Theory of the Origin, Evolution, and Nature of Life †
Abstract
:1. Introduction
| Term | Meaning |
|---|---|
| Alternagyre | A gyrosystem whose gyrapex is not triquantal |
| Dextragyre | A right-handed gyre or gyromodel |
| Focagyre | A gyre that is the focal point of analysis or discussion |
| Gyradaptor | The gyre singularity—a quantum—that exerts all forces on the gyrosystem |
| Gyrapex | The relativistically high potential, excited, unstable, learning state of a particle |
| Gyraxiom | A fact, condition, principle, or rule that constrains and defines the theoretical framework |
| Gyre | The spacetime shape or path of a particle or group of particles; a quantum |
| Gyrequation | Shorthand notation for analysis, discussion, and understanding gyromodels |
| Gyrobase | The relativistically low potential, ground, stable, memory state of a particle |
| Gyrognosis | The thermodynamically demanding process of learning and integrating IEM |
| Gyrolink | The mIEM particle that links two gyromodules in a gyronexus |
| Gyromnemesis | The thermodynamically conserving process of remembering and recovering IEM |
| Gyromodel | The core model undergirding the theoretical framework |
| Gyromodule | A dIEM particle in a gyronexus |
| Gyronexus | A polymer of dIEM particles linked by mIEM particles |
| Gyrostate | The potential and/or kinetic state that a particle occupies in its gyratory path |
| Gyrosystem | A gyromodel with specific IEM composition, organization, and purpose |
| IEM b | Information, energy, and/or matter |
| Levoragyre | A left-handed gyre or gyromodel |
| Majorgyre | A gyrosystem whose gyrapex is triquantal |
| Matrioshkagyre | A model that demonstrates how gyres organize in nested sets |
| Ohiogyre | Higher-order organization in which a gyre gyrates around another gyre |
| Particle | A discrete, finite, empirically definable unit of IEM |
| Quantal | Of or relating to the quantum; tri-, di-, uni- and aquantal gyrostates found in majorgyres |
| Quantum | A capacious, potentially infinite, uncertain unit of IEM; a gyre |
| Subgyre | The gyre that subsumed by the focagyre |
| Supragyre | The gyre that subsumes the focagyre |
| Trimergence | Evolutionary emergence of a triquantal IEM |
| Prefixes c | |
| Amino | Of or relating to sulfur compounds (particles), amino acids, polypeptides |
| Carbo | Of or relating to carbon particles, carbohydrates, hydrocarbons |
| Cellulo | Of or relating to cells, archaebacteria, eubacteria, eukaryotes |
| Electro | Of or relating to visible matter particles, chemical elements, planetary cores |
| Geno | Of or relating to genes, DNA, chromosomes, genomes |
| Oxy | Of or relating to oxygen particles, water, oceans, lunar cores |
| Phospho | Of or relating to phosphate particles, phospholipids, phosphate signaling |
| Ribo | Of or relating to nitrogen particles, nitrogenous bases, RNA |
| Suffixes c | |
| –cycle | The spacetime period to complete a regular series of events in the same order |
| –gyre | Having the quality of a vortex; characterized by cyclical, oscillatory, and unpredictable motion; attractorepulsive, expansocontractive, and creatodestructive |
| –gnose | Characterized by learning or by IEM consideration and integration |
| –helix | Having a three-dimensional twisting, winding shape like that of a spiral staircase |
| –matrix | Having a three-dimensional networked, latticed shape like graphene or an ice crystal |
| –mneme | Characterized by memory or by IEM storage and retrieval |
| –nexus | Being connected or linked in a series |
| –on | Having the quality of a quantum; a particle or an amalgam of such particles |
| –sphere | Having orb-like features and hyperbolic geometry |
2. Model
2.1. Gyre Facts
2.1.1. Gyre Structure
2.1.2. Gyre Qualities
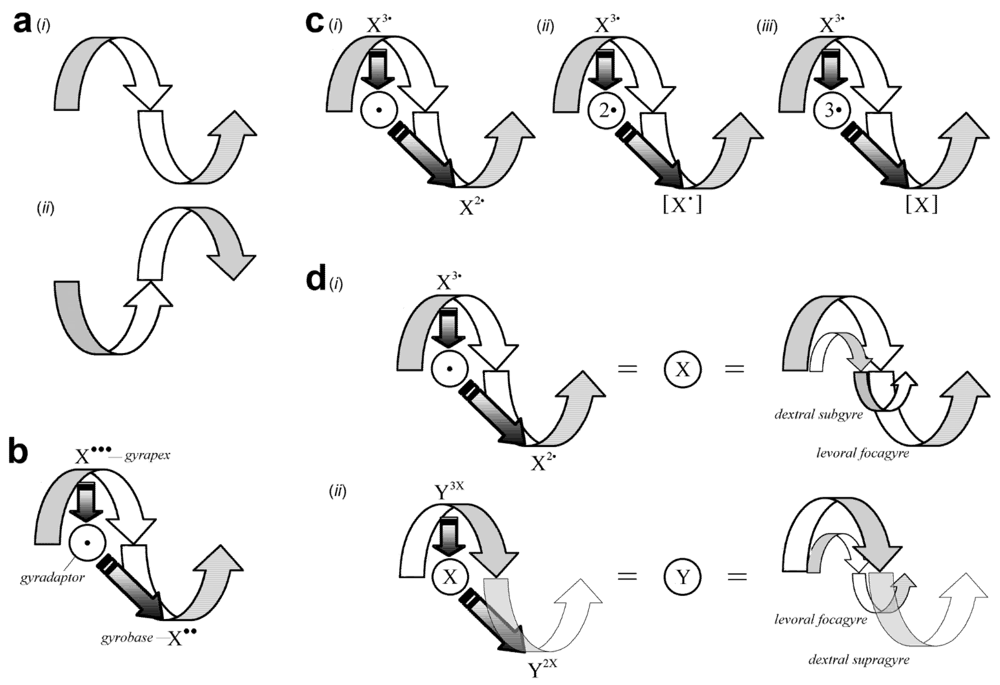
2.1.3. Gyre Thermodynamics
2.1.4. Gyre Forces
2.1.5. Gyre Summary
2.2. Gyromodel Caveats
2.3. Gyromodel Organization
2.3.1. Information, Energy, and Matter
2.3.2. Particle and Quantum
2.3.3. Gyre States
2.3.4. Majorgyres
2.4. Gyromodel Fundamentals
2.4.1. Gyre-Quantum Equality
2.4.2. Wave-Particle Unity
2.4.3. Gyronexus Links and Modules
2.4.4. Gyromodel Learning and Memory
2.4.5. Gyromodel Relativity
2.4.6. Gyromodel Dynamics
2.5. Gyromodel Axioms
3. Theory
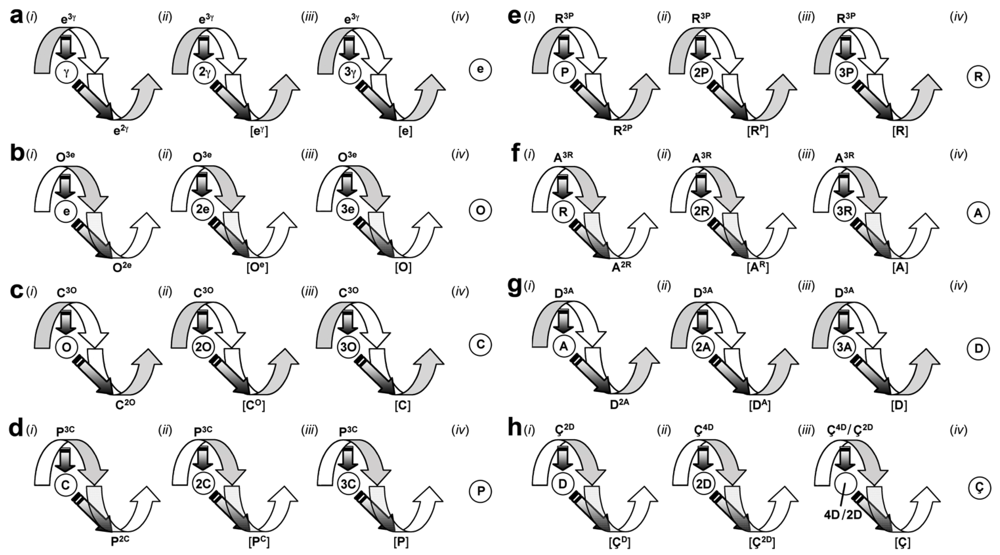
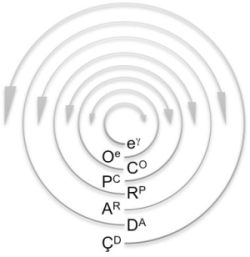
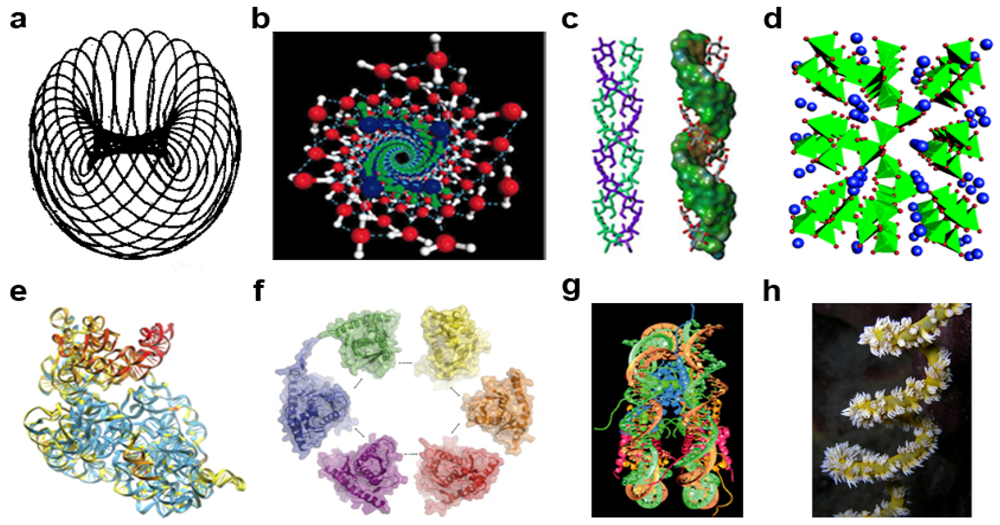
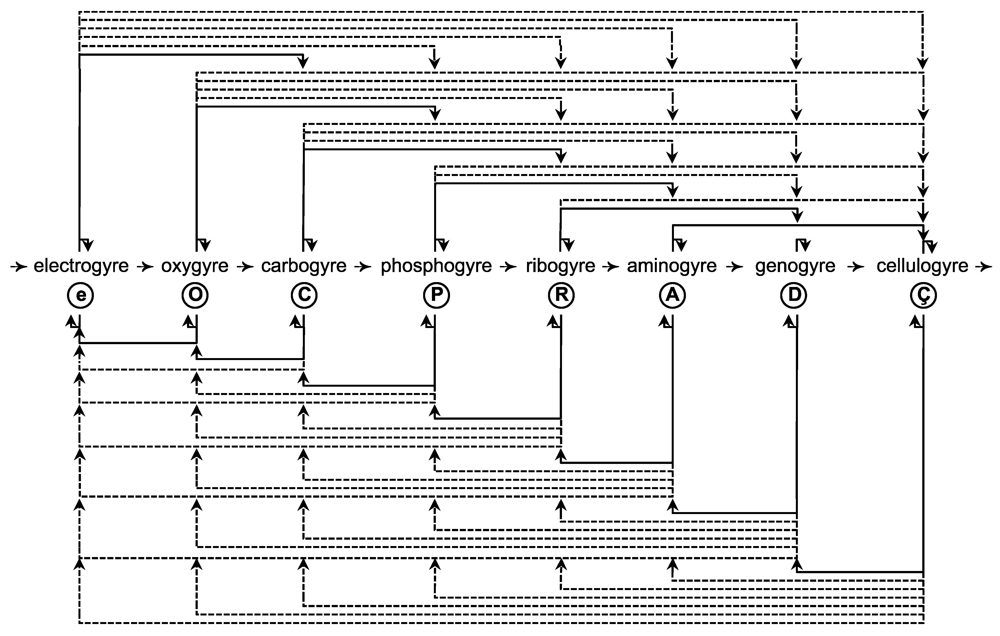
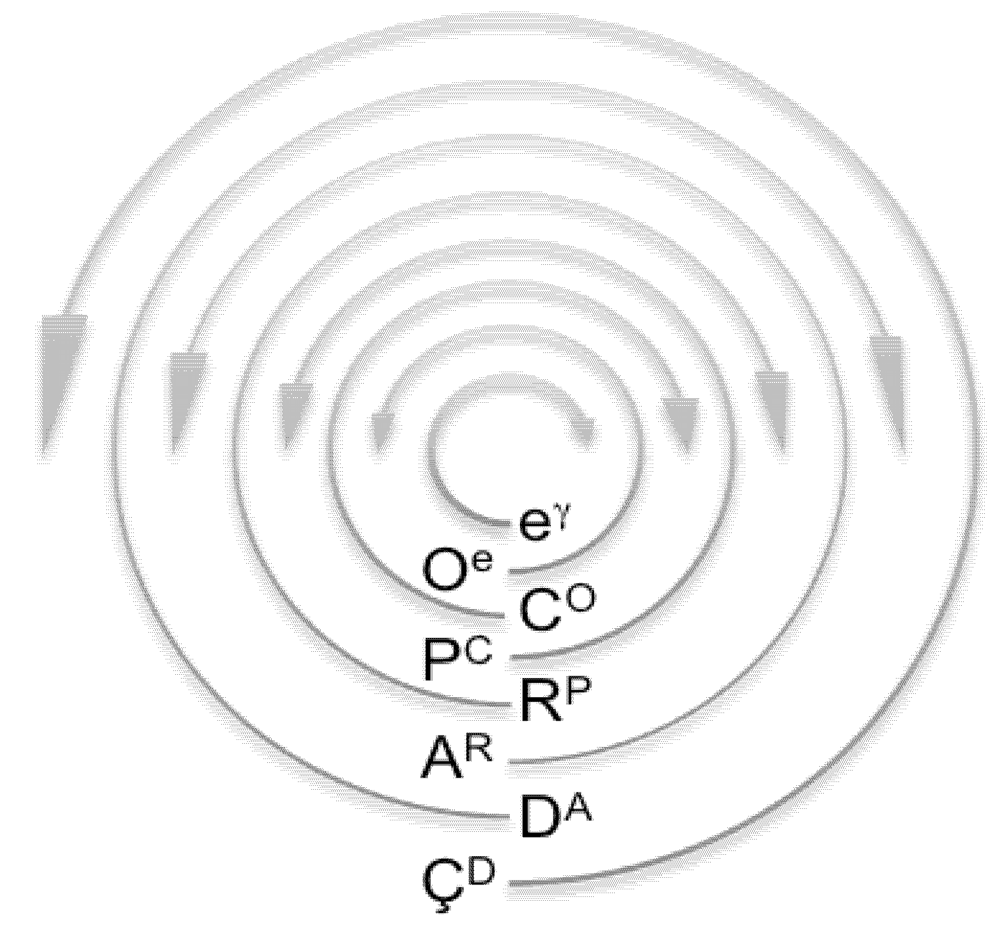
| Gyrostates b | Chirality c | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gyre | Quantum | dIEM | mIEM | Gyradaptor | Gyrapices | Gyrobases | Gyre | 1°/2° e | 3° e |
| Electrogyre | Electron | e | γ d |  | e3γ | e2γ, [eγ], [e] | L | D | L |
| Oxygyre | Oxyon | O | e | ⓔ | O3e | O2e, [Oe], [O] | D | L | D |
| Carbogyre | Carbyon | C | O | Ⓞ | C3O | C2O, [CO], [C] | L | D | L |
| Phosphogyre | Phosphon | P | C | Ⓒ | P3C | P2C, [PC], [P] | D | L | D |
| Ribogyre | Ribon | R | P | Ⓟ | R3P | R2P, [RP], [R] | L | D | L |
| Aminogyre | Aminon | A | R | Ⓡ | A3R | A2R, [AR], [A] | D | L | D |
| Genogyre | Genon | D | A | Ⓐ | D3A | D2A, [DA], [D] | L | D | L |
| Cellulogyre | Cellulon | Ç | D | Ⓓ | Ç4D, Ç2D | [Ç2D], [ÇD], [Ç] | D f | L/D f | D/L f |
3.1. Visible Matter
 ; GVI, GVII, and GXIII). In other words, in reducing its vibrational, rotational, and translational rate, the photon particle literally manifests as the lepton particle.
; GVI, GVII, and GXIII). In other words, in reducing its vibrational, rotational, and translational rate, the photon particle literally manifests as the lepton particle. ; the gyradaptor).
; the gyradaptor). is predicted to be a dextragyre that imparts a dextral spin on e (GXII) whereas gyrosystemic balance occurs by the particle’s trajectory being levoral (GVI). Together, these gyraxioms and this gyrosystem (Figure 2a) explain the chirality of atoms ([73]; Figure 4a). Recall that each symbol in the gyrequation represents one or many particles and that each gyrosystem and -equation can be compressed or expanded accordingly. On this matter, the electrogyre accommodates the three generations of leptons [74], where the proximity to the gyradaptive singularity dictates the stability and energy of the particle.
is predicted to be a dextragyre that imparts a dextral spin on e (GXII) whereas gyrosystemic balance occurs by the particle’s trajectory being levoral (GVI). Together, these gyraxioms and this gyrosystem (Figure 2a) explain the chirality of atoms ([73]; Figure 4a). Recall that each symbol in the gyrequation represents one or many particles and that each gyrosystem and -equation can be compressed or expanded accordingly. On this matter, the electrogyre accommodates the three generations of leptons [74], where the proximity to the gyradaptive singularity dictates the stability and energy of the particle. ⇆ e3γ ⇆ [eγ] + 2
⇆ e3γ ⇆ [eγ] + 2 
 ⇆ [eγ] + 2
⇆ [eγ] + 2 

 ) as metabolizing the electronexus. The further IEM is from the singularity, the lesser the attractorepulsive effect on it. In other words, the more distal IEM has a reduced electronexus cycle rate and forms longer, more stable electronexuses. This gyromodular organization thus explains the origin and emergence of atomic orbitals of all chemical elements and why there exist a greater number of electrons (2, 8, 18, 32…) in outer orbitals, or “shells [77].” The singularity, modeled dextrally to homeostatically balance the left-handed electrogyre, is predicted to induce electronexuses to form right-handed helices (GXII) called electrohelices. Because an electrohelix in one atom exerts attractorepulsive effects (the electrohelix is a gyrating system) on free electrons and electrohelices in other atoms, this gyrosystem explains the emergence of inorganic chemicals that are necessary for the origin and evolution of life [78,79].
) as metabolizing the electronexus. The further IEM is from the singularity, the lesser the attractorepulsive effect on it. In other words, the more distal IEM has a reduced electronexus cycle rate and forms longer, more stable electronexuses. This gyromodular organization thus explains the origin and emergence of atomic orbitals of all chemical elements and why there exist a greater number of electrons (2, 8, 18, 32…) in outer orbitals, or “shells [77].” The singularity, modeled dextrally to homeostatically balance the left-handed electrogyre, is predicted to induce electronexuses to form right-handed helices (GXII) called electrohelices. Because an electrohelix in one atom exerts attractorepulsive effects (the electrohelix is a gyrating system) on free electrons and electrohelices in other atoms, this gyrosystem explains the emergence of inorganic chemicals that are necessary for the origin and evolution of life [78,79]. → e3γ
→ e3γ

 )n and (e3γ ⇆ [eγ] + 2
)n and (e3γ ⇆ [eγ] + 2  )n
)n
 models a macrophoton, and n is an inordinately large number of components in the gyrosystem. This gyrequation reveals that the planetary core (macroelectron) emerges from a macrophoton in a fashion similar to leptonic emergence from a photon. As the macroelectrogyre has the vectorial, spinning, flowing form, it is compatible with the geophysical evidence regarding precession [106]—where a planet rotates on its own axis just like a spinning gyroscope [107]—and the geomagnetic field [108], which is modeled cosmically as it is atomically (Figure 4a). Further, because the excited state of the macroelectrogyre is shared by all the majorgyres, the secondary electrogyre-derived macroelectronexus emerges within and models the inner core of a planet. Being an adaptive supermassive helix, the macroelectronexus is consistent with the notion of a geodynamo in Earth’s core [109] yet diverges from the current idea that the core is liquid iron [110]. Finally, the gyrobase of the tertiary electrogyre (Figure 2a (iii)) models a planetary core, [e], that loses its macrophotonic support. The thermodynamic switch between the two most extreme majorgyrostates (e3γ and e) parsimoniously models geomagnetic reversal, a periodic geophysical event that has not been observed and thus has engendered much speculation [111,112,113].
models a macrophoton, and n is an inordinately large number of components in the gyrosystem. This gyrequation reveals that the planetary core (macroelectron) emerges from a macrophoton in a fashion similar to leptonic emergence from a photon. As the macroelectrogyre has the vectorial, spinning, flowing form, it is compatible with the geophysical evidence regarding precession [106]—where a planet rotates on its own axis just like a spinning gyroscope [107]—and the geomagnetic field [108], which is modeled cosmically as it is atomically (Figure 4a). Further, because the excited state of the macroelectrogyre is shared by all the majorgyres, the secondary electrogyre-derived macroelectronexus emerges within and models the inner core of a planet. Being an adaptive supermassive helix, the macroelectronexus is consistent with the notion of a geodynamo in Earth’s core [109] yet diverges from the current idea that the core is liquid iron [110]. Finally, the gyrobase of the tertiary electrogyre (Figure 2a (iii)) models a planetary core, [e], that loses its macrophotonic support. The thermodynamic switch between the two most extreme majorgyrostates (e3γ and e) parsimoniously models geomagnetic reversal, a periodic geophysical event that has not been observed and thus has engendered much speculation [111,112,113]. ⇆ [e] + 2
⇆ [e] + 2 
3.2. Water
 ). Second, given theoretical expansiveness and particle-quantum relations (GIII), e has alternate applications beyond the hydrogen electron; these are discussed later in this subsection.
). Second, given theoretical expansiveness and particle-quantum relations (GIII), e has alternate applications beyond the hydrogen electron; these are discussed later in this subsection. ) and diphoton (2
) and diphoton (2  ), respectively (Figure 2a (i) and (ii)), the singularity of the secondary electrogyre exerts a greater attractive force on the triquantal excited state electron (e3γ) than does that of the primary electrogyre (recall that majorgyres share the triquantal state). Thus, the electrophilicity of the oxygen atom, like all other electron sinks, is modeled by the relativistically higher magnetism of the secondary electrogyre. Moreover, as gyromodeled, the relative proximity of an electron or electronexus to the singularity affects its metabolic rate.
), respectively (Figure 2a (i) and (ii)), the singularity of the secondary electrogyre exerts a greater attractive force on the triquantal excited state electron (e3γ) than does that of the primary electrogyre (recall that majorgyres share the triquantal state). Thus, the electrophilicity of the oxygen atom, like all other electron sinks, is modeled by the relativistically higher magnetism of the secondary electrogyre. Moreover, as gyromodeled, the relative proximity of an electron or electronexus to the singularity affects its metabolic rate.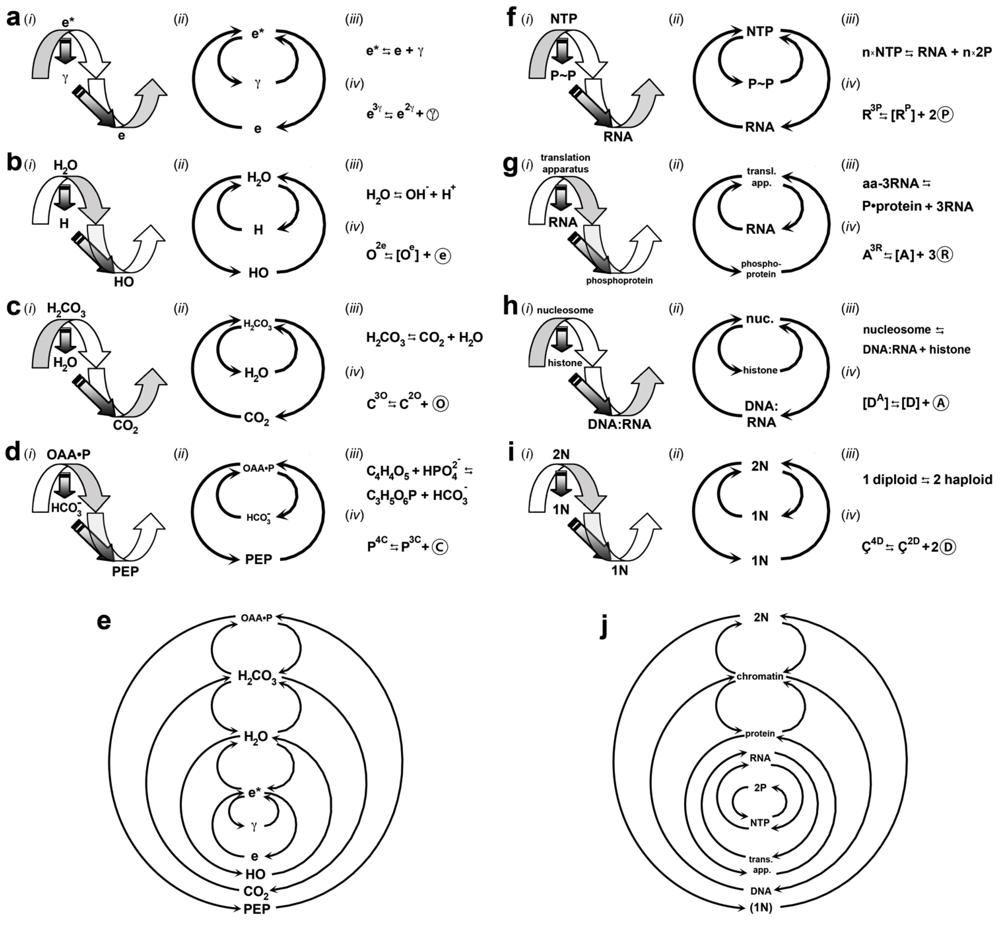
3.3. Organic Matter
 )—even though they are present—for gyromodel consistency and clarity.
)—even though they are present—for gyromodel consistency and clarity. + C3O2e1γ ⇆ C2O2e2γ ⇆ C2O + O2e2γ
+ C3O2e1γ ⇆ C2O2e2γ ⇆ C2O + O2e2γ
3.4. Phosphomembranes
3.5. RNA
3.6. Protein
3.7. DNA
3.8. Cell
4. Conclusions
4.1. Theoretical Solutions, Limitations, and Expectations
4.2. Laws of Nature
4.3. Theoretical Proofs and Implications
4.4. Concluding Remarks
Supplementary Files
Editorial: Publication of Controversial Papers in Life (Shu-Kun Lin) (PDF, 129 KB)
Acknowledgments
References and Notes
- Schrödinger, E. What is Life? The Physical Aspect of the Living Cell; With, Mind And Matter; & Autobiographical Sketches; Cambridge University Press: Cambridge; New York, NY, USA, 1992; p. 184. [Google Scholar]
- Crick, F. Life Itself: Its Origin and Nature; Simon and Schuster: New York, NY, USA, 1981; p. 192. [Google Scholar]
- Hoyle, F.; Wickramasinghe, N.C. Our Place in the Cosmos: The Unfinished Revolution; J.M. Dent: London, UK, 1993; p. 190. [Google Scholar]
- Oparin, A.I.; Morgulis, S. The Origin of Life, 2nd ed.; Dover Publications: Mineola, New York, NY, USA, 2003; p. 270. [Google Scholar]
- Bernal, J.D. The Origin of Life; World Publishing Co.: Cleveland, OH, USA, 1967; p. 345. [Google Scholar]
- Wachtershauser, G. On the chemistry and evolution of the pioneer organism. Chem. Biodivers. 2007, 4, 584–602. [Google Scholar] [CrossRef]
- Gesteland, R.F.; Cech, T.; Atkins, J.F. The RNA World: The Nature of Modern RNA Suggests a Prebiotic RNA, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1999; p. 709. [Google Scholar]
- Gesteland, R.F.; Atkins, J.F. The RNA World: The Nature of Modern RNA Suggests a Prebiotic RNA World; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1993; p. 630. [Google Scholar]
- Gesteland, R.F.; Cech, T.; Atkins, J.F. The RNA World: The Nature of Modern RNA Suggests a Prebiotic RNA World, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2006; p. 768. [Google Scholar]
- Agar, W.E. A Contribution to the Theory of the Living Organism; Melbourne University Press in Association with Oxford University Press: Victoria, Australia, 1943; p. 207. [Google Scholar]
- Cannon, H.G. The Evolution of Living Things; Manchester University Press: Manchester, UK, 1959; p. 152. [Google Scholar]
- De Duve, C. Blueprint for a Cell: The Nature and Origin of Life; N. Patterson: Burlington, NC, USA, 1991; p. 275. [Google Scholar]
- Eigen, M.; Winkler, R. Steps Towards Life: A Perspective on Evolution; Oxford University Press: Oxford; New York, NY, USA, 1992; p. 173. [Google Scholar]
- Elsasser, W.M. Reflections on a Theory of Organisms: Holism in Biology; Published for the Johns Hopkins Dept. of Earth and Planetary Sciences by the Johns Hopkins University Press: Baltimore, MD, USA, 1998; p. 160. [Google Scholar]
- Fry, I. The Emergence of Life on Earth: A Historical and Scientific Overview; Rutgers University Press: New Brunswick, NJ, USA, 2000; p. 327. [Google Scholar]
- Ho, M.-W. The Rainbow and the Worm: The Physics of Organisms; World Scientific: Singapore and River Edge, NJ, USA, 1993; p. 202. [Google Scholar]
- Jacob, F. The Logic of Life: A History of Heredity; Princeton University Press: Princeton, NJ, USA, 1993; p. 348. [Google Scholar]
- Küppers, B.-O. Information and the Origin of Life; MIT Press: Cambridge, MA, USA, 1990; p. 215. [Google Scholar]
- Lahav, N. Biogenesis: Theories of Life’s Origin; Oxford University Press: New York, NY, USA, 1999; p. 349. [Google Scholar]
- Lillie, R.S. General Biology and Philosophy of Organism; University of Chicago Press: Chicago, IL, USA, 1945; p. 215. [Google Scholar]
- Maynard Smith, J.; Szathmáry, E. The Major Transitions in Evolution; W.H. Freeman Spektrum: Oxford, UK and New York, NY, USA, 1995; p. 346. [Google Scholar]
- Weiss, P.A. The Science of Life: The Living System—A System for Living; Futura Publishing Co.: Mount Kisco, NY, USA, 1973; p. 137. [Google Scholar]
- Zubay, G.L. Origins of Life on the Earth and in the Cosmos, 2nd ed.; Academic Press: San Diego, CA, USA, 2000; p. 564. [Google Scholar]
- Davies, P.C.W. The Fifth Miracle: The Search for the Origin and Meaning of Life; Simon & Schuster: New York, NY, USA, 1999; p. 304. [Google Scholar]
- Hargittai, I.; Pickover, C.A. Spiral Symmetry; World Scientific: Singapore and Teaneck, NJ, USA, 1992; p. 449. [Google Scholar]
- Ball, P. The Self-Made Tapestry: Pattern Formation in Nature; Oxford University Press: Oxford, UK and New York, NY, USA, 1999; p. 287. [Google Scholar]
- Ginzburg, V.B.; Ginzburg, T.V. Prime Elements of Ordinary Matter, Dark Matter & Dark Energy: Beyond Standard Model & String Theory, 2nd ed.; Universal Publishers: Boca Raton, FL, USA, 2007; p. 434. [Google Scholar]
- Thompson, D.A.W. On Growth and Form; Dover: New York, NY, USA, 1992; p. 1116. [Google Scholar]
- Bojowald, M. Singularities and quantum gravity. Cosmol. Gravit. 2007, 910, 294–333. [Google Scholar]
- Chrusciel, P.T. Black holes. Conform. Struct. Space Time 2002, 604, 61–102. [Google Scholar] [CrossRef]
- De Duve, C. Singularities: Landmarks on the Pathways of Life; Cambridge University Press: Cambridge, UK and New York, NY, USA, 2005; p. 258. [Google Scholar]
- Blackmond, D.G. The origin of biological homochirality. Cold Spring Harbor Perspect. Biol. 2010, 2, a002147. [Google Scholar] [CrossRef]
- Jantsch, E. The Self-Organizing Universe: Scientific and Human Implications of the Emerging Paradigm of Evolution, 1st ed.; Pergamon Press: Oxford; New York, NY, USA, 1980; p. 343. [Google Scholar]
- Babloyantz, A. Molecules, Dynamics, and Life: An Introduction to Self-Organization of Matter; Wiley: New York, NY, USA, 1986; p. 345. [Google Scholar]
- Solé, R.V.; Bascompte, J. Self-Organization in Complex Ecosystems; Princeton University Press: Princeton, NJ, USA, 2006; p. 373. [Google Scholar]
- Feltz, B.; Crommelinck, M.; Goujon, P. Self-Organization and Emergence in Life Sciences; Springer: Dordrecht, The Netherlands, 2006; p. 360. [Google Scholar]
- McCabe, G. The non-unique universe. Found. Phys. 2010, 40, 629–637. [Google Scholar] [CrossRef]
- Gödel, K. On Formally Undecidable Propositions of Principia Mathematica and Related Systems; Dover: Mineola, NY, USA, 1962. [Google Scholar]
- Hogan, D.A. Talking to themselves: Autoregulation and quorum sensing in fungi. Eukaryot. Cell 2006, 5, 613–619. [Google Scholar] [CrossRef]
- Tappy, L.; Chiolero, R.; Berger, M. Autoregulation of glucose production in health and disease. Curr. Opin. Clin. Metab. Care 1999, 2, 161–164. [Google Scholar] [CrossRef]
- Bateman, E. Autoregulation of eukaryotic transcription factors. Progr. Nucleic Acid Res. Mol. Biol. 1998, 60, 133–168. [Google Scholar] [CrossRef]
- Hajdukovic, D.S. Dark energy, antimatter gravity and geometry of the Universe. Astrophys. Space Sci. 2010, 330, 1–5. [Google Scholar] [CrossRef]
- Bacinich, E.J.; Kriz, T.A. The arrow of time in an expanding 3-sphere. Phys. Essays 1999, 12, 80–91. [Google Scholar] [CrossRef]
- Reid, R.G.B. Biological Emergences: Evolution by Natural Experiment; MIT Press: Cambridge, MA, USA, 2007; p. 517. [Google Scholar]
- Bak, P.; Paczuski, M. Complexity, contingency, and criticality. Proc. Natl. Acad. Sci. USA 1995, 92, 6689–6696. [Google Scholar] [CrossRef]
- Von Bertalanffy, L. General System Theory; Foundations, Development, Applications; G. Braziller: New York, NY, USA, 1969; p. 289. [Google Scholar]
- Kauffman, S.A. The Origins of Order: Self-Organization and Selection in Evolution; Oxford University Press: New York, NY, USA, 1993; p. 709. [Google Scholar]
- Bird, R.J. Chaos and Life: Complexity and Order in Evolution and Thought; Columbia University Press: New York, NY, USA, 2003; p. 322. [Google Scholar]
- Yockey, H.P. Information Theory, Evolution, and the Origin of Life; Cambridge University Press: New York, NY, USA, 2005; p. 259. [Google Scholar]
- Laszlo, E. The Systems View of the World: A Holistic Vision for Our Time; Hampton Press: Cresskill, NJ, USA, 1996; p. 103. [Google Scholar]
- Lorenz, E.N. The Essence of Chaos; University of Washington Press: Seattle, DC, USA, 1993; p. 227. [Google Scholar]
- Standish, R.K. Why Occam’s razor. Found. Phys. Lett. 2004, 17, 255–266. [Google Scholar] [CrossRef]
- Anderson, P.W. More is different. Science 1997, 177, 393–396. [Google Scholar]
- Umpleby, S.A. Physical relationships among matter, energy and information (Reprinted form Cybernetics and Systems ‘04, 2004). Syst. Res. Behav. Sci. 2007, 24, 369–372. [Google Scholar] [CrossRef]
- Smil, V. Energies: An Illustrated Guide to the Biosphere and Civilization; MIT Press: Cambridge, MA, USA, 1999; p. 210. [Google Scholar]
- Wrigglesworth, J.M. Energy and Life; Taylor & Francis: London, UK and Bristol, PA, USA, 1997. [Google Scholar]
- Lehninger, A.L. Bioenergetics; the Molecular Basis of Biological Energy Transformations, 2nd ed.; W.A. Benjamin: Menlo Park, CA, USA, 1971; p. 245. [Google Scholar]
- Von Foerster, H. Understanding Understanding: Essays on Cybernetics and Cognition; Springer: New York, NY, USA, 2003; p. 362. [Google Scholar]
- Dakos, V.; Kefi, S.; Rietkerk, M.; van Nes, E.H.; Scheffer, M. Slowing down in spatially patterned ecosystems at the brink of collapse. Am. Nat. 2011, 177, E153–E166. [Google Scholar] [CrossRef]
- Devreotes, P. Dictyostelium discoideum: A model system for cell-cell interactions in development. Science 1989, 245, 1054–1058. [Google Scholar]
- Kuznetsov, E.A. Wave collapse in plasmas and fluids. Chaos 1996, 6, 381–390. [Google Scholar] [CrossRef]
- Anraku, Y. Bacterial electron transport chains. Annu. Rev. Biochem. 1988, 57, 101–132. [Google Scholar] [CrossRef]
- Kovacic, P.; Pozos, R.S. Bioelectronome. Integrated approach to receptor chemistry, radicals, electrochemistry, cell signaling, and physiological effects based on electron transfer. J. Recept. Signal Transduct. Res. 2007, 27, 261–294. [Google Scholar] [CrossRef]
- Trevors, J.T.; Masson, L. Quantum Microbiology. Curr. Issues Mol. Biol. 2011, 13, 43–49. [Google Scholar]
- Smolin, L. A crisis in fundamental physics. Update, N. Y. Acad. Sci. Mag. 2006, 10–14. [Google Scholar]
- Nagel, S. Physics in crisis. Phys. Today 2002, 55, 55–57. [Google Scholar] [CrossRef]
- Heller, M.; Pysiak, L.; Sasin, W. Fundamental Problems in the unification of physics. Found. Phys. 2011, 41, 905–918. [Google Scholar] [CrossRef]
- Nikolic, H. Quantum mechanics: Myths and facts. Found. Phys. 2007, 37, 1563–1611. [Google Scholar] [CrossRef]
- Turyshev, S.G. Experimental Tests of General Relativity. Annu. Rev. Nuclear Part. Sci. 2008, 58, 207–248. [Google Scholar] [CrossRef]
- Penrose, R. The Road to Reality: A Complete Guide to the Laws of the Universe, 1st Vintage Books ed.; Vintage Books: New York, NY, USA, 2007; p. 1099. [Google Scholar]
- Lyons, L. An introduction to the possible substructure of quarks and leptons. Progr. Part. Nuclear Phys. 1983, 10, 227–304. [Google Scholar] [CrossRef]
- Hegstrom, R.A. Electron Chirality. Theochem J. Mol. Struct. 1991, 78, 17–21. [Google Scholar] [CrossRef]
- Hegstrom, R.A. Weak neutral current and beta-radiolysis effects on the origin of biomolecular chirality. Nature 1985, 315, 749–750. [Google Scholar] [CrossRef]
- Bryman, D. Three generations of quarks and leptons: Who ordered that? Intersect. Part. Nuclear Phys. 2000, 549, 150–163. [Google Scholar]
- Arunan, E.; Raghavendra, B. Unpaired and sigma bond electrons as H, Cl, and Li bond acceptors: An anomalous one-electron blue-shifting chlorine bond. J. Phys. Chem. A 2007, 111, 9699–9706. [Google Scholar] [CrossRef]
- Haviland, D.B.; Delsing, P. Cooper-pair charge solitons: The electrodynamics of localized charge in a superconductor. Phys. Rev. B 1996, 54, R6857–R6860. [Google Scholar] [CrossRef]
- Reiher, M.; Eickerling, G. The shell structure of atoms. J. Chem. Theory Comput. 2008, 4, 286–296. [Google Scholar] [CrossRef]
- Williams, R.J.P. The fundamental nature of life as a chemical system: The part played by inorganic elements. J. Inorg. Biochem. 2002, 88, 241–250. [Google Scholar] [CrossRef]
- Williams, R.J.P.; da Silva, J.J.R.F. Evolution was chemically constrained. J. Theor. Biol. 2003, 220, 323–343. [Google Scholar] [CrossRef]
- Truscott, A.G.; Strecker, K.E.; McAlexander, W.I.; Partridge, G.B.; Hulet, R.G. Observation of Fermi pressure in a gas of trapped atoms. Science 2001, 291, 2570–2572. [Google Scholar]
- Gensemer, S.D.; Jin, D.S. Transition from collisionless to hydrodynamic behavior in an ultracold Fermi gas. Phys. Rev. Lett. 2001, 87. [Google Scholar] [CrossRef]
- Anderson, P.W.; Casey, P.A. Hidden Fermi liquid; the moral: A good effective low-energy theory is worth all of Monte Carlo with Las Vegas thrown in. J. Phys. Condens. Matter 2010, 22. [Google Scholar] [CrossRef]
- Mook, H.A.; Dai, P.; Dogan, F.; Hunt, R.D. One-dimensional nature of the magnetic fluctuations in YBa2Cu3O6.6. Nature 2000, 404, 729–731. [Google Scholar]
- Sharma, R.P.; Ogale, S.B.; Zhang, Z.H.; Liu, J.R.; Chu, W.K.; Veal, B.; Paulikas, A.; Zheng, H.; Venkatesan, T. Phase transitions in the incoherent lattice fluctuations in YBa2Cu3O(7-delta). Nature 2000, 404, 736–740. [Google Scholar]
- Zwierlein, M.W.; Abo-Shaeer, J.R.; Schirotzek, A.; Schunck, C.H.; Ketterle, W. Vortices and superfluidity in a strongly interacting Fermi gas. Nature 2005, 435, 1047–1051. [Google Scholar]
- Galitski, V.M.; Refael, G.; Fisher, M.P.; Senthil, T. Vortices and quasiparticles near the superconductor-insulator transition in thin films. Phys. Rev. Lett. 2005, 95. [Google Scholar] [CrossRef]
- Kopnin, N.B. Vortex dynamics and mutual friction in superconductors and Fermi superfluids. Rep. Prog. Phys. 2002, 65, 1633–1678. [Google Scholar] [CrossRef]
- Carati, A.; Galgani, L.; Giorgilli, A. The Fermi-Pasta-Ulam problem as a challenge for the foundations of physics. Chaos 2005, 15. [Google Scholar] [CrossRef]
- Moreover, given that the electrogyre is a cyclical, periodic system in which all particles within the gyre continually adapt to one another through photon mobilization and storage, this provides a novel solution to the Fermi-Pasta-Ulam problem.
- Kalmus, P.I.P. The forces of nature. Interdiscip. Sci. Rev. 1993, 18, 343–349. [Google Scholar] [CrossRef]
- The frequently presented 2D lengthening wavelengths of the electromagnetic spectrum from gamma rays to radio rays are modeled as the 4D expansion or widening of the electrogyre from the photonic singularity.
- Uman, M.A.; Krider, E.P. A review of natural lightning—Experimental-data and modeling. IEEE Trans. Electromagn. Compat. 1982, 24, 79–112. [Google Scholar] [CrossRef]
- Rowland, H.L. Theories and simulations of elves, sprites and blue jets. J. Atmos. Solar Terr. Phys. 1998, 60, 831–844. [Google Scholar] [CrossRef]
- The electromagnetic spectral signature of the photonic threshold effect is observed in the colored pre-lightning emissions called red sprites and blue jets.
- Stenhoff, M. Ball Lightning: An Unsolved Problem in Atmospheric Physics; Kluwer Academic: New York, NY, USA, 1999; p. 349. [Google Scholar]
- Abrahamson, J.; Dinniss, J. Ball lightning caused by oxidation of nanoparticle networks from normal lightning strikes on soil. Nature 2000, 403, 519–521. [Google Scholar] [CrossRef]
- Bohr, N. On the notions of causality and complementarity. Science 1950, 111, 51–54. [Google Scholar]
- Pearle, P.; Collett, B. Wavefunction collapse and random walk. Found. Phys. 2003, 33, 1495–1541. [Google Scholar] [CrossRef]
- Karakostas, V.; Dickson, M. Decoherence in unorthodox formulations of quantum-mechanics. Synthese 1995, 102, 61–97. [Google Scholar] [CrossRef]
- Busch, P.; Heinonen, T.; Lahti, P. Heisenberg’s uncertainty principle. Phys. Rep. Rev. Sect. Phys. Lett. 2007, 452, 155–176. [Google Scholar]
- Marco, R.; Diaz, C.; Benguria, A.; Mateos, J.; Mas, J.; de Juan, E. The role of gravity in the evolutionary emergence of multicellular complexity: Microgravity effects on arthropod development and aging. Adv. Space Res. 1999, 23, 2075–2082. [Google Scholar]
- Dubinin, N.P.; Vaulina, E.N. The evolutionary role of gravity. Life Sci. Space Res. 1976, 14, 47–55. [Google Scholar]
- Yoshino, T.; Walter, M.J.; Katsura, T. Core formation in planetesimals triggered by permeable flow. Nature 2003, 422, 154–157. [Google Scholar] [CrossRef]
- Stevenson, D.J. Models of the Earth’s Core. Science 1981, 214, 611–619. [Google Scholar]
- Taylor, S.R. The origin of the earth. AGSO J. Aust. Geol. Geophys. 1997, 17, 27–31. [Google Scholar]
- Forte, A.M.; Mitrovica, J.X. A resonance in the Earth’s obliquity and precession over the past 20 Myr driven by mantle convection. Nature 1997, 390, 676–680. [Google Scholar]
- Zhang, Y.Z.; Luo, J.; Nie, Y.X. Gravitational effects of rotating bodies. Mod. Phys. Lett. A 2001, 16, 789–794. [Google Scholar] [CrossRef]
- Sokoloff, D.D. Geodynamo and models of geomagnetic field generation: A review. Geomagn. Aeron. 2004, 44, 533–542. [Google Scholar]
- Buffett, B.A. Earth’s core and the geodynamo. Science 2000, 288, 2007–2012. [Google Scholar] [CrossRef]
- Sreenivasan, B. Modelling the geodynamo: Progress and challenges. Curr. Sci. 2010, 99, 1739–1750. [Google Scholar]
- Olson, P.; Amit, H. Changes in earth’s dipole. Die Naturwissenschaften 2006, 93, 519–542. [Google Scholar] [CrossRef]
- Greff-Lefftz, M.; Legros, H. Core rotational dynamics and geological events. Science 1999, 286, 1707–1709. [Google Scholar] [CrossRef]
- Courtillot, V.; Besse, J. Magnetic field reversals, polar wander, and core-mantle coupling. Science 1987, 237, 1140–1147. [Google Scholar]
- Crary, F.J.; Clarke, J.T.; Dougherty, M.K.; Hanlon, P.G.; Hansen, K.C.; Steinberg, J.T.; Barraclough, B.L.; Coates, A.J.; Gerard, J.C.; Grodent, D.; et al. Solar wind dynamic pressure and electric field as the main factors controlling Saturn’s aurorae. Nature 2005, 433, 720–722. [Google Scholar]
- Smith, E.J.; Davis, L., Jr.; Jones, D.E.; Colburn, D.S.; Coleman, P.J., Jr.; Dyal, P.; Sonett, C.P. Magnetic field of jupiter and its interaction with the solar wind. Science 1974, 183, 305–306. [Google Scholar]
- Tarduno, J.A.; Cottrell, R.D.; Watkeys, M.K.; Hofmann, A.; Doubrovine, P.V.; Mamajek, E.E.; Liu, D.; Sibeck, D.G.; Neukirch, L.P.; Usui, Y. Geodynamo, solar wind, and magnetopause 3.4 to 3.45 billion years ago. Science 2010, 327, 1238–1240. [Google Scholar]
- Khodachenko, M.L.; Ribas, I.; Lammer, H.; Griessmeier, J.M.; Leitner, M.; Selsis, F.; Eiroa, C.; Hanslmeier, A.; Biernat, H.K.; Farrugia, C.J.; et al. Coronal mass ejection (CME) activity of low mass M stars as an important factor for the habitability of terrestrial exoplanets. I. CME impact on expected magnetospheres of Earth-like exoplanets in close-in habitable zones. Astrobiology 2007, 7, 167–184. [Google Scholar] [CrossRef]
- Howard, T. Coronal Mass Ejections: An Introduction; Springer: New York, NY, USA, 2011. [Google Scholar]
- Cho, A. Particle physics. Hints of greater matter-antimatter asymmetry challenge theorists. Science 2010, 328, 1087. [Google Scholar] [CrossRef]
- Ellis, J. Particle physics: Antimatter matters. Nature 2003, 424, 631–634. [Google Scholar] [CrossRef]
- Buser, R. The formation and early evolution of the Milky Way galaxy. Science 2000, 287, 69–74. [Google Scholar] [CrossRef]
- Alibert, Y.; Broeg, C.; Benz, W.; Wuchterl, G.; Grasset, O.; Sotin, C.; Eiroa, C.; Henning, T.; Herbst, T.; Kaltenegger, L.; et al. Origin and formation of planetary systems. Astrobiology 2010, 10, 19–32. [Google Scholar] [CrossRef]
- Craig, N.C.; Gislason, E.A. First law of thermodynamics; Irreversible and reversible processes. J. Chem. Educ. 2002, 79, 193–200. [Google Scholar] [CrossRef]
- Livio, M. The Accelerating Universe: Infinite Expansion, the Cosmological Constant, and the Beauty of the Cosmos; Wiley: New York, NY, USA, 2000; p. 274. [Google Scholar]
- Guth, A.H. The Inflationary Universe: The Quest for a New Theory of Cosmic Origins; Addison-Wesley Publishing: Reading, MA, USA, 1997; p. 358. [Google Scholar]
- Peebles, P.J.E.; Ratra, B. The cosmological constant and dark energy. Rev. Mod. Phys. 2003, 75, 559–606. [Google Scholar] [CrossRef]
- Frieman, J.A.; Turner, M.S.; Huterer, D. Dark energy and the accelerating universe. Annu. Rev. Astron. Astrophys. 2008, 46, 385–432. [Google Scholar] [CrossRef]
- Freese, K. Review of observational evidence for dark matter in the universe and in upcoming searches for dark stars. Cral Ipnl 2009, 36, 113–126. [Google Scholar]
- Morgan, C.L. Emergent Evolution; the Gifford Lectures; Williams and Norgate: London, UK, 1923; p. 313. [Google Scholar]
- Bergson, H. Creative Evolution; University Press of America: Lanham, MD, USA, 1984; p. 407. [Google Scholar]
- Koonin, E.V. The Biological Big Bang model for the major transitions in evolution. Biology Direct 2007, 2, 21–27. [Google Scholar] [CrossRef]
- Brack, A. Liquid water and the origin of life. Orig. Life Evol. Biosph. 1993, 23, 3–10. [Google Scholar] [CrossRef]
- Ball, P. Water: Water—An enduring mystery. Nature 2008, 452, 291–292. [Google Scholar] [CrossRef]
- Pollack, G.H.; Figueroa, X.; Zhao, Q. Molecules, water, and radiant energy: New clues for the origin of life. Int. J. Mol. Sci. 2009, 10, 1419–1429. [Google Scholar] [CrossRef]
- Robert, F. Isotope geochemistry. The origin of water on earth. Science 2001, 293, 1056–1058. [Google Scholar] [CrossRef]
- Truskett, T.M.; Dill, K.A. A simple analytical model of water. Biophys. Chem. 2003, 105, 449–459. [Google Scholar] [CrossRef]
- Ben-Naim, A. Molecular Theory of Water and Aqueous Solutions; World Scientific: Singapore and Hackensack, NJ, USA, 2009; p. 629. [Google Scholar]
- Zachariassen, K.E.; Kristiansen, E. Ice nucleation and antinucleation in nature. Cryobiology 2000, 41, 257–279. [Google Scholar] [CrossRef]
- Dobretsov, N.L. On the early evolutionary stage of the geosphere and biosphere and the problem of early glaciations. Paleontol. J. 2010, 44, 827–838. [Google Scholar] [CrossRef]
- Van Dishoeck, E.F.; Blake, G.A. Chemical evolution of star-forming regions. Annu. Rev. Astron. Astrophys. 1998, 36, 317–368. [Google Scholar] [CrossRef]
- Johari, G.P.; Hallbrucker, A.; Mayer, E. Two calorimetrically distinct states of liquid water below 150 kelvin. Science 1996, 273, 90–92. [Google Scholar]
- Pal, S.; Sankaran, N.B.; Samanta, A. Structure of a self-assembled chain of water molecules in a crystal host. Angew. Chem. 2003, 42, 1741–1743. [Google Scholar] [CrossRef]
- Goldblatt, C.; Zahnle, K.J. Faint young Sun paradox remains. Nature 2011, 474, E3–E4. [Google Scholar]
- Rosing, M.T.; Bird, D.K.; Sleep, N.H.; Bjerrum, C.J. No climate paradox under the faint early Sun. Nature 2010, 464, 744–U117. [Google Scholar] [CrossRef]
- Jacobsen, S.D.; van der Lee, S.F.M. Earth’s Deep Water Cycle; American Geophysical Union: Washington, DC, USA, 2006; p. 313. [Google Scholar]
- Hellevang, H. On the forcing mechanism for the H(2)-driven deep biosphere. Int. J. Astrobiol. 2008, 7, 157–167. [Google Scholar] [CrossRef]
- Oze, C.; Sharma, M. Serpentinization and the inorganic synthesis of H-2 in planetary surfaces. Icarus 2007, 186, 557–561. [Google Scholar] [CrossRef]
- Pierrehumbert, R.; Gaidos, E. Hydrogen greenhouse planets beyond the habitable zone. Astrophys. J. Lett. 2011. [Google Scholar] [CrossRef]
- Tian, F.; Toon, O.B.; Pavlov, A.A.; de Sterck, H. A hydrogen-rich early Earth atmosphere. Science 2005, 308, 1014–1017. [Google Scholar]
- Zakharov, V.V.; Brodskaya, E.N.; Laaksonen, A. Surface tension of water droplets: A molecular dynamics study of model and size dependencies. J. Chem. Phys. 1997, 107, 10675–10683. [Google Scholar]
- Claussen, W.F. Surface tension and surface structure of water. Science 1967, 156, 1226–1227. [Google Scholar]
- Stokes, G. On the theory of oscillatory waves. Trans. Camb. Phil. Soc. 1847, 8, 441–455. [Google Scholar]
- Constantin, A.; Strauss, W. Rotational steady water waves near stagnation. Philos. Trans. Ser. A 2007, 365, 2227–2239. [Google Scholar] [CrossRef]
- Poitevin, B. The continuing mystery of the Memory of Water. Homeopathy 2008, 97, 39–41. [Google Scholar] [CrossRef]
- Davenas, E.; Beauvais, F.; Amara, J.; Oberbaum, M.; Robinzon, B.; Miadonna, A.; Tedeschi, A.; Pomeranz, B.; Fortner, P.; Belon, P.; et al. Human basophil de-granulation triggered by very dilute antiserum against ige. Nature 1988, 333, 816–818. [Google Scholar] [CrossRef]
- Vega, C.; Conde, M.M.; McBride, C.; Abascal, J.L.; Noya, E.G.; Ramirez, R.; Sese, L.M. Heat capacity of water: A signature of nuclear quantum effects. J. Chem. Phys. 2010, 132. [Google Scholar] [CrossRef] [Green Version]
- Wyrtki, K.; Wenzel, J. Possible gyre gyre interaction in the Pacific-Ocean. Nature 1984, 309, 538–540. [Google Scholar] [CrossRef]
- Rypina, I.I.; Pratt, L.J.; Lozier, M.S. Near-surface transport pathways in the north atlantic ocean: Looking for throughput from the subtropical to the subpolar gyre. J. Phys. Oceanogr. 2011, 41, 911–925. [Google Scholar]
- Zeng, X.C.; Bai, J.E.; Wang, J. Multiwalled ice helixes and ice nanotubes. Proc. Natl. Acad. Sci. USA 2006, 103, 19664–19667. [Google Scholar]
- Dismukes, G.C.; Klimov, V.V.; Baranov, S.V.; Kozlov, Y.N.; DasGupta, J.; Tyryshkin, A. The origin of atmospheric oxygen on Earth: The innovation of oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 2001, 98, 2170–2175. [Google Scholar]
- Schafer, G. How did the Earth’s oxygen atmosphere originate? Anasthesiol. Intensivmmed. Notfallmedizin Schmerzther. 2004, 39, S19–S27. [Google Scholar] [CrossRef]
- Thuillier, G. The Sun-Earth relationship. C. R. Acad. Sci. Ser. II 2001, 333, 311–328. [Google Scholar]
- Falkowski, P.G.; Godfrey, L.V. Electrons, life and the evolution of Earth’s oxygen cycle. Philos. Trans. R. Soc. Lond. Ser. B 2008, 363, 2705–2716. [Google Scholar] [CrossRef]
- Wayne, R.P. Atmospheric chemistry—The evolution of our atmosphere. J. Phothchem. Photobiol. A 1992, 62, 379–396. [Google Scholar] [CrossRef]
- Hyde, W.T.; Crowley, T.J.; Baum, S.K.; Peltier, W.R. Neoproterozoic ‘snowball Earth’ simulations with a coupled climate/ice-sheet model. Nature 2000, 405, 425–429. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Gaidos, E.J.; Bertani, L.E.; Beukes, N.J.; Gutzmer, J.; Maepa, L.N.; Steinberger, R.E. Paleoproterozoic snowball earth: Extreme climatic and geochemical global change and its biological consequences. Proc. Natl. Acad. Sci. USA 2000, 97, 1400–1405. [Google Scholar]
- Sessions, A.L.; Doughty, D.M.; Welander, P.V.; Summons, R.E.; Newman, D.K. The continuing puzzle of the great oxidation event. Curr. Biol. 2009, 19, R567–R574. [Google Scholar] [CrossRef]
- Kleidon, A. Life, hierarchy, and the thermodynamic machinery of planet Earth. Phys. Life Rev. 2010, 7, 424–460. [Google Scholar] [CrossRef]
- Brown, G.C.; Mussett, A.E. The Inaccessible Earth: An Integrated View to Its Structue and Composition, 2nd ed.; Chapman & Hall: London; New York, NY, USA, 1993; p. 276. [Google Scholar]
- Alfe, D.; Price, G.D.; Gillan, M.J. Oxygen in the Earth’s core: A first-principles study. Phys. Earth Planet. Inter. 1999, 110, 191–210. [Google Scholar] [CrossRef]
- Dai, W.; Song, X.D. Detection of motion and heterogeneity in Earth’s liquid outer core. Geophys. Res. Lett. 2008. [Google Scholar] [CrossRef]
- Lutgens, F.K.; Tarbuck, E.J. Essentials of Geology, 6th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1998; p. 450. [Google Scholar]
- Jacoby, W.R. Successes and failures in geodynamics: From past to future. J. Geodyn. 2001, 32, 3–27. [Google Scholar] [CrossRef]
- Jordan, P. The Expanding Earth; Some Consequences of Dirac’s Gravitation Hypothesis, 1st ed.; Pergamon Press: Oxford, New York, NY, USA, 1971; p. 202. [Google Scholar]
- Betelev, N.P. The concept of an expanding earth. J. Volcanol. Seismol. 2009, 3, 355–362. [Google Scholar] [CrossRef]
- Van Kranendonk, M.J. Volcanic degassing, hydrothermal circulation and the flourishing of early life on Earth: A review of the evidence from c. 3490–3240 Ma rocks of the Pilbara Supergroup, Pilbara Craton, Western Australia. Earth Sci. Rev. 2006, 74, 197–240. [Google Scholar] [CrossRef]
- Smith, A.D.; Lewis, C. The planet beyond the plume hypothesis. Earth Sci. Rev. 1999, 48, 135–182. [Google Scholar] [CrossRef]
- Rogers, J.J.W.; Santosh, M. Supercontinents in earth history. Gondwana Res. 2003, 6, 357–368. [Google Scholar] [CrossRef]
- Dewit, M.J.; Hart, R.A. Earths earliest continental lithosphere, hydrothermal flux and crustal recycling. Lithos 1993, 30, 309–335. [Google Scholar] [CrossRef]
- de Wit, M.J. On Archean granites, greenstones, cratons and tectonics: Does the evidence demand a verdict? Precambrian Res. 1998, 91, 181–226. [Google Scholar] [CrossRef]
- Perez-Malvaez, C.; Alfredo, B.H.; Manuel, F.O.; Rosaura, R.R. Ninety-four years of the theory of the continental drift of Alfred Lothar Wegener. Interciencia 2006, 31, 536–543. [Google Scholar]
- Varga, P. On origins of geodynamics and of modern seismology. Acta Geod. Geophys. Hungar. 2010, 45, 231–252. [Google Scholar] [CrossRef]
- Ito, K. Towards a new view of earthquake phenomena. Pure Appl. Geophys. 1992, 138, 531–548. [Google Scholar] [CrossRef]
- Freund, F. Pre-earthquake signals: Underlying physical processes. J. Asian Earth Sci. 2011, 41, 383–400. [Google Scholar] [CrossRef]
- Teisseyre, R.; Takeo, M.; Majewski, E. Earthquake Source Asymmetry, Structural Media and Rotation Effects; Springer: Berlin, Germany and New York, NY, USA, 2006; p. 582. [Google Scholar]
- Harris, R.A. Earthquake stress triggers, stress shadows, and seismic hazard. Curr. Sci. 2000, 79, 1215–1225. [Google Scholar]
- Johnston, A.C.; Schweig, E.S. The enigma of the New Madrid earthquakes of 1811–1812. Annu. Rev. Earth Planet. Sci. 1996, 24, 339–384. [Google Scholar] [CrossRef]
- Simpson, J.F. Solar activity as a triggering mechanism for earthquakes. Earth Planet. Sci. Lett. 1967, 3, 417–425. [Google Scholar] [CrossRef]
- Canup, R.M.; Asphaug, E. Origin of the Moon in a giant impact near the end of the Earth’s formation. Nature 2001, 412, 708–712. [Google Scholar]
- Taylor, S.R. Origin of the terrestrial planets and the moon. J. R. Soc. West. Aust. 1996, 79 Pt 1, 59–65. [Google Scholar]
- Singer, S.F.; Bandermann, L.W. Where was the moon formed? Science 1970, 170, 438–439. [Google Scholar]
- Hartmann, W.K.; Phillips, R.J.; Taylor, G.J. Origin of the Moon; Lunar & Planetary Institute: Houston, TX, USA, 1986; p. 781. [Google Scholar]
- Hughes, D.W. The open question in selenology. Nature 1987, 327. [Google Scholar] [CrossRef]
- Prettyman, T.H.; Hagerty, J.J.; Elphic, R.C.; Feldman, W.C.; Lawrence, D.J.; McKinney, G.W.; Vaniman, D.T. Elemental composition of the lunar surface: Analysis of gamma ray spectroscopy data from Lunar Prospector. J. Geophys. Res. Planets 111. [CrossRef]
- Kleine, T.; Palme, H.; Mezger, K.; Halliday, A.N. Hf-W chronometry of lunar metals and the age and early differentiation of the Moon. Science 2005, 310, 1671–1674. [Google Scholar] [CrossRef]
- In addition to resembling the superceded fission hypothesis, this theoretical relationship is spot-on with calculations that time Earth’s origin at ~4.54 billion years ago (bya) and the Moon’s origin at ~4.52 bya, within ~50 million years of Solar System origin.
- Hauri, E.H.; Weinreich, T.; Saal, A.E.; Rutherford, M.C.; van Orman, J.A. High pre-eruptive water contents preserved in lunar melt inclusions. Science 2011, 333, 213–215. [Google Scholar]
- Clark, R.N. Detection of adsorbed water and hydroxyl on the Moon. Science 2009, 326, 562–564. [Google Scholar] [CrossRef]
- Pieters, C.M.; Goswami, J.N.; Clark, R.N.; Annadurai, M.; Boardman, J.; Buratti, B.; Combe, J.P.; Dyar, M.D.; Green, R.; Head, J.W.; et al. Character and spatial distribution of OH/H2O on the surface of the Moon seen by M3 on Chandrayaan-1. Science 2009, 326, 568–572. [Google Scholar] [CrossRef]
- Ward, W.R. Past orientation of the lunar spin axis. Science 1975, 189, 377–379. [Google Scholar]
- Foster, R.G.; Roenneberg, T. Human responses to the geophysical daily, annual and lunar cycles. Curr. Biol. 2008, 18, R784–R794. [Google Scholar] [CrossRef]
- Keeling, C.D.; Whorf, T.P. The 1800-year oceanic tidal cycle: A possible cause of rapid climate change. Proc. Natl. Acad. Sci. USA 2000, 97, 3814–3819. [Google Scholar] [CrossRef]
- Trask, N.J.; Rowan, L.C. Lunar Orbiter Photographs: Some Fundamental Observations: Preliminary study reveals details of craters, crater distributions, and the major types of terrain. Science 1967, 158, 1529–1535. [Google Scholar]
- Alvarez, W.; Claeys, P.; Kieffer, S.W. Emplacement of cretaceous-tertiary boundary shocked quartz from chicxulub crater. Science 1995, 269, 930–935. [Google Scholar]
- Zahnle, K.; Dones, L.; Levison, H.F. Cratering rates on the Galilean satellites. Icarus 1998, 136, 202–222. [Google Scholar] [CrossRef]
- In other words, this theory indicates that craters are not due to impact (from without to within) but rather from “expact” (a cratering force exerted from within to without).
- Catling, D.C.; Glein, C.R.; Zahnle, K.J.; Mckay, C.P. Why O-2 is required by complex life on habitable planets and the concept of planetary “oxygenation time”. Astrobiology 2005, 5, 415–438. [Google Scholar] [CrossRef]
- Farquhar, J.; Johnston, D.T. The oxygen cycle of the terrestrial planets: Insights into the processing and history of oxygen in surface environments. Rev. Mineral. Geochem.. 2008, 68, 463–492. [Google Scholar] [CrossRef]
- De Leeuw, N.H.; Catlow, C.R.; King, H.E.; Putnis, A.; Muralidharan, K.; Deymier, P.; Stimpfl, M.; Drake, M.J. Where on Earth has our water come from? Chem. Commun. 2010, 46, 8923–8925. [Google Scholar]
- Valencia, D.; Sasselov, D.D.; O’Connell, R.J. Radius and structure models of the first super-earth planet. Astrophys. J. 2007, 656, 545–551. [Google Scholar] [CrossRef]
- Segura, A.; Walkowicz, L.M.; Meadows, V.; Kasting, J.; Hawley, S. The effect of a strong stellar flare on the atmospheric chemistry of an earth-like planet orbiting an M dwarf. Astrobiology 2010, 10, 751–771. [Google Scholar] [CrossRef]
- Hegstrom, R.A.; Chamberlain, J.P.; Seto, K.; Watson, R.G. Mapping the weak chirality of atoms. Am.J. Phys. 1988, 56, 1086–1092. [Google Scholar]
- Bai, J.; Wang, J.; Zeng, X.C. Multiwalled ice helixes and ice nanotubes. Proc. Natl. Acad. Sci. USA 2006, 103, 19664–19667. [Google Scholar]
- Perez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch Starke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Wright, A.J.; Jackson, L.E.; Kariuki, B.M.; Smith, M.E.; Barralet, J.E. Synthesis and structure of a calcium polyphosphate with a unique criss-cross arrangement of helical phosphate chains. Chem. Mater. 2005, 17, 4642–4646. [Google Scholar]
- Korostelev, A.; Trakhanov, S.; Laurberg, M.; Noller, H.F. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell 2006, 126, 1065–1077. [Google Scholar] [CrossRef]
- Liu, Q.; Greimann, J.C.; Lima, C.D. Reconstitution, activities, and structure of the eukaryotic RNA exosome. Cell 2006, 127, 1223–1237. [Google Scholar]
- Luger, K.; Mader, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997, 389, 251–260. [Google Scholar]
- Cairns-Smith, A.G. Seven Clues to the Origin of Life: A Scientific Detective Story; Cambridge University Press: Cambridge, UK and New York, NY, USA, 1990; p. 131. [Google Scholar]
- Calusaru, A. Why life can be only a carbon based chemistry. Rev. Roum. Chim. 1989, 34, 1787–1798. [Google Scholar]
- Levine, J.S.; Augustsson, T.R.; Natarajan, M. The prebiological paleoatmosphere: Stability and composition. Orig. Life 1982, 12, 245–259. [Google Scholar] [CrossRef]
- Kasting, J.F. The evolution of the prebiotic atmosphere. Orig. Life 1984, 14, 75–82. [Google Scholar] [CrossRef]
- Chittenden, G.J.; Schwartz, A.W. Prebiotic photosynthetic reactions. Biol. Syst. 1981, 14, 15–32. [Google Scholar]
- Osborne, C.P.; Beerling, D.J. Nature’s green revolution: The remarkable evolutionary rise of C4 plants. Philos. Trans. R. Soc. Lond. Ser. B 2006, 361, 173–194. [Google Scholar] [CrossRef]
- Liedl, K.R.; Hage, W.; Hallbrucker, A.; Mayer, E. Carbonic acid in the gas phase and its astrophysical relevance. Science 1998, 279, 1332–1335. [Google Scholar] [CrossRef]
- Garg, L.C.; Maren, T.H. The rates of hydration of carbon dioxide and dehydration of carbonic acid at 37 degrees. Biochim. Biophys. Acta 1972, 261, 70–76. [Google Scholar] [CrossRef]
- Harris, D.C. Charles David Keeling and the story of atmospheric CO2 measurements. Anal. Chem. 2010, 82, 7865–7870. [Google Scholar] [CrossRef]
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO2 emissions and climate change from existing energy infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef]
- Adams, J.M.; Piovesan, G. Long series relationships between global interannual CO2 increment and climate: Evidence for stability and change in role of the tropical and boreal-temperate zones. Chemosphere 2005, 59, 1595–1612. [Google Scholar] [CrossRef]
- Schubert, E. The theory of and experimentation into respiratory gas exchange—Carl Ludwig and his school. Pflugers Arch. 1996, 432, R111–119. [Google Scholar]
- Piiper, J. Carbon dioxide-oxygen relationships in gas exchange of animals. In memory of Hermann Rahn. Boll. Soc. Ital. Biol. Sper. 1991, 67, 635–658. [Google Scholar]
- Casey, J.R. Why bicarbonate? Biochem. Cell Biol. 2006, 84, 930–939. [Google Scholar] [CrossRef]
- Dore, J.E.; Lukas, R.; Sadler, D.W.; Church, M.J.; Karl, D.M. Physical and biogeochemical modulation of ocean acidification in the central North Pacific. Proc. Natl. Acad. Sci. USA 2009, 106, 12235–12240. [Google Scholar]
- Flores, C.L.; Rodriguez, C.; Petit, T.; Gancedo, C. Carbohydrate and energy-yielding metabolism in non-conventional yeasts. FEMS Microbiol. Rev. 2000, 24, 507–529. [Google Scholar]
- Siebers, B.; Schonheit, P. Unusual pathways and enzymes of central carbohydrate metabolism in Archaea. Curr. Opin. Microbiol. 2005, 8, 695–705. [Google Scholar] [CrossRef]
- Kandler, O.; Gibbs, M. Asymmetric distribution of C in the glucose phosphates formed during photosynthesis. Plant Physiology 1956, 31, 411–412. [Google Scholar] [CrossRef]
- Fraser, N.J.; Hashimoto, H.; Cogdell, R.J. Carotenoids and bacterial photosynthesis: The story so far. Photosynth. Res. 2001, 70, 249–256. [Google Scholar] [CrossRef]
- Cleaves, H.J. The prebiotic geochemistry of formaldehyde. Precambrian Res. 2008, 164, 111–118. [Google Scholar] [CrossRef]
- Kalapos, M.P. A possible evolutionary role of formaldehyde. Exp. Mol. Med. 1999, 31, 1–4. [Google Scholar]
- Feng, S.H.; Tian, G.; He, C.; Yuan, H.M.; Mu, Y.; Wang, Y.W.; Wang, L. Hydrothermal biochemistry: From formaldehyde to oligopeptides. J. Mater. Sci. 2008, 43, 2418–2425. [Google Scholar]
- Sutherland, J.D.; Weaver, G.W. Synthesis of bis(glycoaldehyde) phosphodiester and mixed glycoaldehyde-triose phosphodiesters. Tetrahedron Lett. 1994, 35, 9109–9112. [Google Scholar] [CrossRef]
- Toxvaerd, S. Homochirality in bio-organic systems and glyceraldehyde in the formose reaction. J. Biol. Phys. 2005, 31, 599–606. [Google Scholar] [CrossRef]
- Hazen, R.M.; Deamer, D.W. Hydrothermal reactions of pyruvic acid: Synthesis, selection, and self-assembly of amphiphilic molecules. Orig. Life Evol. Biosph. 2007, 37, 143–152. [Google Scholar]
- Martin, S.T.; Guzman, M.I. Prebiotic metabolism: Production by mineral photoelectrochemistry of alpha-ketocarboxylic acids in the reductive tricarboxylic acid cycle. Astrobiology 2009, 9, 833–842. [Google Scholar] [CrossRef]
- Lazcano, A.; Dworkin, J.P.; Miller, S.L. The roads to and from the RNA world. J. Theor. Biol. 2003, 222, 127–134. [Google Scholar] [CrossRef]
- Bielski, R.; Tencer, M. A possible path to the RNA world: Enantioselective and diastereoselective purification of ribose. Orig. Life Evol. Biosph. 2007, 37, 167–175. [Google Scholar] [CrossRef]
- Fiechter, A.; Fuhrmann, G.F.; Kappeli, O. Regulation of glucose metabolism in growing yeast cells. Adv. Microb. Physiol. 1981, 22, 123–183. [Google Scholar] [CrossRef]
- Fukasawa, T.; Nogi, Y. Molecular genetics of galactose metabolism in yeast. Biotechnology 1989, 13, 1–18. [Google Scholar]
- Benner, S.A.; Kim, H.J.; Ricardo, A.; Illangkoon, H.I.; Kim, M.J.; Carrigan, M.A.; Frye, F. Synthesis of carbohydrates in mineral-guided prebiotic cycles. J. Am. Chem. Soc. 2011, 133, 9457–9468. [Google Scholar]
- Berner, R.A. The long-term carbon cycle, fossil fuels and atmospheric composition. Nature 2003, 426, 323–326. [Google Scholar] [CrossRef]
- Kasting, J.F.; Siefert, J.L. Life and the evolution of Earth’s atmosphere. Science 2002, 296, 1066–1068. [Google Scholar]
- Nunn, J.F. Evolution of the atmosphere. Proc. Geol. Assoc. 1998, 109, 1–13. [Google Scholar] [CrossRef]
- Hohmann-Marriott, M.F.; Blankenship, R.E. Evolution of photosynthesis. Annu. Rev. Plant Biol. 2011, 62, 515–548. [Google Scholar] [CrossRef]
- Xiong, J.; Fischer, W.M.; Inoue, K.; Nakahara, M.; Bauer, C.E. Molecular evidence for the early evolution of photosynthesis. Science 2000, 289, 1724–1730. [Google Scholar]
- Heinrich, R.; Melendez-Hevia, E.; Montero, F.; Nuno, J.C.; Stephani, A.; Waddell, T.G. The structural design of glycolysis: An evolutionary approach. Biochem. Soc. Trans. 1999, 27, 294–298. [Google Scholar]
- Plaxton, W.C. The Organization and Regulation of Plant Glycolysis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 185–214. [Google Scholar] [CrossRef]
- Guest, J.R.; Russell, G.C. Complexes and complexities of the citric acid cycle in Escherichia coli. Curr. Top. Cell. Regul. 1992, 33, 231–247. [Google Scholar]
- Thauer, R.K. Citric-acid cycle, 50 years on. Modifications and an alternative pathway in anaerobic bacteria. Eur. J. Biochem. FEBS 1988, 176, 497–508. [Google Scholar] [CrossRef]
- Martin, W.; Schnarrenberger, C. The evolution of the Calvin cycle from prokaryotic to eukaryotic chromosomes: A case study of functional redundancy in ancient pathways through endosymbiosis. Curr. Genet. 1997, 32, 1–18. [Google Scholar] [CrossRef]
- Orgel, L.E. Self-organizing biochemical cycles. Proc. Natl. Acad. Sci. USA 2000, 97, 12503–12507. [Google Scholar] [CrossRef]
- Holland, H.D. The Chemistry of the Atmosphere and Oceans; Wiley: New York, NY, USA, 1978; p. 351. [Google Scholar]
- Cintas, P. Tracing the origins and evolution of chirality and handedness in chemical language. Angew. Chem. 2007, 46, 4016–4024. [Google Scholar] [CrossRef]
- Ribo, J.M.; Crusats, J.; Sagues, F.; Claret, J.; Rubires, R. Chiral sign induction by vortices during the formation of mesophases in stirred solutions. Science 2001, 292, 2063–2066. [Google Scholar]
- Imberty, A.; Chanzy, H.; Perez, S.; Buleon, A.; Tran, V. The double-helical nature of the crystalline part of A-starch. J. Mol. Biol. 1988, 201, 365–378. [Google Scholar] [CrossRef]
- Vietor, R.J.; Newman, R.H.; Ha, M.A.; Apperley, D.C.; Jarvis, M.C. Conformational features of crystal-surface cellulose from higher plants. Plant J. 2002, 30, 721–731. [Google Scholar] [CrossRef]
- Sikorski, P.; Hori, R.; Wada, M. Revisit of alpha-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 2009, 10, 1100–1105. [Google Scholar] [CrossRef]
- Hirabayashi, J. On the origin of glycome and saccharide recognition. Trends Glycosci. Glycotechnol. 2004, 16, 63–85. [Google Scholar] [CrossRef]
- Head, I.M.; Jones, D.M.; Larter, S.R. Biological activity in the deep subsurface and the origin of heavy oil. Nature 2003, 426, 344–352. [Google Scholar]
- Glasby, G.P. Abiogenic origin of hydrocarbons: An historical overview. Resour. Geol. 2006, 56, 85–98. [Google Scholar]
- Rushdi, A.I.; Simoneit, B.R. Abiotic synthesis of organic compounds from carbon disulfide under hydrothermal conditions. Astrobiology 2005, 5, 749–769. [Google Scholar] [CrossRef]
- McCollom, T.M.; Simoneit, B.R. Abiotic formation of hydrocarbons and oxygenated compounds during thermal decomposition of iron oxalate. Orig. Life Evol. Biosph. 1999, 29, 167–186. [Google Scholar] [CrossRef]
- Sugisaki, R.; Mimura, K. Mantle hydrocarbons: Abiotic or biotic? Geochim. Cosmochim. Acta 1994, 58, 2527–2542. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Kasting, J.F.; Brown, L.L.; Rages, K.A.; Freedman, R. Greenhouse warming by CH4 in the atmosphere of early Earth. J. Geophys. Res. Planets 2000, 105, 11981–11990. [Google Scholar] [CrossRef]
- Sorokhtin, O.G. Generation of abiogenic methane in the earth’s history. Oceanology 2005, 45, 500–510. [Google Scholar]
- Vyshemirskii, V.S.; Kontorovich, A.E. Evolution of the formation of gaseous hydrocarbons in the Earth’s history. Geol. I Geofiz. 1998, 39, 1392–1401. [Google Scholar]
- Matthews, C.N. Hydrogen cyanide polymerization: A preferred cosmochemical pathway. J. Br. Interplanet. Soc. 1992, 45, 43–48. [Google Scholar]
- Colin-Garcia, M.; Negron-Mendoza, A.; Ramos-Bernal, S. Organics produced by irradiation of frozen and liquid HCN solutions: Implications for chemical evolution studies. Astrobiology 2009, 9, 279–288. [Google Scholar] [CrossRef]
- This includes carbides ([C]1 models C4−, [C]2 is C24−, and [C]3 is C32−; note the triquantal form) and cyanides (-CN, where N models the quantized e), the latter being thought especially important to the origin of essential biopolymers. Recall that the particle has quantal potential (GIII-1) such that the link between the C atoms can be e, 2e, 3e. Modeling further, an e (electron) gyrolink between C gyromodules accounts for all the alkanes (linear or cyclic), the single bond between carbon atoms; the 2e (dielectron) gyrolink models alkenes, the double bond; and the 3e (trielectron) gyrolink models alkynes, the triple bond. (Again, please note the triquantal organization.) The cycling and re-organization of electrons is found in any other organic chemicals, for example, as in alkadienes.
- Cheng, Q.; Thomas, S.M.; Rouviere, P. Biological conversion of cyclic alkanes and cyclic alcohols into dicarboxylic acids: Biochemical and molecular basis. Appl. Microbiol. Biotechnol. 2002, 58, 704–711. [Google Scholar]
- Spormann, A.M.; Widdel, F. Metabolism of alkylbenzenes, alkanes, and other hydrocarbons in anaerobic bacteria. Biodegradation 2000, 11, 85–105. [Google Scholar] [CrossRef]
- MacFarland, H.N. Toxicology of petroleum hydrocarbons. Occup. Med. 1988, 3, 445–454. [Google Scholar]
- Catling, D.C.; Zahnle, K.J.; McKay, C. Biogenic methane, hydrogen escape, and the irreversible oxidation of early Earth. Science 2001, 293, 839–843. [Google Scholar] [CrossRef]
- Bender, M.L.; Battle, M.; Keeling, R.F. The O2 balance of the atmosphere: A tool for studying the fate of fossil-fuel CO2. Annu. Rev. Energy Environ. 1998, 23, 207–223. [Google Scholar] [CrossRef]
- Wolfson, R. Energy, Environment, and Climate, 1st ed.; W.W. Norton & Company: New York, NY, USA, 2008; p. 532. [Google Scholar]
- Richmond, G.L.; McFearin, C.L.; Beaman, D.K.; Moore, F.G. From Franklin to today: Toward a molecular level understanding of bonding and adsorption at the oil-water interface. J. Phys. Chem. C 2009, 113, 1171–1188. [Google Scholar]
- Trevors, J.T. Possible origin of a membrane in the subsurface of the Earth. Cell Biol. Int. 2003, 27, 451–457. [Google Scholar] [CrossRef]
- Weber, A.L. Chemical constraints governing the origin of metabolism: The thermodynamic landscape of carbon group transformations under mild aqueous conditions. Orig. Life Evol. Biosph. 2002, 32, 333–357. [Google Scholar] [CrossRef]
- Further, the exchanged electron in these oxygyres can represent any primary, secondary, or tertiary electrogyre or combination thereof. Finally, because a carbyon particle exists in either one gyrostate or another in spacetime (GV), its arrangement within a polymer can undergo gyrostate interconversion depending upon the gyradaptive forces of the oxyon.
- Monnard, P.A.; Maurer, S.E.; Deamer, D.W.; Boncella, J.M. Chemical evolution of amphiphiles: Glycerol monoacyl derivatives stabilize plausible prebiotic membranes. Astrobiology 2009, 9, 979–987. [Google Scholar] [CrossRef]
- Ohlrogge, J.B.; Jaworski, J.G. Regulation of Fatty Acid Synthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 109–136. [Google Scholar] [CrossRef]
- Slabas, A.R.; Brown, A.; Sinden, B.S.; Swinhoe, R.; Simon, J.W.; Ashton, A.R.; Whitfeld, P.R.; Elborough, K.M. Pivotal reactions in fatty acid synthesis. Prog. Lipid Res. 1994, 33, 39–46. [Google Scholar] [CrossRef]
- Weber, A.L. Origin of fatty-acid synthesis—Thermodynamics and kinetics of reaction pathways. J. Mol. Evol. 1991, 32, 93–100. [Google Scholar] [CrossRef]
- Huber, C.; Wachtershauser, G. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science 1997, 276, 245–247. [Google Scholar] [CrossRef]
- Goldanskii, V.I. Cold prebiotic evolution, tunneling, chirality and exobiology. Phys. Orig. Homochirality Life 1996, 211–230. [Google Scholar]
- Venema, K.; Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar] [CrossRef]
- Beauchamp, E.; Rioux, V.; Legrand, P. New regulatory and signal functions for myristic acid. Med. Sci. 2009, 25, 57–63. [Google Scholar]
- Dabadie, H.; Peuchant, E.; Motta, C.; Bernard, M.; Mendy, F. Myristic acid: Effects on HDL, omega3, LDL oxidation and membrane fluidity. Sci. Des Aliment. 2008, 28, 134–142. [Google Scholar] [CrossRef]
- Biochemistry of Lipids, Lipoproteins and Membranes, 5th ed.; Vance, D.E.; Vance, J.E. (Eds.) Elsevier: Amsterdam, The Netherlands and Boston, MA, USA, 2008; p. 631.
- Kuksis, A. Fatty Acids and Glycerides; Plenum Press: New York, NY, USA, 1978; p. 469. [Google Scholar]
- Fujita, Y.; Matsuoka, H.; Hirooka, K. Regulation of fatty acid metabolism in bacteria. Mol. Microbiol. 2007, 66, 829–839. [Google Scholar] [CrossRef]
- Van Roermund, C.W.; Waterham, H.R.; Ijlst, L.; Wanders, R.J. Fatty acid metabolism in Saccharomyces cerevisiae. Cell. Mol. Life Sci. 2003, 60, 1838–1851. [Google Scholar] [CrossRef]
- Small, D.M. The effects of glyceride structure on absorption and metabolism. Annu. Rev. Nutr. 1991, 11, 413–434. [Google Scholar] [CrossRef]
- Hsieh, H.H.; Jewitt, D. A population of comets in the main asteroid belt. Science 2006, 312, 561–563. [Google Scholar] [CrossRef]
- Brown, M.E.; Barkume, K.M.; Ragozzine, D.; Schaller, E.L. A collisional family of icy objects in the Kuiper belt. Nature 2007, 446, 294–296. [Google Scholar]
- Michel, P.; Benz, W.; Richardson, D.C. Disruption of fragmented parent bodies as the origin of asteroid families. Nature 2003, 421, 608–611. [Google Scholar]
- Levison, H.F.; Duncan, M.J.; Brasser, R.; Kaufmann, D.E. Capture of the Sun’s Oort cloud from stars in its birth cluster. Science 2010, 329, 187–190. [Google Scholar]
- Stern, S.A. The evolution of comets in the Oort cloud and Kuiper belt. Nature 2003, 424, 639–642. [Google Scholar] [CrossRef]
- Sunshine, J.M.; A’Hearn, M.F.; Groussin, O.; Li, J.Y.; Belton, M.J.; Delamere, W.A.; Kissel, J.; Klaasen, K.P.; McFadden, L.A.; Meech, K.J.; et al. Exposed water ice deposits on the surface of comet 9P/Tempel 1. Science 2006, 311, 1453–1455. [Google Scholar]
- Mumma, M.J.; DiSanti, M.A.; Dello Russo, N.; Fomenkova, M.; Magee-Sauer, K.; Kaminski, C.D.; Xie, D.X. Detection of abundant ethane and methane, along with carbon monoxide and water, in comet C/1996 B2 Hyakutake: Evidence for interstellar origin. Science 1996, 272, 1310–1314. [Google Scholar]
- Kirschvink, J.L.; Maine, A.T.; Vali, H. Paleomagnetic evidence of a low-temperature origin of carbonate in the Martian meteorite ALH84001. Science 1997, 275, 1629–1633. [Google Scholar]
- Fischer, T.P.; Burnard, P.; Marty, B.; Hilton, D.R.; Furi, E.; Palhol, F.; Sharp, Z.D.; Mangasini, F. Upper-mantle volatile chemistry at Oldoinyo Lengai volcano and the origin of carbonatites. Nature 2009, 459, 77–80. [Google Scholar]
- Cooper, G.; Kimmich, N.; Belisle, W.; Sarinana, J.; Brabham, K.; Garrel, L. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 2001, 414, 879–883. [Google Scholar]
- For example, cometary orbit around planets and their moons is modeled as an ohiogyre, with the binary planet-moon system exerting the attractorepulsive effects that adapt and sustain the cyclical path.
- Free, A.; Barton, N.H. Do evolution and ecology need the Gaia hypothesis? Trends Ecol. Evol. 2007, 22, 611–619. [Google Scholar] [CrossRef]
- Kerr, R.A. No Longer Willful, Gaia Becomes Respectable: The Gaia hypothesis, that Earth is a single huge organism intentionally creating an optimum environment for itself; has been made more palatable; interesting science is coming of it. Science 1988, 240, 393–395. [Google Scholar]
- Lovelock, J. Gaia: The Practical Science of Planetary Medicine; Oxford University Press: Oxford, UK and New York, NY, USA, 2000; p. 192. [Google Scholar]
- Monnard, P.A.; Deamer, D.W. Membrane self-assembly processes: Steps toward the first cellular life. The Anatomical Record 2002, 268, 196–207. [Google Scholar] [CrossRef]
- Norris, V.; Raine, D.J. A fission-fusion origin for life. Orig. Life Evol. Biosph. 1998, 28, 523–537. [Google Scholar] [CrossRef]
- Maddox, J. Origin of the first cell membrane? Nature 1994, 371. [Google Scholar] [CrossRef]
- Lipmann, F. Metabolic Generation and Utilization of Phosphate Bond Energy; Wiley: Hoboken, NJ, USA, 2006; Volume 1. [Google Scholar]
- Pasek, M.A. Rethinking early Earth phosphorus geochemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 853–858. [Google Scholar] [CrossRef]
- Schopf, J.W.; Packer, B.M. Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science 1987, 237, 70–73. [Google Scholar]
- Knoll, A.H. Life on a Young Planet: The First Three Billion Years of Evolution on Earth; Princeton University Press: Princeton, NJ, USA, 2003; p. 277. [Google Scholar]
- Liebl, V.; Novak, V.J.; Masinovsky, Z.; Pacltova, B.; Bejsovcova, L. The evolution of prebiological self-organization: Probable colloid-chemical evolution of first prokaryotic cells. Orig. Life 1984, 14, 323–334. [Google Scholar] [CrossRef]
- Luisi, P.L.; Rasi, P.S.; Mavelli, F. A possible route to prebiotic vesicle reproduction. Artif. Life 2004, 10, 297–308. [Google Scholar] [CrossRef]
- Monnard, P.A.; Ziock, H.J. Question 9: Prospects for the construction of artificial cells or protocells. Orig. Life Evol. Biosph. 2007, 37, 469–472. [Google Scholar] [CrossRef]
- Qian, H.; Beard, D.A. Thermodynamics of stoichiometric biochemical networks in living systems far from equilibrium. Biophys. Chem. 2005, 114, 213–220. [Google Scholar] [CrossRef]
- Alberty, R.A. Biochemical thermodynamics. Biochim. Biophys. Acta 1994, 1207, 1–11. [Google Scholar] [CrossRef]
- Walsh, C.T.; Benson, T.E.; Kim, D.H.; Lees, W.J. The versatility of phosphoenolpyruvate and its vinyl ether products in biosynthesis. Chem. Biol. 1996, 3, 83–91. [Google Scholar] [CrossRef]
- Gabor, E.; Gohler, A.K.; Kosfeld, A.; Staab, A.; Kremling, A.; Jahreis, K. The phosphoenolpyruvate-dependent glucose-phosphotransferase system from Escherichia coli K-12 as the center of a network regulating carbohydrate flux in the cell. Eur. J. Cell Biol. 2011, 90, 711–720. [Google Scholar] [CrossRef]
- McCleary, W.R.; Stock, J.B.; Ninfa, A.J. Is acetyl phosphate a global signal in Escherichia coli? J. Bacteriol. 1993, 175, 2793–2798. [Google Scholar]
- Wolfe, A.J.; Chang, D.E.; Walker, J.D.; Seitz-Partridge, J.E.; Vidaurri, M.D.; Lange, C.F.; Pruss, B.M.; Henk, M.C.; Larkin, J.C.; Conway, T. Evidence that acetyl phosphate functions as a global signal during biofilm development. Mol. Microbiol. 2003, 48, 977–988. [Google Scholar] [CrossRef]
- Hers, H.G.; Hue, L. Gluconeogenesis and related aspects of glycolysis. Annu. Rev. Biochem. 1983, 52, 617–653. [Google Scholar] [CrossRef]
- Mather, M.W.; Gennis, R.B. Kinetic studies of the lipid-activated pyruvate oxidase flavoprotein of Escherichia coli. J. Biol. Chem. 1985, 260, 16148–16155. [Google Scholar]
- Svensson, P.; Blasing, O.E.; Westhoff, P. Evolution of C4 phosphoenolpyruvate carboxylase. Arch. Biochem. Biophys. 2003, 414, 180–188. [Google Scholar] [CrossRef]
- Comte, B.; Vincent, G.; Bouchard, B.; Des Rosiers, C. Probing the origin of acetyl-CoA and oxaloacetate entering the citric acid cycle from the 13C labeling of citrate released by perfused rat hearts. J. Biol. Chem. 1997, 272, 26117–26124. [Google Scholar]
- Kent, C.; Carman, G.M.; Spence, M.W.; Dowhan, W. Regulation of eukaryotic phospholipid metabolism. FASEB J. 1991, 5, 2258–2266. [Google Scholar]
- Alb, J.G., Jr.; Kearns, M.A.; Bankaitis, V.A. Phospholipid metabolism and membrane dynamics. Curr. Opin. Cell. Biol. 1996, 8, 534–541. [Google Scholar] [CrossRef]
- Hamilton, J.A. Fatty acid transport: Difficult or easy? J. Lipid Res. 1998, 39, 467–481. [Google Scholar]
- Walter, A.; Kuehl, G.; Barnes, K.; VanderWaerdt, G. The vesicle-to-micelle transition of phosphatidylcholine vesicles induced by nonionic detergents: Effects of sodium chloride, sucrose and urea. Biochim. Biophys. Acta 2000, 1508, 20–33. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Opatowski, E.; Kozlov, M.M. Phase boundaries in mixtures of membrane-forming amphiphiles and micelle-forming amphiphiles. Biochim. Biophys. Acta 2000, 1508, 1–19. [Google Scholar] [CrossRef]
- Zhang, J.; Jing, B.; Tokutake, N.; Regen, S.L. Transbilayer complementarity of phospholipids. A look beyond the fluid mosaic model. J. Am. Chem. Soc. 2004, 126, 10856–10857. [Google Scholar]
- Wisniewska, A.; Draus, J.; Subczynski, W.K. Is a fluid-mosaic model of biological membranes fully relevant? Studies on lipid organization in model and biological membranes. Cell. Mol. Biol. Lett. 2003, 8, 147–159. [Google Scholar]
- Sinensky, M. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef]
- Williams, N.H. Models for biological phosphoryl transfer. Biochim. Biophys. Acta 2004, 1697, 279–287. [Google Scholar] [CrossRef]
- Florian, J.; Warshel, A. A fundamental assumption about OH- attack in phosphate ester hydrolysis is not fully justified. J. Am. Chem. Soc. 1997, 119, 5473–5474. [Google Scholar] [CrossRef]
- Meister, A. Carboxy phosphate: An intermediate in the enzymatic synthesis of carbamyl phosphate. Trans. N. Y. Acad. Sci. 1983, 41, 117–128. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Zhang, J.; Jing, B.; Janout, V.; Regen, S.L. Detecting cross talk between two halves of a phospholipid bilayer. Langmuir 2007, 23, 8709–8712. [Google Scholar] [CrossRef]
- Klute, M.J.; Melancon, P.; Dacks, J.B. Evolution and diversity of the Golgi. Cold Spring Harbor Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef]
- Sparkes, I.A.; Frigerio, L.; Tolley, N.; Hawes, C. The plant endoplasmic reticulum: A cell-wide web. Biochem. J. 2009, 423, 145–155. [Google Scholar] [CrossRef]
- Mironov, A.A.; Banin, V.V.; Sesorova, I.S.; Dolgikh, V.V.; Luini, A.; Beznoussenko, G.V. Evolution of the endoplasmic reticulum and the Golgi complex. Adv. Exp. Med. Biol. 2007, 607, 61–72. [Google Scholar] [CrossRef]
- Schrader, M.; Fahimi, H.D. The peroxisome: Still a mysterious organelle. Histochem. Cell Biol. 2008, 129, 421–440. [Google Scholar] [CrossRef]
- Weisman, L.S. Organelles on the move: Insights from yeast vacuole inheritance. Nat. Rev. Mol. Cell Biol. 2006, 7, 243–252. [Google Scholar] [CrossRef]
- Dacks, J.B.; Field, M.C. Evolution of the eukaryotic membrane-trafficking system: Origin, tempo and mode. J. Cell Sci. 2007, 120, 2977–2985. [Google Scholar] [CrossRef]
- Nota bene: While these endomembrane systems are theoretically positioned here, the modeling the origin of these systems is premature—evolutionarily consistent modeling requires thermodynamic feedback or shunting of IEM from supervenient gyrosystems (3.5–3.8).
- Homan, R.; Pownall, H.J. Transbilayer diffusion of phospholipids: Dependence on headgroup structure and acyl chain length. Biochim. Biophys. Acta 1988, 938, 155–166. [Google Scholar] [CrossRef]
- Kamp, F.; Zakim, D.; Zhang, F.; Noy, N.; Hamilton, J.A. Fatty acid flip-flop in phospholipid bilayers is extremely fast. Biochemistry 1995, 34, 11928–11937. [Google Scholar] [CrossRef]
- Lamarche, M.G.; Wanner, B.L.; Crepin, S.; Harel, J. The phosphate regulon and bacterial virulence: A regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 2008, 32, 461–473. [Google Scholar] [CrossRef]
- Berndt, T.; Kumar, R. Phosphatonins and the regulation of phosphate homeostasis. Annu. Rev. Physiol. 2007, 69, 341–359. [Google Scholar] [CrossRef]
- Del Popolo, M.G.; Ballone, P. Melting behavior of an idealized membrane model. J. Chem. Phys. 2008, 128. [Google Scholar] [CrossRef]
- Wassall, S.R.; Stillwell, W. Polyunsaturated fatty acid-cholesterol interactions: Domain formation in membranes. Biochim. Biophys. Acta 2009, 1788, 24–32. [Google Scholar] [CrossRef]
- Reinhart, M.P. Intracellular sterol trafficking. Experientia 1990, 46, 599–611. [Google Scholar] [CrossRef]
- Lucy, J.A.; Dingle, J.T. Fat-Soluble Vitamins and Biological Membranes. Nature 1964, 204, 156–160. [Google Scholar]
- Navas, P.; Villalba, J.M.; de Cabo, R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion 2007, 7, S34–S40. [Google Scholar] [CrossRef]
- White, H.B. Coenzymes as fossils of an earlier metabolic state. J. Mol. Evol. 1976, 7, 101–104. [Google Scholar] [CrossRef]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef]
- Lemaire-Ewing, S.; Desrumaux, C.; Neel, D.; Lagrost, L. Vitamin E transport, membrane incorporation and cell metabolism: Is alpha-tocopherol in lipid rafts an oar in the lifeboat? Mol. Nutr. Food Res. 2010, 54, 631–640. [Google Scholar] [CrossRef]
- Kulaev, I.; Kulakovskaya, T. Polyphosphate and phosphate pump. Annu. Rev. Microbiol. 2000, 54, 709–734. [Google Scholar] [CrossRef]
- Achbergerova, L.; Nahalka, J. Polyphosphate—An ancient energy source and active metabolic regulator. Microb. Cell Fact. 2011, 10. [Google Scholar] [CrossRef]
- Kornberg, A. Inorganic polyphosphate: A molecule of many functions. Progr. Mol. Subcell. Biol. 1999, 23, 1–18. [Google Scholar] [CrossRef]
- Crawford, G.E.; Earnshaw, J.C. Phase transitions in monoglyceride bilayers. A light scattering study. Biophys. J. 1986, 49, 869–889. [Google Scholar] [CrossRef]
- Rotering, H.; Raetz, C.R. Appearance of monoglyceride and triglyceride in the cell envelope of Escherichia coli mutants defective in diglyceride kinase. J. Biol. Chem. 1983, 258, 8068–8073. [Google Scholar]
- Carrasco, S.; Merida, I. Diacylglycerol, when simplicity becomes complex. Trends Biochem. Sci. 2007, 32, 27–36. [Google Scholar] [CrossRef]
- Khandelia, H.; Duelund, L.; Pakkanen, K.I.; Ipsen, J.H. Triglyceride blisters in lipid bilayers: Implications for lipid droplet biogenesis and the mobile lipid signal in cancer cell membranes. PLoS One 2010, 5. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, S.O.; Bostrom, P.; Andersson, L.; Rutberg, M.; Levin, M.; Perman, J.; Boren, J. Triglyceride containing lipid droplets and lipid droplet-associated proteins. Curr. Opin. Lipidol. 2008, 19, 441–447. [Google Scholar] [CrossRef]
- Graves, J.D.; Krebs, E.G. Protein phosphorylation and signal transduction. Pharmacol. Ther. 1999, 82, 111–121. [Google Scholar] [CrossRef]
- Towler, D.A. Inorganic pyrophosphate: A paracrine regulator of vascular calcification and smooth muscle phenotype. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 651–654. [Google Scholar] [CrossRef]
- Mansurova, S.E. Inorganic pyrophosphate in mitochondrial metabolism. Biochim. Biophys. Acta 1989, 977, 237–247. [Google Scholar] [CrossRef]
- Reusch, R.N. Transmembrane ion transport by polyphosphate/poly-(R)-3-hydroxybutyrate complexes. Biochem. Biokhimiia 2000, 65, 280–295. [Google Scholar]
- Roels, J.; Verstraete, W. Biological formation of volatile phosphorus compounds. Bioresour. Technol. 2001, 79, 243–250. [Google Scholar] [CrossRef]
- Pasek, M.A.; Lauretta, D.S. Aqueous corrosion of phosphide minerals from iron meteorites: A highly reactive source of prebiotic phosphorus on the surface of the early Earth. Astrobiology 2005, 5, 515–535. [Google Scholar] [CrossRef]
- Schink, B.; Friedrich, M. Phosphite oxidation by sulphate reduction. Nature 2000, 406. [Google Scholar] [CrossRef]
- Based upon thermodynamic relationships of a focagyre with distal subgyrosystems (GXI–1), the tertiary phosphogyre models phosphate relationships with elements in the electrogyre, explaining the bioproduction and bioremediation of volatile phosphorous compounds such as phosphines and phosphides.
- Paytan, A.; McLaughlin, K. The oceanic phosphorus cycle. Curr. Rev. 2007, 107, 563–576. [Google Scholar]
- Cembella, A.D.; Antia, N.J.; Harrison, P.J. The utilization of inorganic and organic phosphorus compounds as nutrients by eukaryotic microalgae: A multidisciplinary perspective. Part 2. Crit. Rev. Microbiol. 1984, 11, 13–81. [Google Scholar] [CrossRef]
- Cembella, A.D.; Antia, N.J.; Harrison, P.J. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: A multidisciplinary perspective: Part 1. Crit. Rev. Microbiol. 1984, 10, 317–391. [Google Scholar]
- Cavalier-Smith, T. Membrane heredity and early chloroplast evolution. Trends Plant Sci. 2000, 5, 174–182. [Google Scholar] [CrossRef]
- Saraste, J.; Goud, B. Functional symmetry of endomembranes. Mol. Biol. Cell 2007, 18, 1430–1436. [Google Scholar] [CrossRef]
- Morowitz, H.J.; Heinz, B.; Deamer, D.W. The chemical logic of a minimum protocell. Orig. Life Evol. Biosph. 1988, 18, 281–287. [Google Scholar] [CrossRef]
- Segre, D.; Ben-Eli, D.; Deamer, D.W.; Lancet, D. The lipid world. Orig. Life Evol. Biosph. 2001, 31, 119–145. [Google Scholar] [CrossRef]
- Kiss, D.L.; Andrulis, E.D. The exozyme model: A continuum of functionally distinct complexes. RNA 2011, 17, 1–13. [Google Scholar] [CrossRef]
- Thieffry, D.; Sarkar, S. Forty years under the central dogma. Trends Biochem. Sci. 1998, 23, 312–316. [Google Scholar] [CrossRef]
- Henikoff, S. Beyond the central dogma. Bioinformatics 2002, 18, 223–225. [Google Scholar]
- Temin, H.M.; Mizutani, S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 1970, 226, 1211–1213. [Google Scholar]
- Baltimore, D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature 1970, 226, 1209–1211. [Google Scholar] [CrossRef]
- Gilbert, W. Origin of life—The rna world. Nature 1986, 319, 618–618. [Google Scholar] [CrossRef]
- Bartel, D.P.; Unrau, P.J. RNA-catalysed nucleotide synthesis. Nature 1998, 395, 260–263. [Google Scholar] [CrossRef]
- Eschenmoser, A. Chemical etiology of nucleic acid structure. Science 1999, 284, 2118–2124. [Google Scholar] [CrossRef]
- Orgel, L.E. The origin of life—A review of facts and speculations. Trends Biochem. Sci. 1998, 23, 491–495. [Google Scholar] [CrossRef]
- Ferris, J. Origins of life. Chemical replication. Nature 1994, 369, 184–185. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho family proteins: Coordinating cell responses. Trends Cell Biol. 2001, 11, 471–477. [Google Scholar] [CrossRef]
- Prodromou, C.; Roe, S.M.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 1997, 90, 65–75. [Google Scholar] [CrossRef]
- Chavrier, P.; Goud, B. The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell. Biol. 1999, 11, 466–475. [Google Scholar] [CrossRef]
- Avis, J.M.; Clarke, P.R. Ran, a GTPase involved in nuclear processes: Its regulators and effectors. J. Cell Sci. 1996, 109, 2423–2427. [Google Scholar]
- Anderson, C.M.; Parkinson, F.E. Potential signalling roles for UTP and UDP: Sources, regulation and release of uracil nucleotides. Trends Pharmacol. Sci. 1997, 18, 387–392. [Google Scholar]
- Renner, A.B.; Rieger, K.; Grunow, D.; Zimmermann-Kordmann, M.; Gohlke, M.; Reutter, W. Liver-specific increase of UTP and UDP-sugar concentrations in rats induced by dietary vitamin B6-deficiency and its relation to complex N-glycan structures of liver membrane-proteins. Glycoconj. J. 2007, 24, 531–541. [Google Scholar] [CrossRef]
- Chang, Y.F.; Carman, G.M. CTP synthetase and its role in phospholipid synthesis in the yeast Saccharomyces cerevisiae. Prog. Lipid Res. 2008, 47, 333–339. [Google Scholar] [CrossRef]
- Turnock, D.C.; Ferguson, M.A. Sugar nucleotide pools of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major. Eukaryot. Cell 2007, 6, 1450–1463. [Google Scholar] [CrossRef]
- Mitchell, P.; Moyle, J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature 1967, 213, 137–139. [Google Scholar] [CrossRef]
- Urata, H.; Shimizu, H.; Akagi, M. Structural studies of heterochiral DNA/DNA, RNA/RNA, AND DNA/RNA duplexes. Nucleosides Nucleotides Nucleic Acids 2006, 25, 359–367. [Google Scholar] [CrossRef]
- Kulaev, I.S.; Mansurova, S.E.; Burlakova, E.B.; Dukhovich, V.F. Why ATP instead of pyrophosphate—interrelation between ATP and pyrophosphate production during evolution and in contemporary organisms. Biosystems 1980, 12, 177–180. [Google Scholar] [CrossRef]
- This spatiotemporal and thermodynamic relationship explains the biomembrane-dependent transition from phosphorylation with pyrophosphate formation to phosphorylation with ATP formation.
- Fuda, N.J.; Ardehali, M.B.; Lis, J.T. Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 2009, 461, 186–192. [Google Scholar]
- Malik, S.; Roeder, R.G. Dynamic regulation of pol II transcription by the mammalian Mediator complex. Trends Biochem. Sci. 2005, 30, 256–263. [Google Scholar] [CrossRef]
- Tjian, R. The biochemistry of transcription in eukaryotes: A paradigm for multisubunit regulatory complexes. Philos. Trans. R. Soc. Lond. Ser. B 1996, 351, 491–499. [Google Scholar] [CrossRef]
- Zamore, P.D.; Tuschl, T.; Sharp, P.A.; Bartel, D.P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000, 101, 25–33. [Google Scholar] [CrossRef]
- Liu, Q.; Paroo, Z. Biochemical principles of small RNA pathways. Annu. Rev. Biochem. 2010, 79, 295–319. [Google Scholar] [CrossRef]
- Pullirsch, D.; Jantsch, M.F. Proteome diversification by adenosine to inosine RNA editing. RNA Biol. 2010, 7, 205–212. [Google Scholar] [CrossRef]
- Gott, J.M. Expanding genome capacity via RNA editing. Comptes Rendus Biol. 2003, 326, 901–908. [Google Scholar] [CrossRef]
- Roy, S.W.; Gilbert, W. The evolution of spliceosomal introns: Patterns, puzzles and progress. Nat. Rev. Genet. 2006, 7, 211–221. [Google Scholar]
- Borek, E.; Baliga, B.S.; Gehrke, C.W.; Kuo, C.W.; Belman, S.; Troll, W.; Waalkes, T.P. High turnover rate of transfer RNA in tumor tissue. Cancer Res. 1977, 37, 3362–3366. [Google Scholar]
- Gill, S.C.; Yager, T.D.; von Hippel, P.H. Thermodynamic analysis of the transcription cycle in E. coli. Biophys. Chem. 1990, 37, 239–250. [Google Scholar] [CrossRef]
- Spellman, P.T.; Sherlock, G.; Zhang, M.Q.; Iyer, V.R.; Anders, K.; Eisen, M.B.; Brown, P.O.; Botstein, D.; Futcher, B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell 1998, 9, 3273–3297. [Google Scholar]
- Krejcova, R.; Horska, K. Nucleoside diphosphate kinases. Chem. Listy 1997, 91, 466–476. [Google Scholar]
- Briggs, A.G.; Bent, A.F. Poly(ADP-ribosyl)ation in plants. Trends Plant Sci. 2011, 16, 372–380. [Google Scholar] [CrossRef]
- Proudfoot, N. Connecting transcription to messenger RNA processing. Trends Biochem. Sci. 2000, 25, 290–293. [Google Scholar] [CrossRef]
- Bentley, D. Coupling RNA polymerase II transcription with pre-mRNA processing. Curr. Opin. Cell Biol. 1999, 11, 347–351. [Google Scholar] [CrossRef]
- McClain, W.H. Transfer RNA identity. FASEB J. 1993, 7, 72–78. [Google Scholar]
- Sun, F.J.; Caetano-Anolles, G. Transfer RNA and the origins of diversified life. Science Progress 2008, 91, 265–284. [Google Scholar] [CrossRef]
- Nazar, R.N. Ribosomal RNA processing and ribosome biogenesis in eukaryotes. IUBMB Life 2004, 56, 457–465. [Google Scholar] [CrossRef]
- Moss, T. At the crossroads of growth control; making ribosomal RNA. Curr. Opin. Genet. Dev. 2004, 14, 210–217. [Google Scholar] [CrossRef]
- Barraud, P.; Schmitt, E.; Mechulam, Y.; Dardel, F.; Tisne, C. A unique conformation of the anticodon stem-loop is associated with the capacity of tRNAfMet to initiate protein synthesis. Nucleic Acids Res. 2008, 36, 4894–4901. [Google Scholar] [CrossRef] [Green Version]
- Rupert, P.B.; Ferre-D’Amare, A.R. Crystal structure of a hairpin ribozyme-inhibitor complex with implications for catalysis. Nature 2001, 410, 780–786. [Google Scholar] [CrossRef]
- Stahley, M.R.; Strobel, S.A. Structural evidence for a two-metal-ion mechanism of group I intron splicing. Science 2005, 309, 1587–1590. [Google Scholar]
- Selmer, M.; Dunham, C.M.; Murphy, F.V., 4th; Weixlbaumer, A.; Petry, S.; Kelley, A.C.; Weir, J.R.; Ramakrishnan, V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 2006, 313, 1935–1942. [Google Scholar] [CrossRef]
- Antoni, F.A. Molecular diversity of cyclic AMP signalling. Front. Neuroendocrinol. 2000, 21, 103–132. [Google Scholar] [CrossRef]
- Bolwell, G.P. Cyclic AMP, the reluctant messenger in plants. Trends Biochem. Sci. 1995, 20, 492–495. [Google Scholar] [CrossRef]
- Roelofs, J.; Smith, J.L.; Van Haastert, P.J. cGMP signalling: Different ways to create a pathway. Trends Genet. 2003, 19, 132–134. [Google Scholar] [CrossRef]
- Ryan, R.P.; Fouhy, Y.; Lucey, J.F.; Jiang, B.L.; He, Y.Q.; Feng, J.X.; Tang, J.L.; Dow, J.M. Cyclic di-GMP signalling in the virulence and environmental adaptation of Xanthomonas campestris. Mol. Microbiol. 2007, 63, 429–442. [Google Scholar]
- Mills, E.; Pultz, I.S.; Kulasekara, H.D.; Miller, S.I. The bacterial second messenger c-di-GMP: Mechanisms of signalling. Cell. Microbiol. 2011, 13, 1122–1129. [Google Scholar] [CrossRef]
- Belenky, P.; Bogan, K.L.; Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 2007, 32, 12–19. [Google Scholar] [CrossRef]
- Muller, F. The flavin redox-system and its biological function. Topics Curr. Chem. 1983, 108, 71–107. [Google Scholar] [CrossRef]
- Given the emergence of vitamins in the carbogyre, and the relativistically strong creatodestructive force it exerts through the phosphogyre on the ribogyre (GXIII), the compounds flavin adenine dinucleotide and flavin mononucleotide are also positioned in the gyrobase of the secondary majorgyre.
- Koonin, E.V.; Novozhilov, A.S. Origin and evolution of the genetic code: The universal enigma. IUBMB Life 2009, 61, 99–111. [Google Scholar] [CrossRef]
- Westover, K.D.; Bushnell, D.A.; Kornberg, R.D. Structural basis of transcription: Nucleotide selection by rotation in the RNA polymerase II active center. Cell 2004, 119, 481–489. [Google Scholar] [CrossRef]
- Anand, V.S.; Patel, S.S. Transient state kinetics of transcription elongation by T7 RNA polymerase. J. Biol. Chem. 2006, 281, 35677–35685. [Google Scholar]
- Alvager, T.; Graham, G.; Hilleke, R.; Hutchison, D.; Westgard, J. On the information content of the genetic code. Biol. Syst. 1989, 22, 189–196. [Google Scholar]
- Crick, F.H. Codon—anticodon pairing: The wobble hypothesis. J. Mol. Biol. 1966, 19, 548–555. [Google Scholar] [CrossRef]
- Forterre, P. Defining life: The virus viewpoint. Orig. Life Evol. Biosph. 2010, 40, 151–160. [Google Scholar] [CrossRef]
- Villarreal, L.P. Viruses and the Evolution of Life; ASM Press: Washington, DC, USA, 2005; p. 395. [Google Scholar]
- Dadalti, P.; Goodheart, C. Did the first virus self-assemble from self-replicating prion proteins and RNA? Med. Hypotheses 2007, 69, 724–730. [Google Scholar] [CrossRef]
- Becker, Y. Molecular evolution of viruses: An interim summary. Virus Genes 1995, 11, 299–302. [Google Scholar] [CrossRef]
- Holmes, E.C. On the origin and evolution of the human immunodeficiency virus (HIV). Biol. Rev. Camb. Philos. Soc. 2001, 76, 239–254. [Google Scholar] [CrossRef]
- Zandi, R.; van der Schoot, P. Size regulation of ss-RNA viruses. Biophys. J. 2009, 96, 9–20. [Google Scholar]
- Basile, B.; Lazcano, A.; Oro, J. Prebiotic syntheses of purines and pyrimidines. Adv. Space Res. 1984, 4, 125–131. [Google Scholar]
- Sutherland, J.D. Ribonucleotides. Cold Spring Harbor Perspect. Biol. 2010, 2, a005439. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Cassone, V.M.; Natesan, A.K. Time and time again: The phylogeny of melatonin as a transducer of biological time. J. Biol. Rhythm. 1997, 12, 489–497. [Google Scholar] [CrossRef]
- Stefano, G.B.; Kream, R.M. Endogenous morphine synthetic pathway preceded and gave rise to catecholamine synthesis in evolution (Review). Int. J. Mol. Med. 2007, 20, 837–841. [Google Scholar]
- Deiters, A.; Martin, S.F. Synthesis of oxygen- and nitrogen-containing heterocycles by ring-closing metathesis. Curr. Rev. 2004, 104, 2199–2238. [Google Scholar]
- Sahr, T.; Ravanel, S.; Rebeille, F. Tetrahydrofolate biosynthesis and distribution in higher plants. Biochem. Soc. Trans. 2005, 33, 758–762. [Google Scholar] [CrossRef]
- Vasileuskaya, Z.; Oster, U.; Beck, C.F. Involvement of tetrapyrroles in inter-organellar signaling in plants and algae. Photosynth. Res. 2004, 82, 289–299. [Google Scholar] [CrossRef]
- Kulikowska, E.; Kierdaszuk, B.; Shugar, D. Xanthine, xanthosine and its nucleotides: Solution structures of neutral and ionic forms, and relevance to substrate properties in various enzyme systems and metabolic pathways. Acta Biochim. Pol. 2004, 51, 493–531. [Google Scholar]
- Lucock, M.; Yates, Z. Folic acid - vitamin and panacea or genetic time bomb? Nat. Rev. Genet. 2005, 6, 235–240. [Google Scholar] [CrossRef]
- Alvarez-Lario, B.; Macarron-Vicente, J. Uric acid and evolution. Rheumatology 2010, 49, 2010–2015. [Google Scholar] [CrossRef]
- Azmitia, E.C. Modern views on an ancient chemical: Serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res. Bull. 2001, 56, 413–424. [Google Scholar] [CrossRef]
- Cuvillier, O. Sphingosine in apoptosis signaling. Biochim. Biophys. Acta 2002, 1585, 153–162. [Google Scholar] [CrossRef]
- Miller, S.L. Production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar]
- Denis, V.; Daignan-Fornier, B. Synthesis of glutamine, glycine and 10-formyl tetrahydrofolate is coregulated with purine biosynthesis in Saccharomyces cerevisiae. Mol. Gen. Genet. 1998, 259, 246–255. [Google Scholar] [CrossRef]
- Boza, J.J.; Moennoz, D.; Bournot, C.E.; Blum, S.; Zbinden, I.; Finot, P.A.; Ballevre, O. Role of glutamine on the de novo purine nucleotide synthesis in Caco-2 cells. Eur. J. Nutr. 2000, 39, 38–46. [Google Scholar] [CrossRef]
- Huang, M.; Graves, L.M. De novo synthesis of pyrimidine nucleotides; emerging interfaces with signal transduction pathways. Cell. Mol. Life Sci. 2003, 60, 321–336. [Google Scholar] [CrossRef]
- Traut, T.W.; Jones, M.E. Uracil metabolism—UMP synthesis from orotic acid or uridine and conversion of uracil to beta-alanine: Enzymes and cDNAs. Prog. Nucleic Acid Res. Mol. Biol. 1996, 53, 1–78. [Google Scholar] [CrossRef]
- Stepansky, A.; Leustek, T. Histidine biosynthesis in plants. Amino Acids 2006, 30, 127–142. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar]
- Navarro-Gonzalez, R.; McKay, C.P.; Mvondo, D.N. A possible nitrogen crisis for Archaean life due to reduced nitrogen fixation by lightning. Nature 2001, 412, 61–64. [Google Scholar] [CrossRef]
- Hamilton, I.R.; Burris, R.H.; Wilson, P.W.; Wang, C.H. Pyruvate metabolism, carbon dioxide assimilation, and nitrogen fixation by an achromobacter species. J. Bacteriol. 1965, 89, 647–653. [Google Scholar]
- Sobko, T.; Reinders, C.I.; Jansson, E.; Norin, E.; Midtvedt, T.; Lundberg, J.O. Gastrointestinal bacteria generate nitric oxide from nitrate and nitrite. Nitric Oxide 2005, 13, 272–278. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Stuehr, D.J. Enzymes of the L-arginine to nitric oxide pathway. J. Nutr. 2004, 134, 2748–2751, discussion 2765S-2767S. [Google Scholar]
- Nyffeler, P.T.; Liang, C.H.; Koeller, K.M.; Wong, C.H. The chemistry of amine-azide interconversion: Catalytic diazotransfer and regioselective azide reduction. J. Am. Chem. Soc. 2002, 124, 10773–10778. [Google Scholar] [CrossRef]
- Brandes, J.A.; Boctor, N.Z.; Cody, G.D.; Cooper, B.A.; Hazen, R.M.; Yoder, H.S., Jr. Abiotic nitrogen reduction on the early Earth. Nature 1998, 395, 365–367. [Google Scholar]
- On this matter, the attractorepulsive effects of the primary electrogyre on the nitrogyre models ammonia, consistent with ideas regarding its formation on the early Earth.
- Doherty, E.A.; Doudna, J.A. Ribozyme structures and mechanisms. Annu. Rev. Biophys. Biomol. Struct. 2001, 30, 457–475. [Google Scholar] [CrossRef]
- Lahav, N. Prebiotic co-evolution of self-replication and translation or RNA world? J. Theor. Biol. 1991, 151, 531–539. [Google Scholar] [CrossRef]
- Lilley, D.M. The ribosome functions as a ribozyme. Chembiolchem 2001, 2, 31–35. [Google Scholar] [CrossRef]
- Wolf, Y.I.; Koonin, E.V. On the origin of the translation system and the genetic code in the RNA world by means of natural selection, exaptation, and subfunctionalization. Biol.Direct 2007, 2. [Google Scholar] [CrossRef]
- Ma, W. The scenario on the origin of translation in the RNA world: In principle of replication parsimony. Biol.Direct 2010, 5. [Google Scholar] [CrossRef]
- Ibba, M.; Soll, D. Aminoacyl-tRNAs: Setting the limits of the genetic code. Genes Dev. 2004, 18, 731–738. [Google Scholar] [CrossRef]
- Wong, J.T. A co-evolution theory of the genetic code. Proc. Natl. Acad. Sci. USA 1975, 72, 1909–1912. [Google Scholar] [CrossRef]
- Wong, J.T. Coevolution theory of the genetic code at age thirty. BioEssays 2005, 27, 416–425. [Google Scholar] [CrossRef]
- Easterwood, T.R.; Major, F.; Malhotra, A.; Harvey, S.C. Orientations of transfer RNA in the ribosomal A and P sites. Nucleic Acids Res. 1994, 22, 3779–3786. [Google Scholar] [CrossRef]
- Schmeing, T.M.; Moore, P.B.; Steitz, T.A. Structures of deacylated tRNA mimics bound to the E site of the large ribosomal subunit. RNA 2003, 9, 1345–1352. [Google Scholar] [CrossRef]
- Tozzi, M.G.; Camici, M.; Mascia, L.; Sgarrella, F.; Ipata, P.L. Pentose phosphates in nucleoside interconversion and catabolism. FEBS J. 2006, 273, 1089–1101. [Google Scholar] [CrossRef]
- Wirtz, M.; Droux, M. Synthesis of the sulfur amino acids: Cysteine and methionine. Photosynth. Res. 2005, 86, 345–362. [Google Scholar] [CrossRef]
- Recall that the electrogyre models the hydrogen cycle (3.1), the oxygyre models the oxygen and water cycles (3.2), the carbogyre models the carbon cycle (3.3), the phosphogyre models the phosphorus cycle (3.4), and the ribogyre and nitrogyre model the nitrogen cycle (3.5).
- Kertesz, M.A. Riding the sulfur cycle—Metabolism of sulfonates and sulfate esters in Gram-negative bacteria. Fems Microbiol. Rev. 2000, 24, 135–175. [Google Scholar]
- Farquhar, J.; Wu, N.P.; Canfield, D.E.; Oduro, H. Connections between sulfur cycle evolution, sulfur isotopes, sediments, and base metal sulfide deposits. Econ. Geol. 2010, 105, 509–533. [Google Scholar] [CrossRef]
- Philippot, P.; van Zuilen, M.; Lepot, K.; Thomazo, C.; Farquhar, J.; van Kranendonk, M.J. Early Archaean microorganisms preferred elemental sulfur, not sulfate. Science 2007, 317, 1534–1537. [Google Scholar] [CrossRef]
- Turchyn, A.V.; Schrag, D.P. Oxygen isotope constraints on the sulfur cycle over the past 10 million years. Science 2004, 303, 2004–2007. [Google Scholar] [CrossRef]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Metabolism of sulfur-containing amino acids. Annu. Rev. Nutr. 1986, 6, 179–209. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636–1640. [Google Scholar]
- Chatterjee, N.K.; Kerwar, S.S.; Weissbach, H. Initiation of protein synthesis in HeLa cells. Proc. Natl. Acad. Sci. USA 1972, 69, 1375–1379. [Google Scholar] [CrossRef]
- Han, D.X.; Wang, H.Y.; Ji, Z.L.; Hu, A.F.; Zhao, Y.F. Amino Acid Homochirality may be Linked to the Origin of Phosphate-Based Life. J. Mol. Evol. 2010, 70, 572–582. [Google Scholar] [CrossRef]
- Tamura, K. Origin of amino acid homochirality: Relationship with the RNA world and origin of tRNA aminoacylation. Biosystems 2008, 92, 91–98. [Google Scholar] [CrossRef]
- Zaher, H.S.; Green, R. Fidelity at the molecular level: Lessons from protein synthesis. Cell 2009, 136, 746–762. [Google Scholar] [CrossRef]
- Blommaart, E.F.; Luiken, J.J.; Meijer, A.J. Autophagic proteolysis: Control and specificity. Histochem. J. 1997, 29, 365–385. [Google Scholar] [CrossRef]
- Hamel, F.G.; Fawcett, J.; Bennett, R.G.; Duckworth, W.C. Control of proteolysis: Hormones, nutrients, and the changing role of the proteasome. Curr. Opin. Clin. Metab. Care 2004, 7, 255–258. [Google Scholar]
- Kadowaki, M.; Kanazawa, T. Amino acids as regulators of proteolysis. J. Nutr. 2003, 133, 2052–2056. [Google Scholar]
- Knaggs, M.; Williams, M.; Goodfellow, J.M. Protein hydration, stability and unfolding. Biochem. Soc. Trans. 1995, 23, 711–715. [Google Scholar]
- Harari-Steinberg, O.; Chamovitz, D.A. The COP9 signalosome: Mediating between kinase signaling and protein degradation. Curr. Protein Pept. Sci. 2004, 5, 185–189. [Google Scholar]
- Fuchs, S.Y.; Fried, V.A.; Ronai, Z. Stress-activated kinases regulate protein stability. Oncogene 1998, 17, 1483–1490. [Google Scholar]
- Buljan, M.; Bateman, A. The evolution of protein domain families. Biochem. Soc. Trans. 2009, 37, 751–755. [Google Scholar]
- Caetano-Anolles, G.; Wang, M.; Caetano-Anolles, D.; Mittenthal, J.E. The origin, evolution and structure of the protein world. Biochem. J. 2009, 417, 621–637. [Google Scholar] [CrossRef]
- Pal, C.; Papp, B.; Lercher, M.J. An integrated view of protein evolution. Nat. Rev. Genet. 2006, 7, 337–348. [Google Scholar] [CrossRef]
- Hausmann, C.D.; Ibba, M. Aminoacyl-tRNA synthetase complexes: Molecular multitasking revealed. FEMS Microbiol. Rev. 2008, 32, 705–721. [Google Scholar] [CrossRef]
- Lipman, R.S.; Hou, Y.M. Aminoacylation of tRNA in the evolution of an aminoacyl-tRNA synthetase. Proc. Natl. Acad. Sci. USA 1998, 95, 13495–13500. [Google Scholar]
- Errington, N.; Iqbalsyah, T.; Doig, A.J. Structure and stability of the alpha-helix: Lessons for design. Methods Mol. Biol. 2006, 340, 3–26. [Google Scholar]
- Toniolo, C.; Benedetti, E. The polypeptide 310-helix. Trends Biochem. Sci. 1991, 16, 350–353. [Google Scholar] [CrossRef]
- Riek, R.P.; Graham, R.M. The elusive pi-helix. J. Struct. Biol. 2011, 173, 153–160. [Google Scholar] [CrossRef]
- Chou, K.C.; Pottle, M.; Nemethy, G.; Ueda, Y.; Scheraga, H.A. Structure of beta-sheets. Origin of the right-handed twist and of the increased stability of antiparallel over parallel sheets. J. Mol. Biol. 1982, 162, 89–112. [Google Scholar] [CrossRef]
- Armen, R.; Alonso, D.O.; Daggett, V. The role of alpha-, 3(10)-, and pi-helix in helix→coil transitions. Protein Sci. 2003, 12, 1145–1157. [Google Scholar] [CrossRef]
- Schutt, C.E.; Myslik, J.C.; Rozycki, M.D.; Goonesekere, N.C.; Lindberg, U. The structure of crystalline profilin-beta-actin. Nature 1993, 365, 810–816. [Google Scholar]
- Nogales, E.; Whittaker, M.; Milligan, R.A.; Downing, K.H. High-resolution model of the microtubule. Cell 1999, 96, 79–88. [Google Scholar] [CrossRef]
- Strelkov, S.V.; Schumacher, J.; Burkhard, P.; Aebi, U.; Herrmann, H. Crystal structure of the human lamin A coil 2B dimer: Implications for the head-to-tail association of nuclear lamins. J. Mol. Biol. 2004, 343, 1067–1080. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Bansal, M. Collagen structure: The Madras triple helix and the current scenario. IUBMB Life 2005, 57, 161–172. [Google Scholar] [CrossRef]
- Wierzbicka-Patynowski, I.; Schwarzbauer, J.E. The ins and outs of fibronectin matrix assembly. J. Cell Sci. 2003, 116, 3269–3276. [Google Scholar] [CrossRef]
- Leahy, D.J.; Aukhil, I.; Erickson, H.P. 2.0 A crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell 1996, 84, 155–164. [Google Scholar] [CrossRef]
- Beck, K.; Hunter, I.; Engel, J. Structure and function of laminin: Anatomy of a multidomain glycoprotein. FASEB J. 1990, 4, 148–160. [Google Scholar]
- Ali, I.; Marenduzzo, D.; Yeomans, J.M. Polymer packaging and ejection in viral capsids: Shape matters. Phys. Rev. Lett. 2006, 96. [Google Scholar] [CrossRef]
- Zampighi, G.A.; Fisher, R.S. Polyhedral protein cages encase synaptic vesicles and participate in their attachment to the active zone. J. Struct. Biol. 1997, 119, 347–359. [Google Scholar] [CrossRef]
- Anfinsen, C.B. Studies on the reduction and re-formation of protein disulfide bonds. J. Biol. Chem. 1961, 236, 1361–1363. [Google Scholar]
- Anfinsen, C.B. Principles that govern folding of protein chains. Science 1973, 181, 223–230. [Google Scholar]
- Ptitsyn, O.B. A determinable but unresolved problem. FASEB J. 1996, 10, 3–4. [Google Scholar]
- I have modeled that the aminonexus is composed of amino acids (ribogyre (3.5) and aminogyre (3.6)) that evolutionarily emerge from organic matter and oxides and are bathed in a water solution (oxygyre (3.2) and carbogyre (3.3)). Furthermore, the aminonexus is replete with electrons (electrogyre (3.1)) and frequently modified with phosphate groups (phosphogyre (3.4)).
- Nordlund, P.; Reichard, P. Ribonucleotide reductases. Annu. Rev. Biochem. 2006, 75, 681–706. [Google Scholar] [CrossRef]
- Kennelly, P.J. Protein kinases and protein phosphatases in prokaryotes: A genomic perspective. FEMS Microbiol. Lett. 2002, 206, 1–8. [Google Scholar] [CrossRef]
- Taylor, S.S.; Kornev, A.P. Protein kinases: Evolution of dynamic regulatory proteins. Trends Biochem. Sci. 2011, 36, 65–77. [Google Scholar] [CrossRef]
- Kennelly, P.J. Protein phosphatases—A phylogenetic perspective. Curr. Rev. 2001, 101, 2291–2312. [Google Scholar]
- Kwapisz, M.; Beckouet, F.; Thuriaux, P. Early evolution of eukaryotic DNA-dependent RNA polymerases. Trends Genet. 2008, 24, 211–215. [Google Scholar] [CrossRef]
- Sonntag, K.C.; Darai, G. Evolution of viral DNA-dependent RNA polymerases. Virus Genes 1995, 11, 271–284. [Google Scholar] [CrossRef]
- Werner, F.; Grohmann, D. Evolution of multisubunit RNA polymerases in the three domains of life. Nat. Rev. Microbiol. 2011, 9, 85–98. [Google Scholar] [CrossRef]
- Makeyev, E.V.; Grimes, J.M. RNA-dependent RNA polymerases of dsRNA bacteriophages. Virus Res. 2004, 101, 45–55. [Google Scholar] [CrossRef]
- Sorrentino, S. The eight human “canonical” ribonucleases: Molecular diversity, catalytic properties, and special biological actions of the enzyme proteins. FEBS Lett. 2010, 584, 2194–2200. [Google Scholar] [CrossRef]
- Danchin, A. A phylogenetic view of bacterial ribonucleases. Progress Mol. Biol. Transl. Sci. 2009, 85, 1–41. [Google Scholar] [CrossRef]
- Evans, C.J.; Aguilera, R.J. DNase II: Genes, enzymes and function. Gene 2003, 322, 1–15. [Google Scholar] [CrossRef]
- Pyle, A.M. Translocation and unwinding mechanisms of RNA and DNA helicases. Annu. Rev. Biophys. 2008, 37, 317–336. [Google Scholar] [CrossRef]
- Tan, S.; Richmond, T.J. Eukaryotic transcription factors. Curr. Opin. Struct. Biol. 1998, 8, 41–48. [Google Scholar] [CrossRef]
- Reeve, J.N.; Bailey, K.A.; Li, W.T.; Marc, F.; Sandman, K.; Soares, D.J. Archaeal histones: Structures, stability and DNA binding. Biochem. Soc. Trans. 2004, 32, 227–230. [Google Scholar] [CrossRef]
- Johnson, L.N. The regulation of protein phosphorylation. Biochem. Soc. Trans. 2009, 37, 627–641. [Google Scholar] [CrossRef]
- Bayle, J.H.; Crabtree, G.R. Protein acetylation: More than chromatin modification to regulate transcription. Chem. Biol. 1997, 4, 885–888. [Google Scholar]
- McIlhinney, R.A. The fats of life: The importance and function of protein acylation. Trends Biochem. Sci. 1990, 15, 387–391. [Google Scholar] [CrossRef]
- Paik, W.K.; Paik, D.C.; Kim, S. Historical review: The field of protein methylation. Trends Biochem. Sci. 2007, 32, 146–152. [Google Scholar] [CrossRef]
- Fang, S.; Weissman, A.M. A field guide to ubiquitylation. Cell. Mol. Life Sci. 2004, 61, 1546–1561. [Google Scholar]
- Wilkinson, K.A.; Henley, J.M. Mechanisms, regulation and consequences of protein SUMOylation. Biochem. J. 2010, 428, 133–145. [Google Scholar] [CrossRef]
- Protein phosphorylation is modeled as thermodynamic relationships between the ribogyre (NTPs) and aminogyre; protein acetylation, acylation, and methylation are modeled as relationships between the carbogyre and aminogyre; and protein ubiquitylation and SUMOylation occur via aminogyre autoregulation.
- Wendler, P.; Ciniawsky, S.; Kock, M.; Kube, S. Structure and function of the AAA+ nucleotide binding pocket. Biochim. Biophys. Acta 2011, in press. [Google Scholar]
- Blower, M.D.; Nachury, M.; Heald, R.; Weis, K. A Rae1-containing ribonucleoprotein complex is required for mitotic spindle assembly. Cell 2005, 121, 223–234. [Google Scholar] [CrossRef]
- Van Hooser, A.A.; Yuh, P.; Heald, R. The perichromosomal layer. Chromosoma 2005, 114, 377–388. [Google Scholar] [CrossRef]
- Cole, C.N.; Scarcelli, J.J. Transport of messenger RNA from the nucleus to the cytoplasm. Curr. Opin. Cell. Biol. 2006, 18, 299–306. [Google Scholar] [CrossRef]
- Maniatis, T.; Reed, R. An extensive network of coupling among gene expression machines. Nature 2002, 416, 499–506. [Google Scholar] [CrossRef]
- Azubel, M.; Wolf, S.G.; Sperling, J.; Sperling, R. Three-dimensional structure of the native spliceosome by cryo-electron microscopy. Mol. Cell 2004, 15, 833–839. [Google Scholar] [CrossRef]
- Matera, A.G.; Shpargel, K.B. Pumping RNA: Nuclear bodybuilding along the RNP pipeline. Curr. Opin. Cell. Biol. 2006, 18, 317–324. [Google Scholar] [CrossRef]
- Parker, R.; Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 2007, 25, 635–646. [Google Scholar] [CrossRef]
- Erickson, S.L.; Lykke-Andersen, J. Cytoplasmic mRNP granules at a glance. J. Cell Sci. 2011, 124, 293–297. [Google Scholar] [CrossRef]
- Examples of these gyrapical RNA-protein complexes are the spindle ribonucleoprotein, perichromosomal sheath, nascent and nucleocytoplasmically transported mRNAs, spliceosome, Cajal bodies, and cytoplasmic P-bodies and stress granules.
- Bowie, J.U. Solving the membrane protein folding problem. Nature 2005, 438, 581–589. [Google Scholar] [CrossRef]
- Pohorille, A.; Schweighofer, K.; Wilson, M.A. The origin and early evolution of membrane channels. Astrobiology 2005, 5, 1–17. [Google Scholar] [CrossRef]
- Debler, E.W.; Ma, Y.; Seo, H.S.; Hsia, K.C.; Noriega, T.R.; Blobel, G.; Hoelz, A. A fence-like coat for the nuclear pore membrane. Mol. Cell 2008, 32, 815–826. [Google Scholar] [CrossRef]
- Albrecht-Buehler, G. The iris diaphragm model of centriole and basal body formation. Cell Motil.Cytoskelet. 1990, 17, 197–213. [Google Scholar] [CrossRef]
- Note that each of these aminons originate and evolve in response to changes in subsumed gyrosystems; this should remind the reader of gyraxioms that dictate interconnectivity of the gyromodel (GIV, GIV–1): Although these phenomena—polypeptide synthesis, protein complex structure and function, and membrane insertion—can be studied independently, they cannot be understood without losing information related to the processes that precede them evolutionarily, atomically, and metabolically.
- Walsh, C. Antibiotics: Actions, Origins, Resistance; ASM Press: Washington, DC, USA, 2003; p. 335. [Google Scholar]
- Yim, G.; Wang, H.H.; Davies, J. Antibiotics as signalling molecules. Philos. Trans. R. Soc. Lond. Ser. B 2007, 362, 1195–1200. [Google Scholar] [CrossRef]
- Kleinkauf, H.; von Dohren, H. A nonribosomal system of peptide biosynthesis. Eur. J. Biochem. FEBS 1996, 236, 335–351. [Google Scholar]
- Von Dohren, H.; Dieckmann, R.; Pavela-Vrancic, M. The nonribosomal code. Chem. Biol. 1999, 6, R273–R279. [Google Scholar] [CrossRef]
- Caboche, S.; Leclere, V.; Pupin, M.; Kucherov, G.; Jacques, P. Diversity of monomers in nonribosomal peptides: Towards the prediction of origin and biological activity. J. Bacteriol. 2010, 192, 5143–5150. [Google Scholar] [CrossRef] [Green Version]
- French, G.L. The continuing crisis in antibiotic resistance. Int. J. Antimicrob. Agents 2010, 36, S3–S7. [Google Scholar] [CrossRef]
- Shimojima, M. Biosynthesis and functions of the plant sulfolipid. Progress in Lipid Research. 2011, 50, 234–239. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; de Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef]
- Irvine, W.M. Chemistry between the stars. Planet. Rep. 1987, 7, 6–9. [Google Scholar]
- Kolberg, M.; Strand, K.R.; Graff, P.; Andersson, K.K. Structure, function, and mechanism of ribonucleotide reductases. Biochim. Biophys. Acta 1699, 1–34. [Google Scholar]
- Stubbe, J. Ribonucleotide reductases: The link between an RNA and a DNA world? Curr. Opin. Struct. Biol. 2000, 10, 731–736. [Google Scholar] [CrossRef]
- Forterre, P. The two ages of the RNA world, and the transition to the DNA world: A story of viruses and cells. Biochimie 2005, 87, 793–803. [Google Scholar] [CrossRef]
- Reichard, P. From RNA to DNA, why so many ribonucleotide reductases? Science 1993, 260, 1773–1777. [Google Scholar]
- Watson, J.D.; Berry, A. DNA: The Secret of Life, 1st ed.; Alfred A. Knopf: New York, NY, USA, 2003; p. 446. [Google Scholar]
- Witkowski, J.A. The Inside Story: DNA to RNA to Protein; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2005; p. 382. [Google Scholar]
- Van Rompay, A.R.; Johansson, M.; Karlsson, A. Phosphorylation of nucleosides and nucleoside analogs by mammalian nucleoside monophosphate kinases. Pharmacol. Ther. 2000, 87, 189–198. [Google Scholar] [CrossRef]
- Chakrabarty, A.M. Nucleoside diphosphate kinase: Role in bacterial growth, virulence, cell signalling and polysaccharide synthesis. Mol. Microbiol. 1998, 28, 875–882. [Google Scholar] [CrossRef]
- Itzkovitz, S.; Tlusty, T.; Alon, U. Coding limits on the number of transcription factors. BMC Genomics 2006, 7. [Google Scholar] [CrossRef]
- Karin, M. Too many transcription factors: Positive and negative interactions. New Biol. 1990, 2, 126–131. [Google Scholar]
- Georges, A.B.; Benayoun, B.A.; Caburet, S.; Veitia, R.A. Generic binding sites, generic DNA-binding domains: Where does specific promoter recognition come from? FASEB J. 2010, 24, 346–356. [Google Scholar] [CrossRef]
- Bergman, C.M.; Pfeiffer, B.D.; Rincon-Limas, D.E.; Hoskins, R.A.; Gnirke, A.; Mungall, C.J.; Wang, A.M.; Kronmiller, B.; Pacleb, J.; Park, S.; et al. Assessing the impact of comparative genomic sequence data on the functional annotation of the Drosophila genome. Genome Biol. 2002, 3, 1–20. [Google Scholar]
- Cameron, R.A.; Chow, S.H.; Berney, K.; Chiu, T.Y.; Yuan, Q.A.; Kramer, A.; Helguero, A.; Ransick, A.; Yun, M.; Davidson, E.H. An evolutionary constraint: Strongly disfavored class of change in DNA sequence during divergence of cis-regulatory modules. Proc. Natl. Acad. Sci. USA 2005, 102, 11769–11774. [Google Scholar]
- Carninci, P.; Sandelin, A.; Lenhard, B.; Katayama, S.; Shimokawa, K.; Ponjavic, J.; Semple, C.A.; Taylor, M.S.; Engstrom, P.G.; Frith, M.C.; et al. Genome-wide analysis of mammalian promoter architecture and evolution. Nat. Genet. 2006, 38, 626–635. [Google Scholar]
- Castillo-Davis, C.I. The evolution of noncoding DNA: How much junk, how much func? Trends Genet. 2005, 21, 533–536. [Google Scholar] [CrossRef]
- Dillon, S.C.; Dorman, C.J. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 2010, 8, 185–195. [Google Scholar] [CrossRef]
- Thatcher, T.H.; Gorovsky, M.A. Phylogenetic analysis of the core histones H2A, H2B, H3, and H4. Nucleic Acids Res. 1994, 22, 174–179. [Google Scholar]
- Kato, M.; Onishi, Y.; Wada-Kiyama, Y.; Kiyama, R. Biochemical screening of stable dinucleosomes using DNA fragments from a dinucleosome DNA library. J. Mol. Biol. 2005, 350, 215–227. [Google Scholar] [CrossRef]
- Sollner-Webb, B.; Felsenfeld, G. A comparison of the digestion of nuclei and chromatin by staphylococcal nuclease. Biochemistry 1975, 14, 2915–2920. [Google Scholar] [CrossRef]
- Lucchesi, J.C. Dosage compensation in Drosophila and the “complex’ world of transcriptional regulation. BioEssays 1996, 18, 541–547. [Google Scholar] [CrossRef]
- Winston, F.; Carlson, M. Yeast SNF/SWI transcriptional activators and the SPT/SIN chromatin connection. Trends Genet. 1992, 8, 387–391. [Google Scholar]
- Liu, Z.; Karmarkar, V. Groucho/Tup1 family co-repressors in plant development. Trends Plant Sci. 2008, 13, 137–144. [Google Scholar] [CrossRef]
- Melendy, T.; Li, R. Chromatin remodeling and initiation of DNA replication. Front. Biosci. 2001, 6, D1048–D1053. [Google Scholar] [CrossRef]
- Friedberg, E.C. DNA damage and repair. Nature 2003, 421, 436–440. [Google Scholar] [CrossRef]
- Coghlan, A.; Eichler, E.E.; Oliver, S.G.; Paterson, A.H.; Stein, L. Chromosome evolution in eukaryotes: A multi-kingdom perspective. Trends Genet. 2005, 21, 673–682. [Google Scholar] [CrossRef]
- Feschotte, C.; Pritham, E.J. DNA transposons and the evolution of eukaryotic genomes. Annu. Rev. Genet. 2007, 41, 331–368. [Google Scholar] [CrossRef]
- McClintock, B. The origin and behavior of mutable loci in maize. Proc. Natl. Acad. Sci. USA 1950, 36, 344–355. [Google Scholar] [CrossRef]
- Stankiewicz, P.; Lupski, J.R. Genome architecture, rearrangements and genomic disorders. Trends Genet. 2002, 18, 74–82. [Google Scholar] [CrossRef]
- Zhao, J.; Bacolla, A.; Wang, G.; Vasquez, K.M. Non-B DNA structure-induced genetic instability and evolution. Cell. Mol. Life Sci. 2010, 67, 43–62. [Google Scholar] [CrossRef]
- Ghosh, A.; Bansal, M. A glossary of DNA structures from A to Z. Acta Crystallogr. Sect. D 2003, 59, 620–626. [Google Scholar] [CrossRef]
- Watson, J.D.; Crick, F.H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef]
- McGhee, J.D.; Nickol, J.M.; Felsenfeld, G.; Rau, D.C. Higher order structure of chromatin: Orientation of nucleosomes within the 30 nm chromatin solenoid is independent of species and spacer length. Cell 1983, 33, 831–841. [Google Scholar] [CrossRef]
- Filipski, J.; Leblanc, J.; Youdale, T.; Sikorska, M.; Walker, P.R. Periodicity of DNA folding in higher order chromatin structures. EMBO J. 1990, 9, 1319–1327. [Google Scholar]
- Swedlow, J.R.; Hirano, T. The making of the mitotic chromosome: Modern insights into classical questions. Mol. Cell 2003, 11, 557–569. [Google Scholar] [CrossRef]
- Forterre, P. The origin of viruses and their possible roles in major evolutionary transitions. Virus Res. 2006, 117, 5–16. [Google Scholar] [CrossRef]
- Long, M.; Betran, E.; Thornton, K.; Wang, W. The origin of new genes: Glimpses from the young and old. Nat. Rev. Genet. 2003, 4, 865–875. [Google Scholar]
- Zhou, Q.; Wang, W. On the origin and evolution of new genes—A genomic and experimental perspective. J. Genet. Genomics 2008, 35, 639–648. [Google Scholar] [CrossRef]
- Hittinger, C.T.; Carroll, S.B. Gene duplication and the adaptive evolution of a classic genetic switch. Nature 2007, 449, 677–681. [Google Scholar] [CrossRef]
- Ohno, S. Evolution by Gene Duplication; Springer-Verlag: Berlin, Germany and New York, NY, USA, 1970; p. 160. [Google Scholar]
- Gilbert, W.; de Souza, S.J.; Long, M. Origin of genes. Proc. Natl. Acad. Sci. USA 1997, 94, 7698–7703. [Google Scholar] [CrossRef]
- Swanson, W.J. Adaptive evolution of genes and gene families. Curr. Opin. Genet. Dev. 2003, 13, 617–622. [Google Scholar] [CrossRef]
- Rodriguez-Trelles, F.; Tarrio, R.; Ayala, F.J. Origins and evolution of spliceosomal introns. Annu. Rev. Genet. 2006, 40, 47–76. [Google Scholar] [CrossRef]
- Catania, F.; Lynch, M. Where do introns come from? PLoS Biol. 2008, 6. [Google Scholar] [CrossRef]
- Brisson, D. The directed mutation controversy in an evolutionary context. Crit. Rev. Microbiol. 2003, 29, 25–35. [Google Scholar] [CrossRef]
- Cairns, J.; Overbaugh, J.; Miller, S. The origin of mutants. Nature 1988, 335, 142–145. [Google Scholar] [CrossRef]
- Lenski, R.E.; Mittler, J.E. The directed mutation controversy and neo-Darwinism. Science 1993, 259, 188–194. [Google Scholar]
- Cheng, J.; Kapranov, P.; Drenkow, J.; Dike, S.; Brubaker, S.; Patel, S.; Long, J.; Stern, D.; Tammana, H.; Helt, G.; et al. Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. Science 2005, 308, 1149–1154. [Google Scholar]
- Huertas, P.; Aguilera, A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol. Cell 2003, 12, 711–721. [Google Scholar] [CrossRef]
- Nowacki, M.; Vijayan, V.; Zhou, Y.; Schotanus, K.; Doak, T.G.; Landweber, L.F. RNA-mediated epigenetic programming of a genome-rearrangement pathway. Nature 2008, 451, 153–158. [Google Scholar]
- Storici, F.; Bebenek, K.; Kunkel, T.A.; Gordenin, D.A.; Resnick, M.A. RNA-templated DNA repair. Nature 2007, 447, 338–341. [Google Scholar] [CrossRef]
- Jenkins, F.J.; Roizman, B. Site-specific mutagenesis of large DNA viral genomes. BioEssays 1986, 5, 244–247. [Google Scholar] [CrossRef]
- Duffy, S.; Shackelton, L.A.; Holmes, E.C. Rates of evolutionary change in viruses: Patterns and determinants. Nat. Rev. Genet. 2008, 9, 267–276. [Google Scholar]
- Dunning Hotopp, J.C.; Clark, M.E.; Oliveira, D.C.; Foster, J.M.; Fischer, P.; Munoz Torres, M.C.; Giebel, J.D.; Kumar, N.; Ishmael, N.; Wang, S.; et al. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science 2007, 317, 1753–1756. [Google Scholar]
- Timmis, J.N.; Ayliffe, M.A.; Huang, C.Y.; Martin, W. Endosymbiotic gene transfer: Organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 2004, 5, 123–135. [Google Scholar]
- Bergthorsson, U.; Adams, K.L.; Thomason, B.; Palmer, J.D. Widespread horizontal transfer of mitochondrial genes in flowering plants. Nature 2003, 424, 197–201. [Google Scholar] [CrossRef]
- Mathews, C.K. DNA precursor metabolism and genomic stability. FASEB J. 2006, 20, 1300–1314. [Google Scholar] [CrossRef]
- Niida, H.; Shimada, M.; Murakami, H.; Nakanishi, M. Mechanisms of dNTP supply that play an essential role in maintaining genome integrity in eukaryotic cells. Cancer Sci. 2010, 101, 2505–2509. [Google Scholar] [CrossRef]
- Hakansson, P.; Hofer, A.; Thelander, L. Regulation of mammalian ribonucleotide reduction and dNTP pools after DNA damage and in resting cells. J. Biol. Chem. 2006, 281, 7834–7841. [Google Scholar]
- Chabes, A.; Stillman, B. Constitutively high dNTP concentration inhibits cell cycle progression and the DNA damage checkpoint in yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2007, 104, 1183–1188. [Google Scholar] [CrossRef]
- Harper, J.W.; Elledge, S.J. The DNA damage response: Ten years after. Mol. Cell 2007, 28, 739–745. [Google Scholar] [CrossRef]
- Zou, L.; Elledge, S.J. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 2003, 300, 1542–1548. [Google Scholar] [CrossRef]
- El-Hani, C.N. Between the cross and the sword: The crisis of the gene concept. Genet. Mol. Biol. 2007, 30, 297–307. [Google Scholar] [CrossRef]
- Tautz, D. Redundancies, development and the flow of information. BioEssays 1992, 14, 263–266. [Google Scholar] [CrossRef]
- Keller, E.F. The century beyond the gene. J. Biosci. 2005, 30, 3–10. [Google Scholar] [CrossRef]
- Biro, J.C. Seven fundamental, unsolved questions in molecular biology. Cooperative storage and bi-directional transfer of biological information by nucleic acids and proteins: An alternative to “central dogma”. Med. Hypotheses 2004, 63, 951–962. [Google Scholar] [CrossRef]
- Falk, R. The rise and fall of dominance. Biol. Philos. 2001, 16, 285–323. [Google Scholar] [CrossRef]
- Ubeda, F. Why Mendelian segregation? Biochem. Soc. Trans. 2006, 34, 566–568. [Google Scholar] [CrossRef]
- Ayala, F.J. Darwin’s greatest discovery: Design without designer. Proc. Natl. Acad. Sci. USA 2007, 104, 8567–8573. [Google Scholar] [CrossRef]
- Dawkins, R. The Selfish Gene, 30th Anniversary ed.; Oxford University Press: Oxford, UK and New York, NY, USA, 2006; p. 360. [Google Scholar]
- Lombard, D.B.; Chua, K.F.; Mostoslavsky, R.; Franco, S.; Gostissa, M.; Alt, F.W. DNA repair, genome stability, and aging. Cell 2005, 120, 497–512. [Google Scholar] [CrossRef]
- Kolodner, R.D.; Putnam, C.D.; Myung, K. Maintenance of genome stability in Saccharomyces cerevisiae. Science 2002, 297, 552–557. [Google Scholar] [CrossRef]
- Orr, H.A. The genetic theory of adaptation: A brief history. Nat. Rev. Genet. 2005, 6, 119–127. [Google Scholar] [CrossRef]
- Visscher, P.M.; Hill, W.G.; Wray, N.R. Heritability in the genomics era—concepts and misconceptions. Nat. Rev. Genet. 2008, 9, 255–266. [Google Scholar]
- Cobb, A.B. Cell Theory; Chelsea House: New York, NY, USA, 2011; p. 104. [Google Scholar]
- Cano, R.J.; Borucki, M.K. Revival and identification of bacterial spores in 25- to 40-million-year-old Dominican amber. Science 1995, 268, 1060–1064. [Google Scholar]
- Sargent, M.G. Control of membrane protein synthesis in Bacillus subtilis. Biochim. Biophys. Acta 1975, 406, 564–574. [Google Scholar] [CrossRef]
- Cadenas, E.; Garland, P.B. Synthesis of cytoplasmic membrane during growth and division of Escherichia coli. Dispersive behaviour of respiratory nitrate reductase. Biochem. J. 1979, 184, 45–50. [Google Scholar]
- Diffley, J.F. Eukaryotic DNA replication. Curr. Opin. Cell. Biol. 1994, 6, 368–372. [Google Scholar] [CrossRef]
- Leipe, D.D.; Aravind, L.; Koonin, E.V. Did DNA replication evolve twice independently? Nucleic Acids Res. 1999, 27, 3389–3401. [Google Scholar] [CrossRef]
- Harry, E.; Monahan, L.; Thompson, L. Bacterial cell division: The mechanism and its precison. Int. Rev. Cytol. 2006, 253, 27–94. [Google Scholar] [CrossRef]
- Uhlmann, F. Chromosome cohesion and segregation in mitosis and meiosis. Curr. Opin. Cell. Biol. 2001, 13, 754–761. [Google Scholar] [CrossRef]
- Woese, C.R.; Magrum, L.J.; Fox, G.E. Archaebacteria. J. Mol. Evol. 1978, 11, 245–251. [Google Scholar] [CrossRef]
- Galtier, N.; Gouy, M. Molecular phylogeny of Eubacteria: A new multiple tree analysis method applied to 15 sequence data sets questions the monophyly of gram-positive bacteria. Res. Microbiol. 1994, 145, 531–541. [Google Scholar]
- Pace, N.R. Time for a change. Nature 2006, 441, 289–289. [Google Scholar] [CrossRef]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–560. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Kuroiwa, H.; Sakai, A.; Takahashi, H.; Toda, K.; Itoh, R. The division apparatus of plastids and mitochondria. Int. Rev. Cytol. 1998, 181, 1–41. [Google Scholar] [CrossRef]
- Cooper, S. On G0 and cell cycle controls. BioEssays 1987, 7, 220–223. [Google Scholar] [CrossRef]
- Coelho, S.M.; Peters, A.F.; Charrier, B.; Roze, D.; Destombe, C.; Valero, M.; Cock, J.M. Complex life cycles of multicellular eukaryotes: New approaches based on the use of model organisms. Gene 2007, 406, 152–170. [Google Scholar] [CrossRef]
- Martin, W.F.; Müller, M. Origin of Mitochondria and Hydrogenosomes; Springer: New York, NY, USA, 2007. [Google Scholar]
- Yoon, H.S.; Hackett, J.D.; Pinto, G.; Bhattacharya, D. The single, ancient origin of chromist plastids. Proc. Natl. Acad. Sci. USA 2002, 99, 15507–15512. [Google Scholar] [CrossRef]
- Mahler, H.R.; Raff, R.A. Evolutionary origin of mitochondrion—non-symbiotic model. Int. Rev. Cytol. 1975, 43, 1–124. [Google Scholar] [CrossRef]
- Sagan, L. On origin of mitosing cells. J. Theor. Biol. 1967, 14, 225–274. [Google Scholar] [CrossRef]
- Cotton, D.W. Intimate relations: The serial endosymbiotic theory of the origin of eukaryotes. J. Pathol. 1993, 169, 189–190. [Google Scholar] [CrossRef]
- Martin, W.; Muller, M. The hydrogen hypothesis for the first eukaryote. Nature 1998, 392, 37–41. [Google Scholar] [CrossRef]
- Torres, E.M.; Williams, B.R.; Amon, A. Aneuploidy: Cells losing their balance. Genetics 2008, 179, 737–746. [Google Scholar] [CrossRef]
- Chen, X.J.; Clark-Walker, G.D. The petite mutation in yeasts: 50 years on. Int. Rev. Cytol. 2000, 194, 197–238. [Google Scholar]
- Lindemann, S.; Gawaz, M. The active platelet: Translation and protein synthesis in an anucleate cell. Semin. Thromb. Hemost. 2007, 33, 144–150. [Google Scholar] [CrossRef]
- Palis, J.; Segel, G.B. Developmental biology of erythropoiesis. Blood Rev. 1998, 12, 106–114. [Google Scholar] [CrossRef]
- Nagata, S. DNA degradation in development and programmed cell death. Annu. Rev. Immunol. 2005, 23, 853–875. [Google Scholar] [CrossRef]
- Counis, M.F.; Chaudun, E.; Arruti, C.; Oliver, L.; Sanwal, M.; Courtois, Y.; Torriglia, A. Analysis of nuclear degradation during lens cell differentiation. Cell Death Differ. 1998, 5, 251–261. [Google Scholar]
- Blagosklonny, M.V.; Pardee, A.B. The restriction point of the cell cycle. Cell Cycle 2002, 1, 103–110. [Google Scholar]
- Donjerkovic, D.; Scott, D.W. Regulation of the G1 phase of the mammalian cell cycle. Cell Res. 2000, 10, 1–16. [Google Scholar] [CrossRef]
- Laskey, R.A.; Fairman, M.P.; Blow, J.J. S phase of the cell cycle. Science 1989, 246, 609–614. [Google Scholar]
- Cuddihy, A.R.; O’Connell, M.J. Cell-cycle responses to DNA damage in G2. Int. Rev. Cytol. 2003, 222, 99–140. [Google Scholar] [CrossRef]
- Gorbsky, G.J. Cell cycle checkpoints: Arresting progress in mitosis. BioEssays 1997, 19, 193–197. [Google Scholar] [CrossRef]
- Chang, F.; Nurse, P. Finishing the cell cycle: Control of mitosis and cytokinesis in fission yeast. Trends Genet. 1993, 9, 333–335. [Google Scholar] [CrossRef]
- Rabinowitz, M. Studies on the cytology and early embryology of the egg of Drosophila melanogaster. J. Morphol. 1941, 69, 1–49. [Google Scholar] [CrossRef]
- Edgar, B.A.; O’Farrell, P.H. Genetic control of cell division patterns in the Drosophila embryo. Cell 1989, 57, 177–187. [Google Scholar] [CrossRef]
- Lloyd, D.; Poole, R.K.; Edwards, S.W. The Cell Division Cycle: Temporal Organization and Control of Cellular Growth and Reproduction; Academic Press: New York, NY, USA, 1982; p. 523. [Google Scholar]
- Nasmyth, K. Evolution of the cell-cycle. Philos. Trans. R. Soc. Lond. Ser. B 1995, 349, 271–281. [Google Scholar] [CrossRef]
- Haeckel, E. Art Forms in Nature; Dover Publications: New York, NY, USA, 1974. [Google Scholar]
- Biggs, D.S. 3D deconvolution microscopy. Curr. Protoc. Cytom. 2010, Chapter 12. 12.19:1–12.19:20. [Google Scholar]
- Diaspro, A.; Chirico, G.; Collini, M. Two-photon fluorescence excitation and related techniques in biological microscopy. Q. Rev. Biophys. 2005, 38, 97–166. [Google Scholar] [CrossRef]
- Horwitz, R. Cell biology as the centuries change—About as good as it gets. J. Cell Sci. 2000, 113, 906–908. [Google Scholar]
- Moon, J.; Hake, S. How a leaf gets its shape. Curr. Opin. Plant Biol. 2011, 14, 24–30. [Google Scholar] [CrossRef]
- Stolz, J.F.; Franks, J. Flat laminated microbial mat communities. Earth Sci. Rev. 2009, 96, 163–172. [Google Scholar] [CrossRef]
- Jelsbak, L.; Sogaard-Andersen, L. Cell behavior and cell-cell communication during fruiting body morphogenesis in Myxococcus xanthus. J. Microbiol. Methods 2003, 55, 829–839. [Google Scholar] [CrossRef]
- Tice, M.M.; Lowe, D.R. Photosynthetic microbial mats in the 3,416-Myr-old ocean. Nature 2004, 431, 549–552. [Google Scholar] [CrossRef]
- Lopez-Garcia, P.; Moreira, D.; Douzery, E.; Forterre, P.; van Zuilen, M.; Claeys, P.; Prieur, D. Ancient fossil record and early evolution (ca. 3.8 to 0.5 Ga). Earth Moon Planets 2006, 98, 247–290. [Google Scholar] [CrossRef]
- Thery, M.; Bornens, M. Cell shape and cell division. Curr. Opin. Cell. Biol. 2006, 18, 648–657. [Google Scholar] [CrossRef]
- Cabeen, M.T.; Jacobs-Wagner, C. Bacterial cell shape. Nat. Rev. Microbiol. 2005, 3, 601–610. [Google Scholar] [CrossRef]
- Pajerowski, J.D.; Dahl, K.N.; Zhong, F.L.; Sammak, P.J.; Discher, D.E. Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl. Acad. Sci. USA 2007, 104, 15619–15624. [Google Scholar]
- Guo, X.; Goessl, E.; Jin, G.; Collie-Duguid, E.S.; Cassidy, J.; Wang, W.; O’Brien, V. Cell cycle perturbation and acquired 5-fluorouracil chemoresistance. Anticancer Res. 2008, 28, 9–14. [Google Scholar]
- Taraschi, T.F.; Parashar, A.; Hooks, M.; Rubin, H. Perturbation of red cell membrane structure during intracellular maturation of Plasmodium falciparum. Science 1986, 232, 102–104. [Google Scholar]
- Powell, C.D.; Quain, D.E.; Smart, K.A. Chitin scar breaks in aged Saccharomyces cerevisiae. Microbiology 2003, 149, 3129–3137. [Google Scholar] [CrossRef]
- For example, the yeast bud scar, an end result of cell division, is a near-perfect circle of chitin on the cell surface; please note how the bud scar mirrors the craters on celestial surfaces (3.2).
- Williams, G.C. Pleiotropy, natural selection and the evolution of senescence. Evolution 1957, 11, 398–411. [Google Scholar] [CrossRef]
- Lane, N. Power, Sex, Suicide: Mitochondria and the Meaning of Life; Oxford University Press: Oxford, UK and New York, NY, USA, 2005; p. 354. [Google Scholar]
- Clark, W.R. A Means to an End: The Biological Basis of Aging and Death; Oxford University Press: New York, NY, USA, 1999; p. 234. [Google Scholar]
- Hay, M.E.; Rasher, D.B. Coral reefs in crisis: Reversing the biotic death spiral. F1000 Biol. Rep. 2010, 2. [Google Scholar] [CrossRef]
- Vesteg, M.; Krajcovic, J. On the origin of meiosis and sex. Riv. Biol. 2007, 100, 147–161. [Google Scholar]
- Bernstein, H.; Byerly, H.C.; Hopf, F.A.; Michod, R.E. Origin of sex. J. Theor. Biol. 1984, 110, 323–351. [Google Scholar] [CrossRef]
- Kondrashov, A.S. The asexual ploidy cycle and the origin of sex. Nature 1994, 370, 213–216. [Google Scholar] [CrossRef]
- Otto, S.P.; Mable, B.K. The evolution of life cycles with haploid and diploid phases. BioEssays 1998, 20, 453–462. [Google Scholar] [CrossRef]
- Rodrigues, P.; Limback, D.; McGinnis, L.K.; Plancha, C.E.; Albertini, D.F. Oogenesis: Prospects and challenges for the future. J. Cell. Physiol. 2008, 216, 355–365. [Google Scholar] [CrossRef]
- Verlhac, M.-H.; Villeneuve, A. Oogenesis: The Universal Process; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Moore, I.T.; Lerner, J.P.; Lerner, D.T.; Mason, R.T. Relationships between annual cycles of testosterone, corticosterone, and body condition in male red-spotted garter snakes, Thamnophis sirtalis concinnus. Physiol. Biochem. Zool. 2000, 73, 307–312. [Google Scholar] [CrossRef]
- Tricas, T.C.; Maruska, K.P.; Rasmussen, L.E. Annual cycles of steroid hormone production, gonad development, and reproductive behavior in the Atlantic stingray. Gen. Comp. Endocrinol. 2000, 118, 209–225. [Google Scholar] [CrossRef]
- Hines, G.A.; Watts, S.A.; Sower, S.A.; Walker, C.W. Sex steroid levels in the testes, ovaries, and pyloric caeca during gametogenesis in the sea star Asterias vulgaris. Gen. Comp. Endocrinol. 1992, 87, 451–460. [Google Scholar] [CrossRef]
- Irianni, F.; Hodgen, G.D. Mechanism of ovulation. Endocrinol. Metab. Clin. N. Am. 1992, 21, 19–38. [Google Scholar]
- Chabbert Buffet, N.; Djakoure, C.; Maitre, S.C.; Bouchard, P. Regulation of the human menstrual cycle. Front. Neuroendocrinol. 1998, 19, 151–186. [Google Scholar] [CrossRef]
- These cycles are nested (androgens are modeled by the carbogyre (3.3) and both lutenizing hormone and follicle-stimulating hormone are modeled by the aminogyre (3.5)) within the dip- and hapcellulogyre.
- Mayr, E. Weismann and evolution. J. Hist. Biol. 1985, 18, 295–329. [Google Scholar] [CrossRef]
- Gregory, T.R. The Evolution of the Genome; Elsevier Academic: Burlington, MA, USA, 2005; p. 740. [Google Scholar]
- Cavalier-Smith, T. Nuclear volume control by nucleoskeletal DNA, selection for cell volume and cell growth rate, and the solution of the DNA C-value paradox. J. Cell Sci. 1978, 34, 247–278. [Google Scholar]
- Ozbek, S.; Balasubramanian, P.G.; Chiquet-Ehrismann, R.; Tucker, R.P.; Adams, J.C. The evolution of extracellular matrix. Mol. Biol. Cell 2010, 21, 4300–4305. [Google Scholar] [CrossRef]
- Huxley-Jones, J.; Pinney, J.W.; Archer, J.; Robertson, D.L.; Boot-Handford, R.P. Back to basics—How the evolution of the extracellular matrix underpinned vertebrate evolution. Int. J. Exp. Pathol. 2009, 90, 95–100. [Google Scholar] [CrossRef]
- Bereiter-Hahn, J.; Matoltsy, A.G.; Richards, K.S. Biology of the Integument; Springer-Verlag: Berlin, Germany and New York, NY, USA, 1984. [Google Scholar]
- Gorb, S. Functional Surfaces in Biology; Springer: Dordrecht, The Netherlands, 2009; p. 678. [Google Scholar]
- Findlay, G.H.; Harris, W.F. The topology of hair streams and whorls in man, with an observation on their relationship to epidermal ridge patterns. Am. J. Phys. Anthropol. 1977, 46, 427–437. [Google Scholar] [CrossRef]
- Williams, A. Spiral growth of the laminar shell of the brachiopod Crania. Calcif. Tissue Res. 1970, 6, 11–19. [Google Scholar] [CrossRef]
- Loza-Correa, M.; Gomez-Valero, L.; Buchrieser, C. Circadian clock proteins in prokaryotes: Hidden rhythms? Front. Microbiol. 2010, 1. [Google Scholar] [CrossRef]
- Panda, S.; Hogenesch, J.B.; Kay, S.A. Circadian rhythms from flies to human. Nature 2002, 417, 329–335. [Google Scholar] [CrossRef]
- Park, D.H.; Somers, D.E.; Kim, Y.S.; Choy, Y.H.; Lim, H.K.; Soh, M.S.; Kim, H.J.; Kay, S.A.; Nam, H.G. Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science 1999, 285, 1579–1582. [Google Scholar] [CrossRef]
- Rothenfluh, A.; Abodeely, M.; Price, J.L.; Young, M.W. Isolation and analysis of six timeless alleles that cause short- or long-period circadian rhythms in Drosophila. Genetics 2000, 156, 665–675. [Google Scholar]
- Cyran, S.A.; Buchsbaum, A.M.; Reddy, K.L.; Lin, M.C.; Glossop, N.R.; Hardin, P.E.; Young, M.W.; Storti, R.V.; Blau, J. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell 2003, 112, 329–341. [Google Scholar] [CrossRef]
- Gonze, D.; Leloup, J.C.; Goldbeter, A. Theoretical models for circadian rhythms in Neurospora and Drosophila. Comptes Rendus Acad. Sci. Ser. III 2000, 323, 57–67. [Google Scholar]
- Yang, Q.; Pando, B.F.; Dong, G.; Golden, S.S.; van Oudenaarden, A. Circadian gating of the cell cycle revealed in single cyanobacterial cells. Science 2010, 327, 1522–1526. [Google Scholar]
- Slavov, N.; Botstein, D. Coupling among growth rate response, metabolic cycle, and cell division cycle in yeast. Mol. Biol. Cell 2011, 22, 1997–2009. [Google Scholar] [CrossRef]
- Karsenti, E. Self-organization in cell biology: A brief history. Nat. Rev. Mol. Cell Biol. 2008, 9, 255–262. [Google Scholar] [CrossRef]
- Sapp, J. Microbial Phylogeny and Evolution: Concepts and Controversies; Oxford University Press: New York, NY, USA, 2005; p. 362. [Google Scholar]
- Franc, N.C. Phagocytosis of apoptotic cells in mammals, caenorhabditis elegans and Drosophila melanogaster: Molecular mechanisms and physiological consequences. Front. Biosci. 2002, 7, d1298–1313. [Google Scholar] [CrossRef]
- Soldati, T.; Schliwa, M. Powering membrane traffic in endocytosis and recycling. Nat. Rev. Mol. Cell Biol. 2006, 7, 897–908. [Google Scholar] [CrossRef]
- Black, S. A theory on the origin of life. Adv. Enzymol. Relat. Areas Mol. Biol. 1973, 38, 193–234. [Google Scholar]
- Wolkenhauer, O.; Hofmeyr, J.H.S. A contribution towards a theory of living cells. At-Automatisierungstechnik 2008, 56, 225–232. [Google Scholar] [CrossRef]
- Olson, C.B. A theory of the origin of life. Orig. Life Evol. Biosph. 1981, 11, 353–368. [Google Scholar] [CrossRef]
- Lucido, G. Life out of magma: A new theory for the origin of life. Nuovo Cim. Della Soc. Ital. Fis. D 1998, 20, 2575–2591. [Google Scholar]
- Snooks, G.D. The origin of life on earth: A new general dynamic theory. Space Life Sci. 2005, 36, 226–234. [Google Scholar]
- Martin, W.; Russell, M.J. On the origins of cells: A hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. Lond. Ser. B 2003, 358, 59–83. [Google Scholar] [CrossRef]
- Kennedy, D. 125. Science 2005, 309, 15. [Google Scholar]
- Kennedy, D.; Norman, C. What don’t we know? Science 2005, 309. [Google Scholar] [CrossRef]
- Beyond the standard model. Eff. Field Theor. Flavor Phys. 2004, 203, 157–166.
- Gold, T. The Deep Hot Biosphere; Copernicus: New York, NY, USA, 1999; p. 235. [Google Scholar]
- Asimov, I. Fact and Fancy, 1st ed.; Doubleday: Garden City, NY, USA, 1962; p. 264. [Google Scholar]
- Blackburn, G.M.; Bowler, M.W.; Cliff, M.J.; Waltho, J.P. Why did Nature select phosphate for its dominant roles in biology? New J. Chem. 2010, 34, 784–794. [Google Scholar] [CrossRef]
- Lahav, N.; Nir, S.; Elitzur, A.C. The emergence of life on Earth. Progress Biophys. Mol. Biol. 2001, 75, 75–120. [Google Scholar] [CrossRef]
- Jukes, T.H. Possible evolutionary steps in the genetic code. Biochem. Biophys. Res. Commun. 1982, 107, 225–228. [Google Scholar] [CrossRef]
- Osawa, S. Evolution of the Genetic Code; Oxford University Press: Oxford: New York, NY, USA, 1995; p. 205. [Google Scholar]
- Goldman, A.D.; Samudrala, R.; Baross, J.A. The evolution and functional repertoire of translation proteins following the origin of life. Biol. Direct 2010, 5. [Google Scholar] [CrossRef]
- Central dogma reversed. Nature 1970, 226, 1198–1199. [CrossRef]
- Wag the dogma. Nat. Genet. 2002, 30, 343–344.
- Chaisson, E.J. The cosmic environment for the growth of complexity. Biol. Syst. 1998, 46, 13–19. [Google Scholar]
- Ball, P. Physics: Quantum all the way. Nature 2008, 453, 22–25. [Google Scholar] [CrossRef]
- Waldrop, M.M. Complexity: The Emerging Science at the Edge of Order and Chaos; Simon & Schuster: New York, NY, USA, 1992; p. 380. [Google Scholar]
- Lewin, R. Complexity: Life at the Edge of Chaos, 2nd ed.; University of Chicago Press: Chicago, IL, USA, 1999; p. 234. [Google Scholar]
- Ellis, N.C.; Larsen-Freeman, D. Research Club in Language Learning (Ann Arbor Mich.). In Language as a Complex Adaptive System; Wiley-Blackwell: Chichester, West Sussex, UK and Malden, MA, USA, 2009; p. 275. [Google Scholar]
- Armstrong, D.M. What Is a Law of Nature? Cambridge University Press: Cambridge Cambridgeshire, MA and New York, NY, USA, 1983; p. 180. [Google Scholar]
- Lange, M. Laws and Lawmakers: Science, Metaphysics, and the Laws of Nature; Oxford University Press: Oxford, UK and New York, NY, USA, 2009; p. 257. [Google Scholar]
- Morowitz, H.J. Energy Flow in Biology; Biological Organization as a Problem in Thermal Physics; Academic Press: New York, NY, USA, 1968; p. 179. [Google Scholar]
- Ferris, J.P.; Hill, A.R., Jr.; Liu, R.; Orgel, L.E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 1996, 381, 59–61. [Google Scholar]
- Bohr, N.; Rosenfeld, L. Collected Works; North-Holland Publishing Co.: Amsterdam, Holland, 1972. [Google Scholar]
- Sciama, D.W. The Unity of the Universe, 1st ed.; Doubleday: Garden City, NY, USA, 1959; p. 228. [Google Scholar]
- Weizsäcker, C.F. The Unity of Nature; Farrar Straus Giroux: New York, NY, USA, 1980; p. 406. [Google Scholar]
- Weinberg, S. Dreams of a Final Theory, 1st Vintage Books ed.; Vintage Books: New York, NY, USA, 1994; p. 340. [Google Scholar]
- Prigogine, I.; Stengers, I. The End of Certainty: Time, Chaos, and the New Laws of Nature, 1st Free Press ed.; Free Press: New York, NY, USA, 1997; p. 228. [Google Scholar]
- Wallace, D.F. Everything and More: A Compact History of Infinity, 1st ed.; Atlas Book: New York, NY, USA, 2003; p. 319. [Google Scholar]
- Rucker, R. Infinity and the Mind: The Science and Philosophy of the Infinite; Princeton University Press: Princeton, NJ, USA, 1995; p. 342. [Google Scholar]
- Blum, H.F. Time’s Arrow and Evolution, 2nd ed.; Harper: New York, NY, USA, 1962; p. 220. [Google Scholar]
- Wacey, D.; Kilburn, M.R.; Saunders, M.; Cliff, J.; Brasier, M.D. Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia. Nat. Geosci. 2011, 4, 698–702. [Google Scholar] [CrossRef]
- Eddington, A.S. The Nature of the Physical World; University Press: Cambridge, UK, 1928; p. 361. [Google Scholar]
- Williams, G.C. Adaptation and Natural Selection; a Critique of Some Current Evolutionary Thought; Princeton University Press: Princeton, NJ, USA, 1966; p. 307. [Google Scholar]
- Cannon, W.B. The Wisdom of the Body; W.W. Norton & Company: New York, NY, USA, 1939; p. 333. [Google Scholar]
- Muller, G.B.; Wagner, G.P. Novelty in evolution—Restructuring the concept. Annu. Rev. Ecol. Syst. 1991, 22, 229–256. [Google Scholar]
- Darwin, C. The Origin of Species: Complete and Fully Illustrated; Gramercy Books: New York, NY, USA, 1979; p. 460. [Google Scholar]
- Bak, P. How Nature Works: The Science of Self-Organized Criticality; Copernicus: New York, NY, USA, 1996; p. 212. [Google Scholar]
- Gould, S.J.; Eldredge, N. Punctuated equilibrium comes of age. Nature 1993, 366, 223–227. [Google Scholar] [CrossRef]
- Henderson, L.J. The Fitness of the Environment; an Inquiry into the Biological Significance of the Properties of Matter; P. Smith: Gloucester, MA, USA, 1970; p. 317. [Google Scholar]
- Vernadsky, V.I. The Biosphere; Copernicus: New York, NY, USA, 1998; p. 192. [Google Scholar]
- Cleland, C.E.; Chyba, C.F. Defining ‘life’. Orig. Life Evol. Biosph. 2002, 32, 387–393. [Google Scholar] [CrossRef]
- Des Marais, D.J.; Walter, M.R. Astrobiology: Exploring the origins, evolution, and distribution of life in the Universe. Annu. Rev. Ecol. Syst. 1999, 30, 397–420. [Google Scholar] [CrossRef]
- Javaux, E.J.; Dehant, V. Habitability: From stars to cells. Astron. Astrophys. Rev. 2010, 18, 383–416. [Google Scholar] [CrossRef]
- Horneck, G. Exobiology, the study of the origin, evolution and distribution of life within the context of cosmic evolution: A review. Planet. Space Sci. 1995, 43, 189–217. [Google Scholar] [CrossRef]
- Monod, J. Chance and Necessity; an Essay on the Natural Philosophy of Modern Biology, 1st American ed.; Knopf: New York, NY, USA, 1971; p. 198. [Google Scholar]
- Aristotle; Lawson-Tancred, H. Metaphysics; Penguin Books: London, UK and New York, NY, USA, 1998; p. 459. [Google Scholar]
- Bachelard, G. The Formation of the Scientific Mind: A Contribution to a Psychoanalysis of Objective Knowledge; Clinamen Press Ltd.: Manchester, UK, 2006; p. 266. [Google Scholar]
- Kuhn, T.S. The Structure of Scientific Revolutions; University of Chicago Press: Chicago, IL, USA, 1962; p. 172. [Google Scholar]
- Strohman, R.C. The coming Kuhnian revolution in biology. Nat. Biotechnol. 1997, 15, 194–200. [Google Scholar] [CrossRef]
- Stent, G.S. American Museum of Natural History. In The Coming of the Golden Age; a View of the End of Progress, 1st ed.; Published for the American Museum of Natural History by the Natural History Press: Garden City, NY, USA, 1969; p. 146. [Google Scholar]
- Horgan, J. The End of Science: Facing the Limits of Knowledge in the Twilight of the Scientific Age; Abacus: London, UK, 1998; p. 324. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Andrulis, E.D. Theory of the Origin, Evolution, and Nature of Life. Life 2012, 2, 1-105. https://doi.org/10.3390/life2010001
Andrulis ED. Theory of the Origin, Evolution, and Nature of Life. Life. 2012; 2(1):1-105. https://doi.org/10.3390/life2010001
Chicago/Turabian StyleAndrulis, Erik D. 2012. "Theory of the Origin, Evolution, and Nature of Life" Life 2, no. 1: 1-105. https://doi.org/10.3390/life2010001
APA StyleAndrulis, E. D. (2012). Theory of the Origin, Evolution, and Nature of Life. Life, 2(1), 1-105. https://doi.org/10.3390/life2010001