A Model of Filamentous Cyanobacteria Leading to Reticulate Pattern Formation
Abstract
:1. Introduction
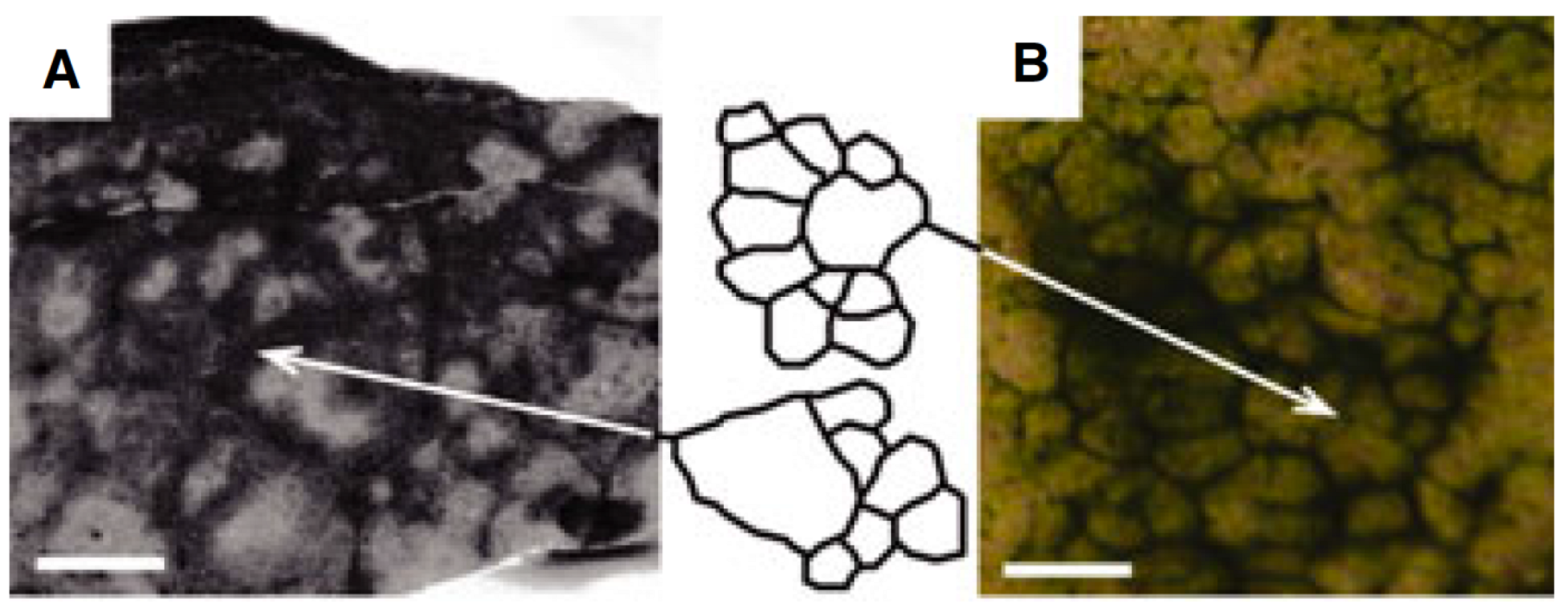
2. Methods
2.1. Biological Background
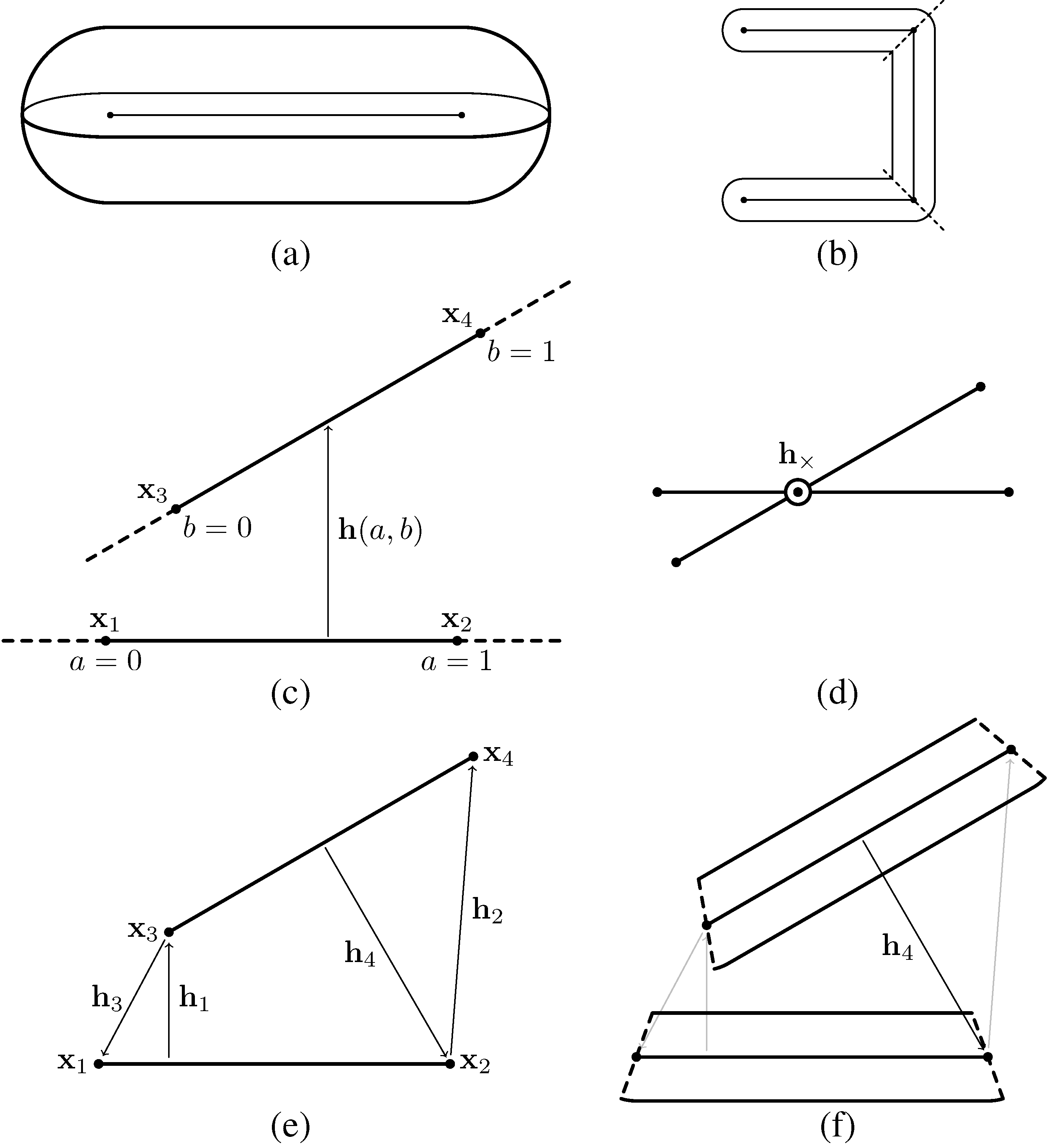
2.2. Model Geometry
2.3. Elasticity
2.4. Gliding
2.5. Contact Interaction
2.6. Boundary and Initial Conditions
2.7. Nondimensionalization
2.8. Parameters
| Type | Symbol | Description | Value | Refs. |
|---|---|---|---|---|
| Numerical Integration | Absolute accuracy | 0.1 µm | ||
| Domain | W | Width | 3,500 µm | |
| H | Height | 7.5 µm | ||
| D | Depth | 3,500 µm | ||
| µ | Viscosity | 1 Pa s | [38] | |
| ρ | Trichome density | 2.5% vol | ||
| Trichomes | Θ | Diameter | 1.5 µm | [7] |
| Λ | Length | 750 µm | [7] | |
| l | Segment length | ∼6 µm | ||
| α | Bending modulus | 2 × 10−12erg·cm−1 | ♭ | |
| v | Gliding speed | 1.3 µm/s | [7] | |
| ω | Reversal frequency | 1/300 s −1 | [29,50] |
2.9. Algorithms and Statistics
3. Results
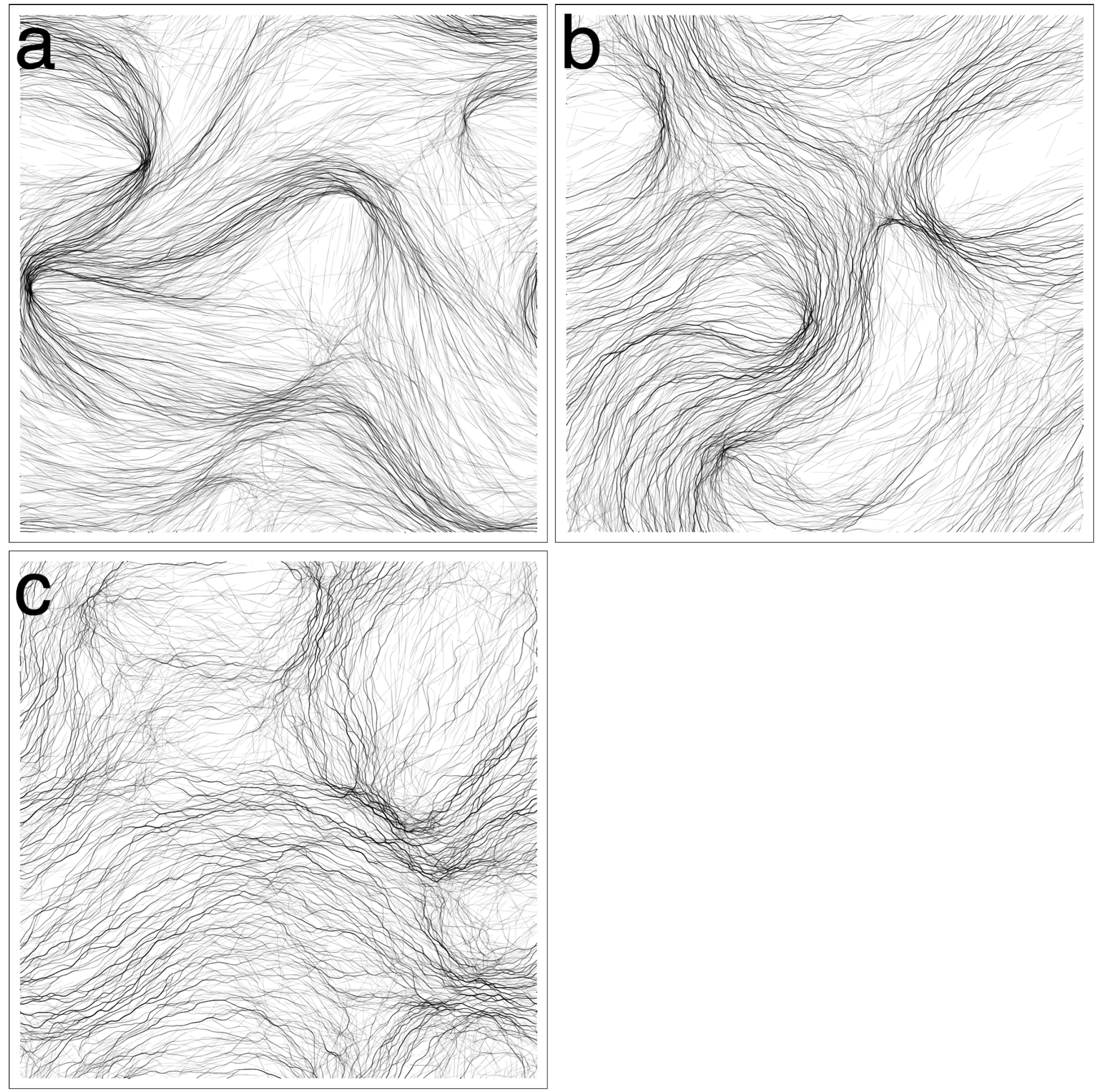
3.1. Varying Cohesion Strength
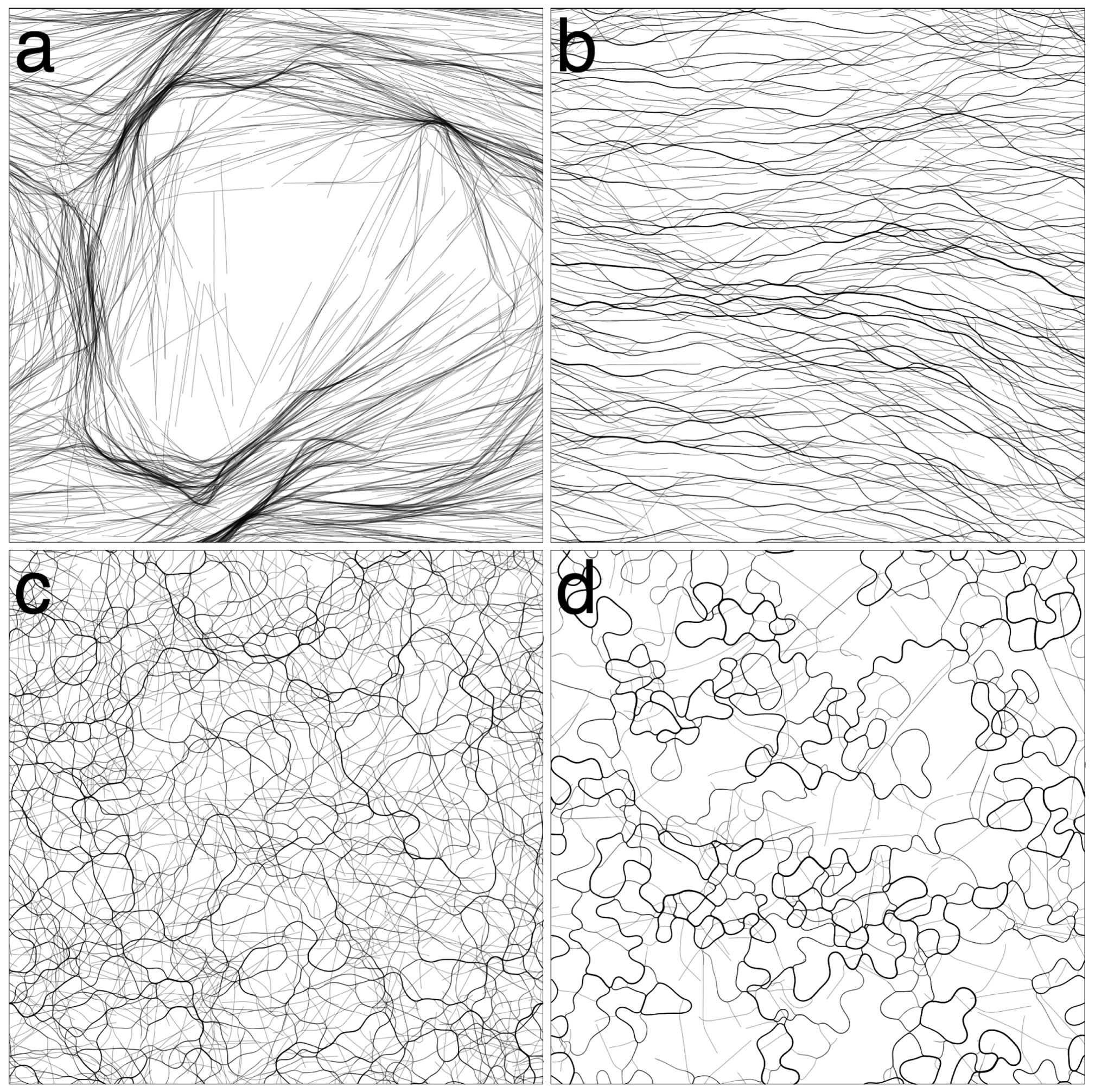
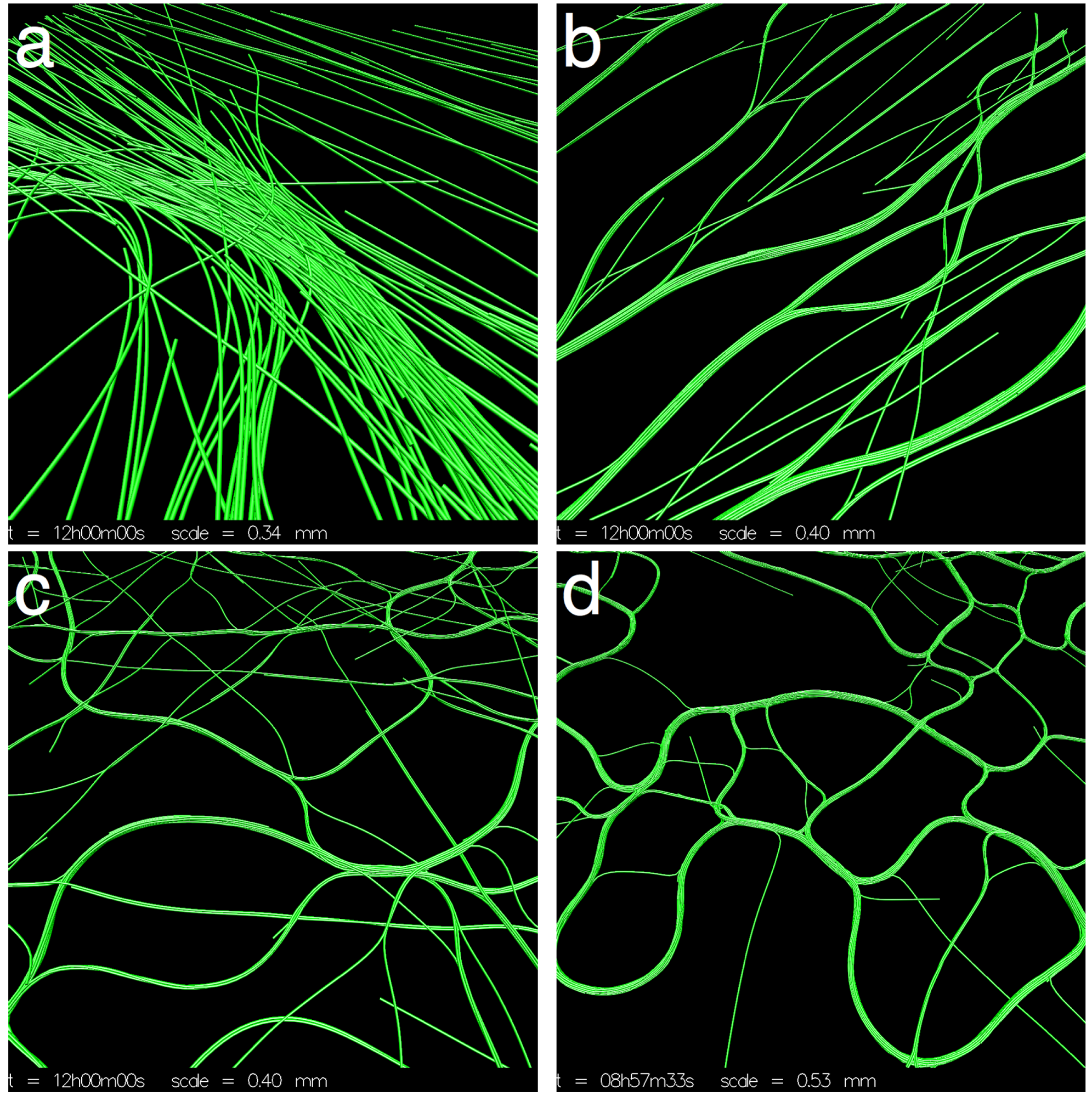
 ) was quantified by calculating the length of the mean direction vector:
) was quantified by calculating the length of the mean direction vector:
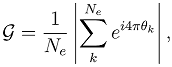
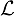 ) for each edge was calculated by taking the norm of the resultant of all the relative orientations of the edges within a Θ radius:
) for each edge was calculated by taking the norm of the resultant of all the relative orientations of the edges within a Θ radius:


3.2. Domain Size, Density and Reversal Frequency

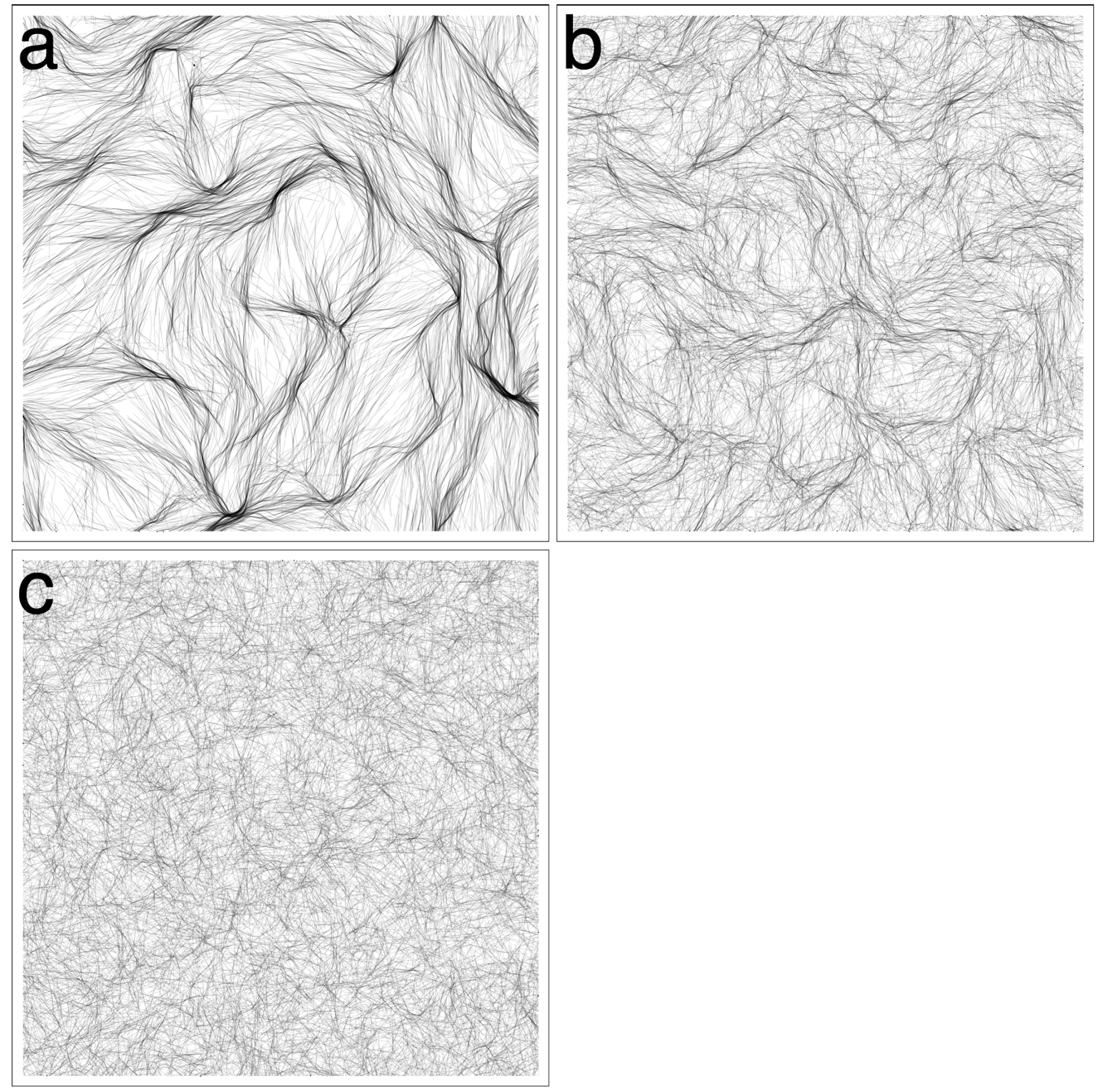
3.3. Revisiting Low Cohesion
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Supplementary Materials
Supplementary Files
Supplementary File 2Conflicts of Interest
References
- Kaiser, D. Coupling cell movement to multicellular development in myxobacteria. Nat. Rev. Microbiol. 2003, 1, 45–54. [Google Scholar]
- Firtel, R.A.; Meili, R. Dictyostelium: A model for regulated cell movement during morphogenesis. Curr. Opin. Genet. Dev. 2000, 10, 421–427. [Google Scholar]
- Rippka, R.; Deruelles, J.; Waterbury, J.; Herdman, M.; Stanier, R. Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar]
- Hoiczyk, E. Gliding motility in Cyanobacteria: Observations and possible explanations. Arch. Microbiol. 2000, 174, 11–17. [Google Scholar]
- Walter, M.R.; Bauld, J.; Brock, T.D. Microbiology and morphogenesis of columnar stromatolites (Conophyton, Vacerrilla) from Hot Springs in Yellowstone National Park. In Stromatolites; Walter, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1976; pp. 273–310. [Google Scholar]
- Petroff, A.P.; Sim, M.S.; Maslov, A.; Krupenin, M.; Rothman, D.H.; Bosak, T. Biophysical basis for the geometry of conical stromatolites. Proc. Natl. Acad. Sci. USA 2010, 107, 9956–9961. [Google Scholar]
- Shepard, R.N.; Sumner, D.Y. Undirected motility of filamentous cyanobacteria produces reticulate mats. Geobiology 2010, 8, 179–190. [Google Scholar]
- Allwood, A.C.; Walter, M.R.; Kamber, B.S.; Marshall, C.P.; Burch, I.W. Stromatolite reef from the early archaean era of Australia. Nature 2006, 441, 714–718. [Google Scholar]
- Jones, B.; Renaut, R.; Rosen, M.; Ansdell, K. Coniform stromatolites from geothermal systems, North Island, New Zealan. Palaios 2002, 17, 84–103. [Google Scholar]
- Wharton, R.; Parker, B.; Simmons, G. Distribution, species composition and morphology of algal mats in Antarctic Dry Valley Lakes. Phycologia 1983, 22, 355–365. [Google Scholar]
- Stal, L. Cyanobacterial Mats and Stromatolites. In The Ecology of Cyanobacteria; Whitton, B.A., Potts, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 61–120. [Google Scholar]
- Allwood, A.C.; Grotzinger, J.P.; Knoll, A.H.; Burch, I.W.; Anderson, M.S.; Coleman, M.L.; Kanik, I. Controls on development and diversity of early archean stromatolites. Proc. Natl. Acad. Sci. USA 2009, 106, 9548–9555. [Google Scholar]
- Grotzinger, J.; Knoll, A. Stromatolites in precambrian carbonates: Evolutionary mileposts or environmental dipsticks? Ann. Rev. Earth Planet. Sci. 1999, 27, 313–358. [Google Scholar]
- Tamulonis, C.; Postma, M.; Kaandorp, J. Modeling filamentous cyanobacteria reveals the advantages of long and fast trichomes for optimizing light exposure. PLoS One 2011, 6, e22084. [Google Scholar]
- Harvey, C.W.; Morcos, F.; Sweet, C.R.; Kaiser, D.; Chatterjee, S.; Liu, X.; Chen, D.Z.; Alber, M. Study of elastic collisions of Myxococcus xanthus in swarms. Phys. Biol. 2011, 8, 026016. [Google Scholar]
- Janulevicius, A.; van Loosdrecht, M.C.M.; Simone, A.; Picioreanu, C. Cell flexibility affects the alignment of model myxobacteria. Biophys. J. 2010, 99, 3129–3138. [Google Scholar]
- Sozinova, O.; Jiang, Y.; Kaiser, D.; Alber, M. A three-dimensional model of myxobacterial aggregation by contact-mediated interactions. Proc. Natl. Acad. Sci. USA 2005, 102, 11308–11312. [Google Scholar]
- Angelani, L.; Di Leonardo, R.; Ruocco, G. Self-starting micromotors in a bacterial bath. Phys. Rev. Lett. 2009, 102, 048104. [Google Scholar]
- Picioreanu, C.; van Loosdrecht, M.C.; Heijnen, J.J. A new combined differential-discrete cellular automaton approach for biofilm modeling: Application for growth in gel beads. Biotechnol. Bioeng. 1998, 57, 718–731. [Google Scholar]
- Ginelli, F.; Peruani, F.; Bär, M.; Chaté, H. Large-scale collective properties of self-propelled rods. Phys. Rev. Lett. 2010, 104, 184502. [Google Scholar]
- Peruani, F.; Klauss, T.; Deutsch, A.; Voss-Boehme, A. Traffic jams, gliders, and bands in the quest for collective motion of self-propelled particle. Phys. Rev. Lett. 2011, 106, 128101. [Google Scholar]
- Marée, A.F.; Hogeweg, P. How amoeboids self-organize into a fruiting body: Multicellular coordination in Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 2001, 98, 3879–3883. [Google Scholar]
- Sozinova, O.; Jiang, Y.; Kaiser, D.; Alber, M. A three-dimensional model of myxobacterial fruiting-body formation. Proc. Natl. Acad. Sci. USA 2006, 103, 17255–17259. [Google Scholar]
- McBride, M.J. Bacterial gliding motility: Multiple mechanisms for cell movement over surfaces. Ann. Rev. Microbiol. 2001, 55, 49–75. [Google Scholar]
- Häder, D.P. Photomovemen. In The Cyanobacteria; Elsevier Science Publishers: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Reichenbach, H. Taxonomy of the gliding bacteria. Annu. Rev. Microbiol. 1981, 35, 339–364. [Google Scholar]
- Hoiczyk, E.; Baumeister, W. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr. Biol. 1998, 8, 1161–1168. [Google Scholar]
- Hader, D.; Burkart, U. Enhanced model for photophobic responses of the Blue-Green Alga, phormidium uncinatum. Plant Cell Physiol. 1982, 23, 1391–1400. [Google Scholar]
- Gabai, V. A one-instant mechanism of phototaxis in the cyanobacterium Phormidium uncinatum. FEMS Microbiol. Lett. 1985, 30, 125–129. [Google Scholar]
- Nultsch, W.; Hader, D. Photomovement of motile microorganisms II. Photochem. Photobiol. 1988, 47, 837–869. [Google Scholar]
- Checcucci, G.; Sgarbossa, A.; Lenci, F. Photomovements of microorganisms: An introduction. In CRC Handbook of Organic Photochemistry and Photobiology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Donkor, V.; Hader, D.P. Effects of solar and ultraviolet radiation on motility, photomovement and pigmentation in filamentous, gliding cyanobacteria. FEMS Microbiol. Lett. 1991, 86, 159–168. [Google Scholar]
- Donkor, V.; Amewowor, D.; Häder, D.P. Effects of tropical solar radiation on the motility of filamentous cyanobacteria. FEMS Microbiol. Ecol. 1993, 12, 143–148. [Google Scholar]
- Garcia-Pichel, F.; Mechling, M.; Castenholz, R.W. Diel migrations of microorganisms within a benthic, hypersaline mat community. Appl. Environ. Microbiol. 1994, 60, 1500–1511. [Google Scholar]
- Fourcans, A.; Sole, A.; Diestra, E.; Ranchou-Peyruse, A.; Esteve, I.; Caumette, P.; Duran, R. Vertical migration of phototrophic bacterial populations in a hypersaline microbial mat from Salins-de-Giraud (Camargue, France). FEMS Microbiol. Ecol. 2006, 57, 367–377. [Google Scholar]
- Lowe, C. Dynamics of filaments: Modelling the dynamics of driven microfilaments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 1543–1550. [Google Scholar]
- Bergou, M.; Wardetzky, M.; Robinson, S.; Audoly, B.; Grinspun, E. Discrete elastic rods. ACM Trans. Graph. 2008, 27. [Google Scholar] [CrossRef]
- Wolgemuth, C. Force and flexibility of flailing myxobacteria. Biophys. J. 2005, 89, 945–950. [Google Scholar]
- Hess, B.; Bekker, H.; Berendsen, H.; Fraaije, J. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar]
- Yu, N.; Polycarpou, A.A. Adhesive contact based on the Lennard-Jones potential: A correction to the value of the equilibrium distance as used in the potential. J. Coll. Interface Sci. 2004, 278, 428–435. [Google Scholar]
- Lamont, H. Sacrificial cell death and trichome breakage in an oscillatoriacean blue-green alga: The role of murein. Arch. Microbiol. 1969, 69, 237–259. [Google Scholar]
- Glagoleva, T.N.; Glagolev, A.N.; Gusev, M.V.; Nikitina, K.A. Protonmotive force supports gliding in cyanobacteria. FEBS Lett. 1980, 117, 49–53. [Google Scholar]
- Robinson, W.B.; Mealor, A.E.; Stevens, S.E., Jr.; Ospeck, M. Measuring the force production of the hormogonia of mastigocladus laminosus. Biophys. J. 2007, 93, 699–703. [Google Scholar]
- Wiggins, C.; Riveline, D.; Ott, A.; Goldstein, R. Trapping and wiggling: Elastohydrodynamics of driven microfilaments. Biophys. J. 1998, 74, 1043–1060. [Google Scholar]
- Boal, D.; Ng, R. Shape analysis of filamentous Precambrian microfossils and modern cyanobacteria. Paleobiology 2010, 36, 555–572. [Google Scholar]
- White, D.; Dworkin, M.; Tipper, D. Peptidoglycan of myxococcus xanthus: Structure and relation to morphogenesis. J. Bacteriol. 1968, 95, 2186–2197. [Google Scholar]
- Hoiczyk, E.; Hansel, A. Cyanobacterial cell walls: News from an unusual prokaryotic envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar]
- Lan, G.; Wolgemuth, C.; Sun, S. Z-ring force and cell shape during division in rod-like bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 16110–16115. [Google Scholar]
- Yao, X.; Jericho, M.; Pink, D.; Beveridge, T. Thickness and elasticity of gram-negative murein sacculi measured by atomic force microscopy. J. Bacteriol. 1999, 181, 6865–6875. [Google Scholar]
- Häder, D.P. Evidence of electrical potential changes in photophobically reacting blue-green algae. Arch. Microbiol. 1978, 118, 115–119. [Google Scholar]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes: The Art of Scientific Computing, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Castenholz, R. Aggregation in a thermophilic oscillatoria. Nature 1967, 215, 1285–1286. [Google Scholar]
- Castenholz, R.W. The behavior of oscillatoria terebriformis in Hot Springs. J. Phycol. 1968, 4, 132–139. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tamulonis, C.; Kaandorp, J. A Model of Filamentous Cyanobacteria Leading to Reticulate Pattern Formation. Life 2014, 4, 433-456. https://doi.org/10.3390/life4030433
Tamulonis C, Kaandorp J. A Model of Filamentous Cyanobacteria Leading to Reticulate Pattern Formation. Life. 2014; 4(3):433-456. https://doi.org/10.3390/life4030433
Chicago/Turabian StyleTamulonis, Carlos, and Jaap Kaandorp. 2014. "A Model of Filamentous Cyanobacteria Leading to Reticulate Pattern Formation" Life 4, no. 3: 433-456. https://doi.org/10.3390/life4030433
APA StyleTamulonis, C., & Kaandorp, J. (2014). A Model of Filamentous Cyanobacteria Leading to Reticulate Pattern Formation. Life, 4(3), 433-456. https://doi.org/10.3390/life4030433




