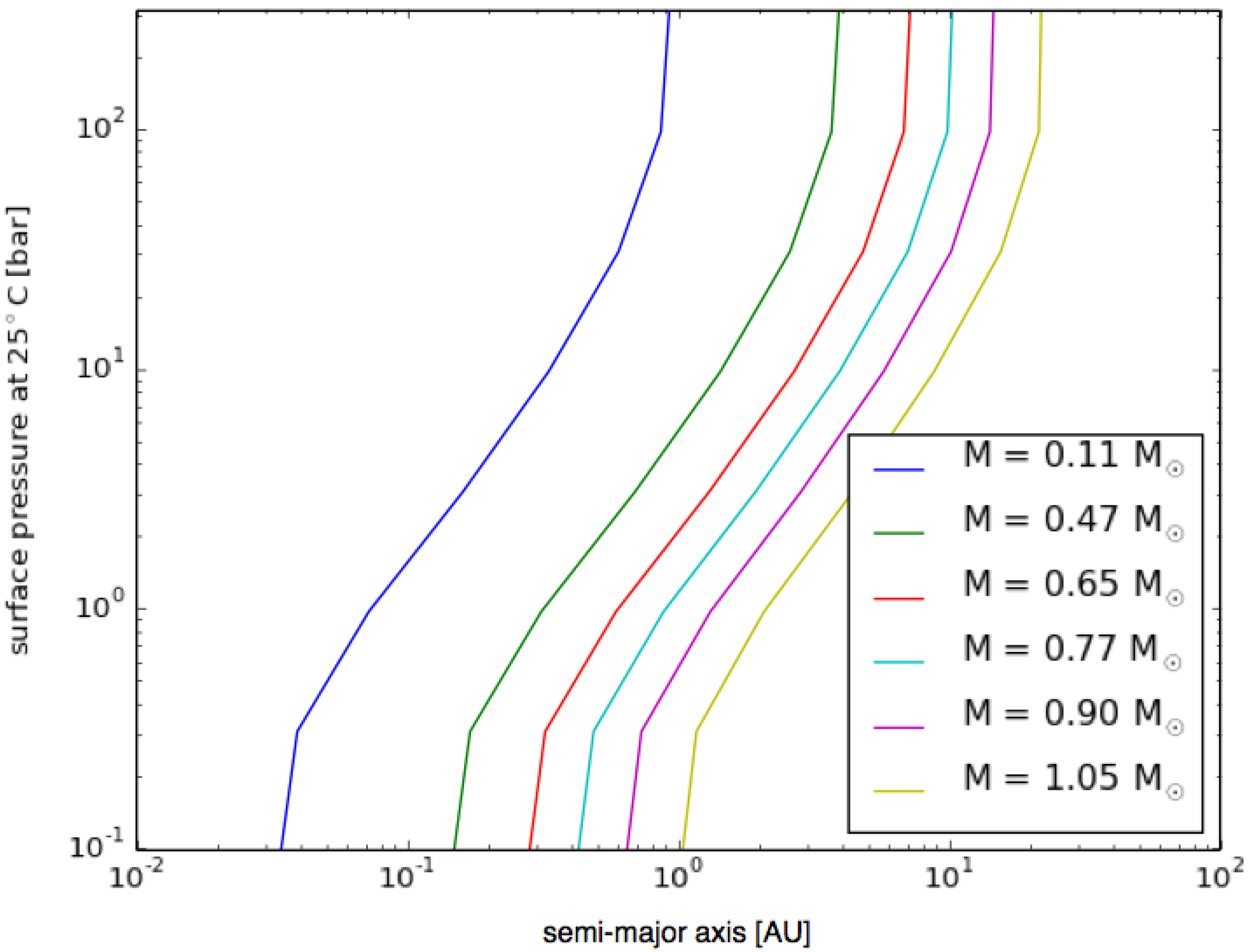
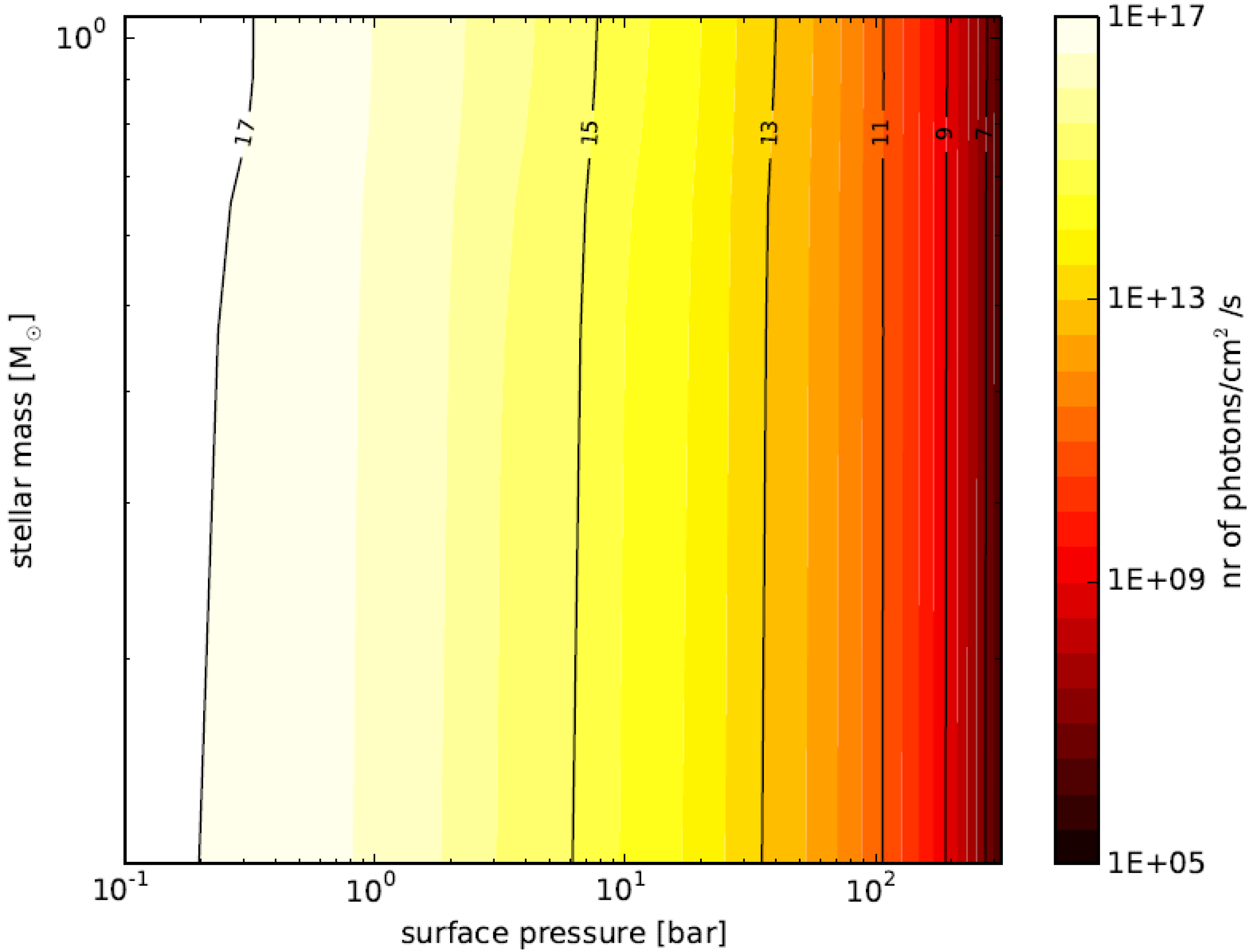
3.6. Planetary Environments for Hydrogenic Photosynthesis
4. Discussions
- (1)
- Hydrogenic photosynthesis requires 5 to 10 times less energy to build a given mass of biomass from methane as oxygenic photosynthesis requires to build the same amount of biomass from carbon dioxide;
- (2)
- Hydrogenic photosynthesis could be driven by photons into the near-infrared—1500 nm—whereas oxygenic photosynthesis is observed to be powered only by red photons of a wavelength ≤720 nm.
- (3)
- Planets with surface conditions suitable for hydrogenic photosynthesis may exist over a much wider range of orbital parameters than the conventional habitable zone;
- (4)
- Hydrogen gas is the most plausible reduced product of methane-oxidizing photosynthesis. Ammonia gas may also be a photosynthetic waste product. Other waste products can be suggested, but either require more energy to make or require rare starting materials and, in any case are very unlikely to be detectable remotely.
4.1. Limited Biosignature Gases from Hydrogenic Photosynthesis
4.2. Evolution of Photosynthesis in an H2-Dominated Environment
- (1)
- Capture of light energy and its use to generate H2 gas;
- (2)
- Oxidation of methane to generate H2.
5. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Blankenship, R.E. Early Evolution of photosynthesis. Plant Physiol. 2010, 154, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Hohmann-Marriott, M.F.; Blankenship, R.E. Evolution of Photosynthesis. Ann. Rev. Plant Biol. 2011, 62, 515–548. [Google Scholar] [CrossRef]
- Xiong, J.; Bauer, C.E. Complex. evolution of photosynthesis. Ann. Rev. Plant Biol. 2002, 53, 503–521. [Google Scholar] [CrossRef]
- Lozier, R.H.; Bogomolni, R.A.; Stoeckenius, W. Bacteriorhodopsin: A light-driven proton pump in Halobacterium Halobium. Biophys. J. 1975, 15, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Stoeckenius, W.; Bogomolni, R.A. Bacteriorhodopsin and related pigments of halobacteria. Ann. Rev. Biochem. 1982, 52, 587–616. [Google Scholar] [CrossRef]
- Béjà, O.; Aravind, L.; Koonin, E.V.; Suzuki1, M.T.; Hadd, A.; Nguyen, L.P.; Jovanovich, S.B.; Gates, C.M.; Feldman, R.A.; Spudich, J.L.; et al. Bacterial Rhodopsin: Evidence for a New Type of Phototrophy in the Sea. Science 2000, 289, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Béjà, O.; Spudich, E.N.; Spudich, J.L.; Leclerc, M.; DeLong, E.F. Proteorhodopsin phototrophy in the ocean. Nature 2001, 411, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Dadachova, E.; Casadevall, A. Ionizing radiation: How fungi cope, adapt, and exploit with the help of melanin. Curr. Opin. Microbiol. 2008, 11, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Turick, C.E.; Ekechukwu, A.A.; Milliken, C.E.; Casadevall, A.; Dadachova, E. Gamma radiation interacts with melanin to alter its oxidation-reduction potential and results in electric current production. Bioelectrochemistry 2011, 82, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Bryan, R.; Jiang, Z.; Friedman, M.; Dadachova, E. The effects of gamma radiation, UV and visible light on ATP levels in yeast cells depend on cellular melanization. Fungal Biol. 2011, 115, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, R.E.; Hartman, H. The origin and evolution of oxygenic photosynthesis. Trends Biochem. Sci. 1998, 23, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Schidlowski, M. A 3800-million-year isotopic record of life from carbon in sedimentary rocks. Nature 1988, 333, 313–318. [Google Scholar] [CrossRef]
- Bains, W.; Seager, S. A Combinatorial Approach to Biochemical Space: Description and Application to the Redox Distribution of Metabolism. Astrobiology 2012, 12, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Canfield, D.E. The early history of atmospheric oxygen. Ann. Rev. Earth Planet. Sci. 2005, 33, 1–36. [Google Scholar] [CrossRef]
- Catling, D.C.; Glein, C.R.; Zahnle, K.J.; McKay, C.P. Why O2 Is Required by Complex Life on Habitable Planets and the Concept of Planetary “Oxygenation Time”. Astrobiology 2005, 5, 415–438. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S. Biological Effects of High. Ultraviolet Radiation on Early Earth—A Theoretical Evaluation. J. Theor. Biol. 1998, 193, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S. The ultraviolet history of the terrestrial planets—Implications for biological evolution. Planet. Space Sci. 2000, 48, 203–214. [Google Scholar] [CrossRef]
- Segura, A.; Krelove, K.; Kasting, J.F.; Sommerlatt, D.; Meadows, V.; Crisp, D.; Cohen, M.; Mlawer, E. Ozone Concentrations and Ultraviolet Fluxes on Earth-Like Planets Around Other Stars. Astrobiology 2003, 3, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Kasting, J.F.; Meadows, V.; Cohen, M.; Scalo, J.; Crisp, D.; Butler, R.A.H.; Tinetti, G. Biosignatures from Earth-Like Planets Around M Dwarfs. Astrobiology 2005, 5, 706–725. [Google Scholar] [CrossRef] [PubMed]
- Scalo, J.; Kaltenegger, L.; Segura, A.; Fridlund, M.; Ribas, I.; Kulikov, Y.N.; Grenfell, J.L.; Rauer, H.; Odert, P.; Leitzinger, M.; et al. M Stars as Targets for Terrestrial Exoplanet Searches and Biosignature Detection. Astrobiology 2007, 7, 85–166. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S.; Raven, J.A.; Kaltenegger, L.; Logan, R.C. Planetary targets in the search for extrasolar oxygenic photosynthesis. Plant Ecol. Divers. 2009, 2, 207–219. [Google Scholar] [CrossRef]
- O’Malley-James, J.T.; Raven, J.A.; Cockell, C.S.; Greaves, J.S. Life and Light: Exotic Photosynthesis in Binary and Multiple-Star Systems. Astrobiology 2012, 12, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.P.; Mead, A.J.; Forgan, D.H.; Raven, J.A.; Cockell, C.S. Photosynthetic Potential of Planets in 3:2 Spin Orbit Resonances. Int. J. Astrobiol. 2014, 13, 279–289. [Google Scholar] [CrossRef]
- Raven, J.A.; Cockell, C.S. Influence on Photosynthesis of Starlight, Moonlight, Planetlight, and Light Pollution (Reflections on Photosynthetically Active Radiation in the Universe). Astrobiology 2006, 6, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Wolstencroft, R.D.; Raven, J.A. Photosynthesis: Likelihood of Occurrence and Possibility of Detection on Earth-like Planets. Icarus 2002, 157, 535–548. [Google Scholar] [CrossRef]
- Kiang, N.Y.; Segura, A.; Tinetti, G.; Govindjee; Blankenship, R.E.; Cohen, M.; Siefert, J.; Crisp, D.; Meadows, V.S. Spectral Signatures of Photosynthesis II Coevolution with Other Stars and the Atmosphere on Extrasolar Worlds. Astrobiology 2007, 7, 252–274. [Google Scholar] [CrossRef] [PubMed]
- Catling, D.C.; Claire, M.W. How Earth’s atmosphere evolved to an oxic state: A status report. Earth Planet. Sci. Lett. 2005, 237, 1–20. [Google Scholar] [CrossRef]
- Sleep, N.H. The Hadean-Archaean Environment. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Dismukes, G.C.; Klimov, V.V.; Baranov, S.V.; Kozlov, Y.N.; DasGupta, J.; Tyryshkin, A. The origin of atmospheric oxygen on Earth: The innovation of oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 2001, 98, 2170–2175. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, L.J. The evolution of photosynthesis ... again? Philos. Trans. R. Soc. B 2008, 363, 2787–2801. [Google Scholar] [CrossRef]
- Kasting, J.F.; Ono, S. Palaeoclimates: The first two billion years. Philos. Trans. R. Soc. B 2012, 361, 917–929. [Google Scholar] [CrossRef]
- Jakosky, B.M.; Shock, E.L. The biological potential of Mars, the early Earth, and Europa. J. Geophys. Res. Planets 1998, 103, 19359–19364. [Google Scholar] [CrossRef]
- Lederberg, J. Signs of life: Criterion-system of exobiology. Nature 1965, 207, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, J.E. A physical basis for life detection experiments. Nature 1965, 207, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, J.E. Thermodynamics and the recognition of alien biospheres. Proc. R. Soc. Lond. B 1975, 189, 167–181. [Google Scholar] [CrossRef]
- Kaltenegger, L.; Selsis, F.; Fridlund, M.; Lammer, H. Darwin Science Team. Deciphering Spectral Fingerprints of Habitable Exoplanets. Astrobiology 2010, 10, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Kaltenegger, L.; Traub, W.A.; Jucks, K.W. Spectral Evolution of an Earth-like Planet. Astrophys. J. 2007, 658, 598–616. [Google Scholar] [CrossRef]
- Seager, S.; Schrenk, M.; Bains, W. An Astrophysical View of Earth-Based Metabolic Biosignature Gases. Astrobiology 2012, 12, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Seager, S.; Turner, E.L.; Schafer, J.; Ford, E.B. Vegetation’s Red Edge: A Possible Spectroscopic Biosignature of Extraterrestrial Plants. Astrobiology 2005, 5, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, G. Characterizing Extrasolar Terrestrial Planets with Reflected, Emitted and Transmitted Spectra. Orig. Life Evol. Biospheres 2006, 36, 541–547. [Google Scholar] [CrossRef]
- Kiang, N.Y.; Siefert, J.; Govindjee; Robert, E. Spectral Signatures of Photosynthesis. I. Review of Earth Organisms. Astrobiology 2007, 7, 222–251. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S.; Kaltenegger, L.; Raven, J.A. Cryptic Photosynthesis—Extrasolar Planetary Oxygen Without a Surface Biological Signature. Astrobiology 2009, 9, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L. Earthshine Observation of Vegetation and Implication for Life Detection on Other Planets. In Strategies of Life Detection; Botta, O., Bada, J.L., Gomez-Elvira, J., Javaux, E., Selsis, F., Summons, R., Eds.; Springer: New York, NY, USA, 2008; pp. 323–333. [Google Scholar]
- Lammer, H.; Stökl, A.; Erkaev, N.V.; Dorfi, E.A.; Odert, P.; Güdel, M.; Kulikov, Y.N.; Kislyakova, K.G.; Leitzinger, M. Origin and loss of nebula-captured hydrogen envelopes from “sub”- to “super-Earths” in the habitable zone of Sun-like stars. Mon. Not. R. Astron. Soc. 2014, 439, 3225–3238. [Google Scholar] [CrossRef]
- Elkins-Tanton, L.T.; Seager, S. Ranges of Atmospheric Mass and Composition of Super-Earth Exoplanets. Astrophys. J. 2008, 685, 1237–1246. [Google Scholar] [CrossRef]
- Schaefer, L.; Fegley, B. Chemistry of atmospheres formed during accretion of the Earth and other terrestrial planets. Icarus 2010, 208, 438–448. [Google Scholar] [CrossRef]
- Pierrehumbert, R.; Gaidos, E. Hydrogen greenhouse planets beyond the habitable zone. J. Geophys Res. 2011, 86. [Google Scholar] [CrossRef]
- Borysow, A. Collision-induced absorption coefficients of H2 pairs at temperatures from 60 K to 1000 K. Astronomy and Astrophysics 2002, 390, 779–782. [Google Scholar] [CrossRef]
- Seager, S. Exoplanet Habitability. Science 2013, 340, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Seager, S.; Bains, W. Photochemistry in terrestrial exoplanet atmospheres. I. photochemistry model and benchmark cases. Astrophys. J. 2012, 761. [Google Scholar] [CrossRef]
- Hu, R.; Seager, S.; Bains, W. Photochemistry in Terrestrial Exoplanet Atmospheres II: H2S and SO2 Photochemistry in Anoxic Atmospheres. Astrophys. J. 2013, 769. [Google Scholar] [CrossRef]
- Seager, S.; Bains, W.; Hu, R. Biosignature Gases in H2-dominated Atmospheres on Rocky Exoplanets. Astrophys. J. 2013, 777. [Google Scholar] [CrossRef]
- Barber, J. Photosynthetic energy conversion: natural and artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Some groups, such as quaternary carbon centres and quaternary amines, are under-represented in the Combimol output, as noted in [13]
- Stewart, J.P. MOPAC: A semiempirical molecular orbital program. J. Comput. Aided Mol. Des. 1990, 4, 1–103. [Google Scholar] [CrossRef] [PubMed]
- Stull, D.R.; Westrum, E.F.J.; Sinke, G.C. The Chemical Thermodynamics of Organic Compounds; Wiley: New York, NY, USA, 1969. [Google Scholar]
- Hu, R.; Seager, S. Photochemistry in Terrestrial Exoplanet Atmospheres III: Photochemistry and Thermochemistry in Thick Atmospheres on Super Earths and Mini Neptunes. Astrophys. J. 2014, 784. [Google Scholar] [CrossRef]
- Wen, J.-S.; Pinto, J.P.; Yung, Y.L. Photochemistry of CO and H2O: Analysis of laboratory experiments and applications to the prebiotic Earth’s atmosphere. J. Geophys. Res. Atmospheres 1989, 94, 14957–14970. [Google Scholar] [CrossRef]
- Le Guern, F.; Gerlach, T.M.; Nohl, A. Field gas chromatograph analyses of gases from a glowing dome at Merapi volcano, Java, Indonesia, 1977, 1978, 1979. J. Volcanol. Geotherm. Res. 1982, 14, 223–245. [Google Scholar]
- Lee, H.-F.; Yang, T.F.; Lan, T.F.; Song, S.-R.; Tsao, S. Fumarolic gas composition of the Tatun volcano group, Northern Taiwan. Terr. Atmos. Ocean. Sci. 2005, 16, 843–864. [Google Scholar]
- Hoehler, T.M.; Alperin, M.J.; Albert, D.B.; Martens, C.S. Apparent minimum free energy requirements for methanogenic Archaea and sulfate-reducing bacteria in an anoxic marine sediment. FEMS Microbiol. Ecol. 2001, 38, 33–41. [Google Scholar] [CrossRef]
- Hoehler, T.M. Biological energy requirements as quantitative boundary conditions for life in the subsurface. Geobiology 2004, 2, 205–215. [Google Scholar] [CrossRef]
- Haqq-Misra, J.D.; Domagal-Goldman, S.D.; Kasting, P.J.; Kasting, J.F. A Revised, Hazy Methane Greenhouse for the Archean Earth. Astrobiology 2008, 8, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Ehrenreich, A.; Widdel, F. Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl. Environ. Microbiol. 1994, 60, 4517–4526. [Google Scholar] [PubMed]
- Amend, J.P.; Shock, E.L. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Ecol. 2001, 25, 175–243. [Google Scholar] [CrossRef]
- Bains, W. A trip through chemical space: Why life has evolved the chemistry that it has. In Evolutionary Biology: Genome Evolution, Speciation, Co-Evolution and Origin of Life; Pontarotti, P., Ed.; Springer: Lausanne, Switzerland, 2014. [Google Scholar]
- We distinguish here from the origin of metabolism and its subsequent evolution. Terrestrial bacteria living in highly anoxic, reducing environments do not change their core metabolism to be more reduced. The core metabolism of all modern life-forms is fixed by its mutual inter-dependence: DNA cannot be changed for a more reduced molecule in order to be more energy efficient. The arguments here address the origins of metabolism rather than their subsequent adaptation to extreme environments
- Seager, S.; Bains, W.; Hu, R. A Biomass-based Model to Estimate the Plausibility of Exoplanet Biosignature Gases. Astrophys. J. 2013, 775, 104. [Google Scholar] [CrossRef]
- Van der Star, W.R.L.; van de Graaf, M.J.; Kartal, B.; Picioreanu, C.; Jetten, M.S.; van Loosdrecht, M.C. Response of Anaerobic Ammonium-Oxidizing Bacteria to Hydroxylamine. Appl. Environ. Microbiol. 2008, 74, 4417–4426. [Google Scholar]
- Van Wazer, J.R. Phosphorus and Its Compounds; Interscience Publishers Inc.: New York, NY, USA, 1958; Volume 1. [Google Scholar]
- Lide, D. Standard Thermodynamic Properties of Chemical Substances. In CRC Handbook of Chemistry and Physics, 89th ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Wall, F.T. Chemical Thermodynamics; Freeman and Co.: San Francisco, CA, USA, 1965. [Google Scholar]
- Palme, H.; O’Neill, H.S.C. Cosmological estimates of mantle composition. Treatise Geochem. 2004, 2, 1–38. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. Treatise Geochem. 2003, 3, 1–64. [Google Scholar]
- Asimov, I. The elementary composition of the earth’s crust. J. Chem. Educ. 1954, 31, 70–71. [Google Scholar] [CrossRef]
- Disposal of Sodium. Available online: https://www.youtube.com/watch?v=HY7mTCMvpEM (accessed on 12 November 2014).
- Roels, J.; Verstraete, W. Biological formation of volatile phosphorus compounds. Bioresour. Technol. 2001, 79, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Glindemann, D.; Edwards, M.; Liu, J.; Kuschk, P. Phosphine in soils, sludges, biogases and atmospheric implications—A review. Ecol. Eng. 2005, 24, 457–463. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Ferguson, S.J. Bioenergetics 2; Academic Press: London, UK, 1992. [Google Scholar]
- Wanders, R.J.A.; Westerhoff, H.V. Sigmoidal relation between mitochondrial respiration and log ([ATP]/[ADP])out under conditions of extramitochondrial ATP utilization. Implications for the control and thermodynamics of oxidative phosphorylation. Biochemistry 1988, 27, 7832–7840. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R. The quantum yield of photosynthesis. Ann. Rev. Plant Physiol. 1958, 9, 1–24. [Google Scholar] [CrossRef]
- Hu, Q.; Miyashita, H.; Iwasaki, I.; Kurano, N.; Miyachi, S.; Iwaki, M.; Itoh, S. A photosystem I reaction center driven by chlorophyll d in oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13319–13323. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.T.; Overmann, J.; Lince, M.T.; Manske, A.K.; Lang, A.S.; Blankenship, R.E.; van Dover, C.L.; Martinson, T.A.; Plumley, F.G. An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. Proc. Natl Acad. Sci. USA 2005, 102, 9306–9310. [Google Scholar] [CrossRef] [PubMed]
- Manske, A.K.; Glaeser, J.; Kuypers, M.M.M.; Overmann, J. Physiology and Phylogeny of Green Sulfur Bacteria Forming a Monospecific Phototrophic Assemblage at a Depth of 100 Meters in the Black Sea. Appl. Environ. Microbiol. 2005, 71, 8049–8060. [Google Scholar] [CrossRef] [PubMed]
- Zsom, A.; Seager, S.; de Wit, J.; Stamenkovic, V. Toward the Minimum Inner Edge Distance of the Habitable Zone. Astrophys. J. 2013, 778. [Google Scholar] [CrossRef]
- Michell, E.C. Volatile Chemistry and Nitrogen Sources and Fluxes in Subduction Zones: Insights from Izu-Bonin-Mariana Arc. In Earth and Planetary Sciences; University of Liverpool: Liverpool, UK, 2005. [Google Scholar]
- Christenson, B.W.; Wood, C.P. Evolution of a vent-hosted hydrothermal system beneath Ruapehu Crater Lake, New Zealand. Bull. Volcanol. 1993, 55, 547–565. [Google Scholar] [CrossRef]
- Clor, L.E.; Fischer, T.P.; Hilton, D.R. Volatile and N isotope chemistry of the Molucca Sea collision zone: tracing source components along the Sangihe Arc, Indonesia. Geochem. Geophys. Geosyst. 2005, 6, Q03J14. [Google Scholar] [CrossRef]
- Weaver, P.F.; Lien, S.; Seibert, M. Photobiological production of hydrogen. Sol. Energy 1980, 24, 3–45. [Google Scholar] [CrossRef]
- Nandi, R.; Sengupta, S. Microbial Production of Hydrogen: An Overview. Crit. Rev. Microbiol. 1998, 24, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Prince, R.C.; Kheshgi, H.S. The Photobiological Production of Hydrogen: Potential Efficiency and Effectiveness as a Renewable Fuel. Crit. Rev. Microbiol. 2005, 31, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Gest, H.; Kamen, M.D.; Bregoff, H.M. Studies on the Metabolism of Photosynthetic Bacteria: V. Photoproduction of Hydrogen and Nitrogen Fixation by Rhodospirillum Rubrum. J. Biol. Chem. 1950, 182, 153–170. [Google Scholar]
- Hoehler, T.M. Biogeochemistry of dihydrogen (H2). Metal Ions Biol. Syst. 2005, 43, 9–48. [Google Scholar]
- Hoehler, T.M.; Alperin, M.J.; Albert, D.B.; Martens, C.S. Field and laboratory studies of methane oxidation in an anoxic marine sediment: Evidence for a methanogen-sulfate reducer consortium. Glob. Biogeochem. Cycles 1994, 8, 451–463. [Google Scholar] [CrossRef]
- Hoehler, T.; Albert, D.B.; Alperin, M.J.; Bebout, B.M.; Martens, C.S.; des Marais, D.J. Comparative ecology of H2 cycling in sedimentary and phototrophic ecosystems. Antonie van Leeuwenhoek 2002, 81, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.W.; Regnier, P.; van Cappellen, P. Bioenergetic Controls on Anaerobic Oxidation of Methane (AOM) in Coastal Marine Sediments: A Theoretical Analysis. Am. J. Sci. 2006, 306, 246–294. [Google Scholar] [CrossRef]
- Knittel, K.; Boetius, A. Anaerobic Oxidation of Methane: Progress with an Unknown Process. Ann. Rev. Microbiol. 2009, 63, 311–334. [Google Scholar] [CrossRef]
- Wagner, M.; Roger, A.J.; Flax, J.L.; Brusseau, G.A.; Stahl, D.A. Phylogeny of Dissimilatory Sulfite Reductases Supports an Early Origin of Sulfate Respiration. J. Bacteriol. 1998, 180, 2975–2982. [Google Scholar] [PubMed]
- Mentel, M.; Martin, W. Anaerobic animals from an ancient, anoxic ecological niche. BMC Biol. 2012, 8. [Google Scholar] [CrossRef]
- Bryant, C. Metazoan Life without Oxygen; Chapman and Hall: London, UK, 1991. [Google Scholar]
- Danovaro, R.; Dell’Anno, A.; Pusceddu, A.; Gambi, C.; Heiner, I.; Kristensen, R.M. The first metazoa living in permanently anoxic conditions. BMC Biol. 2012, 8. [Google Scholar] [CrossRef] [Green Version]
- Watts, S.F. The mass budget of carbonyl sulphide, dimethyl sulphide, carbon disulphide and hydrogen sulphide. Atmos. Environ. 2000, 34, 761–779. [Google Scholar] [CrossRef]
- Kiene, R.P.; Linn, L.J.; Bruton, J.A. New and important roles for DMSP in marine microbial communities. J. Sea Res. 2000, 43, 209–224. [Google Scholar] [CrossRef]
- Kirst, G.O.; Thiel, C.; Wolff, H.; Nothnagel, J.; Wanzek, M.; Ulmke, R. Dimethylsulfoniopropionate (DMSP) in icealgae and its possible biological role. Mar. Chem. 1991, 35, 381–388. [Google Scholar] [CrossRef]
- Stefels, J. Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. J. Sea Res. 2000, 43, 183–197. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bains, W.; Seager, S.; Zsom, A. Photosynthesis in Hydrogen-Dominated Atmospheres. Life 2014, 4, 716-744. https://doi.org/10.3390/life4040716
Bains W, Seager S, Zsom A. Photosynthesis in Hydrogen-Dominated Atmospheres. Life. 2014; 4(4):716-744. https://doi.org/10.3390/life4040716
Chicago/Turabian StyleBains, William, Sara Seager, and Andras Zsom. 2014. "Photosynthesis in Hydrogen-Dominated Atmospheres" Life 4, no. 4: 716-744. https://doi.org/10.3390/life4040716
APA StyleBains, W., Seager, S., & Zsom, A. (2014). Photosynthesis in Hydrogen-Dominated Atmospheres. Life, 4(4), 716-744. https://doi.org/10.3390/life4040716






