Dietetic Prescriptions in Bipolar Disorder: Nutritional Strategies to Support Mood Stability and Reduce Relapse Risk—A Narrative Review
Abstract
1. Introduction
1.1. Rationale for Dietary Approaches in Bipolar Disorder
1.2. Current Gaps in Treatment of Bipolar Disorder and Unmet Needs
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Synthesis
3. Pathophysiological Rationale
3.1. Inflammation, Oxidative Stress, and Metabolic Dysfunction
3.2. Gut–Brain Axis and Microbiome Considerations
4. Dietary Patterns and Nutritional Interventions
4.1. Mediterranean Diet and Anti-Inflammatory Approaches
4.2. Ketogenic and Low-Carbohydrate Diets
4.3. Plant-Based Diets
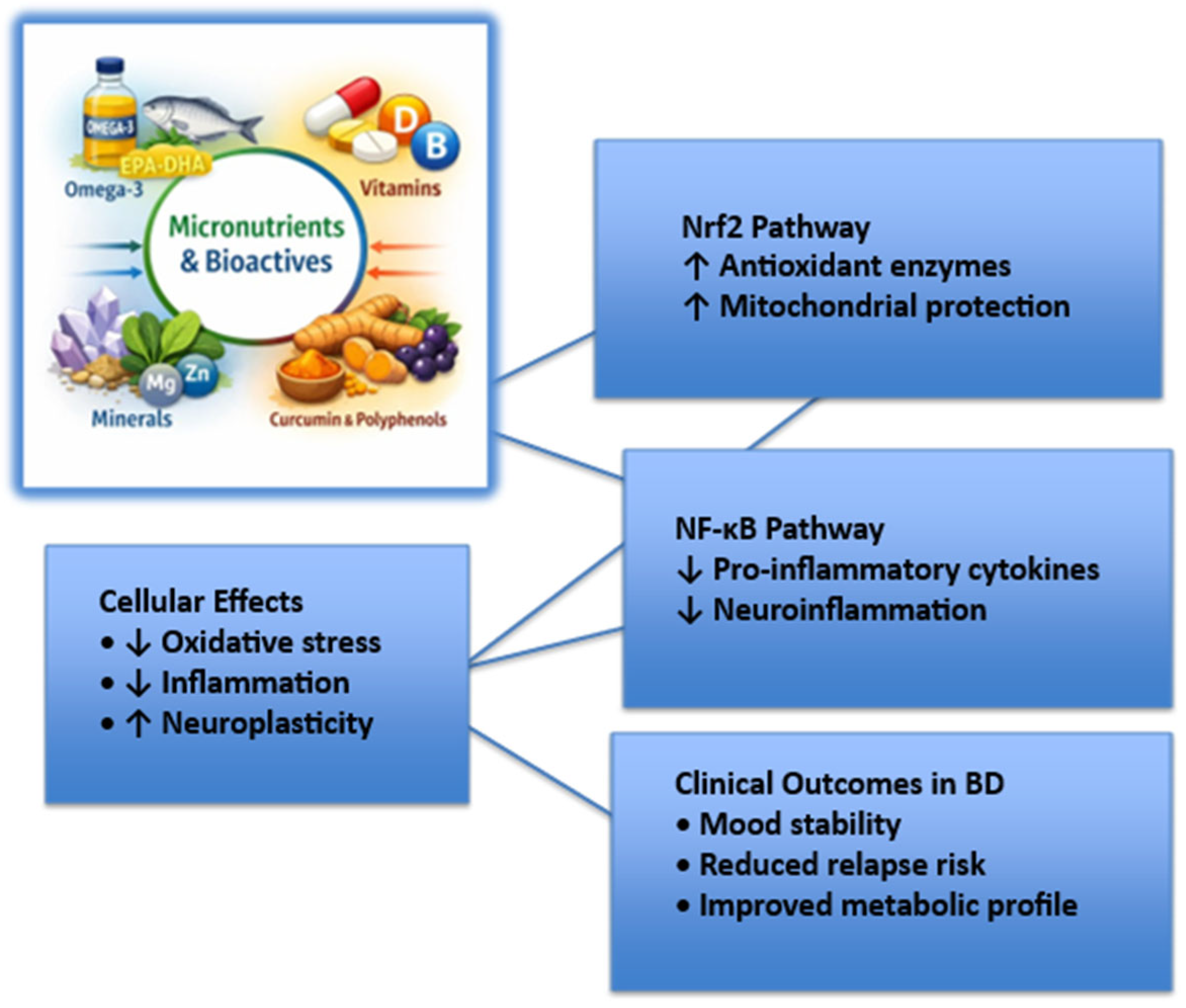
5. Specific Nutrients and Supplementation
5.1. Omega-3 Fatty Acids
5.2. Vitamins (e.g., Folate, Vitamin D, B-Complex)
5.3. Minerals (e.g., Magnesium, Zinc, Selenium)
5.4. Curcumin
6. Integration into Clinical Practice
6.1. Dietary Counseling and Collaboration with Dietitians
6.2. Patient Adherence and Lifestyle Barriers
6.3. Personalized and Precision Nutrition Approaches
6.4. Nutritional Assessment Tools in Serious Mental Illnesses
6.5. Food–Medication and Micronutrient–Medication Interactions
6.6. Digital Health Technologies
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N. Nutritional psychiatry: Where to next? eBioMedicine 2017, 17, 24–29. [Google Scholar] [CrossRef]
- Lai, J.S.; Hiles, S.; Bisquera, A.; Hure, A.J.; McEvoy, M.; Attia, J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2014, 99, 181–197. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, A.; Quirk, S.E.; Housden, S.; Brennan, S.L.; Williams, L.J.; Pasco, J.A.; Berk, M.; Jacka, F.N. Relationship between diet and mental health in children and adolescents: A systematic review. Am. J. Public Health 2014, 104, e31–e42. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Twizeyemariya, A.; Zarnowiecki, D.; Niyonsenga, T.; Bogomolova, S.; Wilson, A.; O’Dea, K.; Parletta, N. Cost effectiveness and cost-utility analysis of a group-based diet intervention for treating major depression: The HELFIMED trial. Nutr. Neurosci. 2020, 23, 770–778. [Google Scholar] [CrossRef]
- Firth, J.; Teasdale, S.B.; Allott, K.; Siskind, D.; Marx, W.; Cotter, J.; Veronese, N.; Schuch, F.; Smith, L.; Solmi, M.; et al. The efficacy and safety of nutrient supplements in the treatment of mental disorders: A meta-review of meta-analyses of randomized controlled trials. World Psychiatry 2019, 18, 308–324. [Google Scholar] [CrossRef]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Platzer, M.; Fellendorf, F.T.; Bengesser, S.A.; Birner, A.; Dalkner, N.; Hamm, C.; Lenger, M.; Maget, A.; Pilz, R.; Queissner, R.; et al. The relationship between food craving, appetite-related hormones and clinical parameters in bipolar disorder. Nutrients 2021, 13, 76. [Google Scholar] [CrossRef]
- Mansur, R.B.; Lee, Y.; Subramaniapillai, M.; Cha, D.S.; Brietzke, E.; McIntyre, R.S. Parsing metabolic heterogeneity in mood disorders: A hypothesis-driven cluster analysis of glucose and insulin abnormalities. Bipolar Disord. 2020, 22, 79–88. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef]
- Shariq, A.S.; Brietzke, E.; Rosenblat, J.D.; Barendra, V.; Pan, Z.; McIntyre, R.S. Targeting cytokines in reduction of depressive symptoms: A comprehensive review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 83, 86–91. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Jacka, F.N. Diet and bipolar disorder: A review of its relationship and potential therapeutic mechanisms of action. J. Altern. Complement. Med. 2015, 21, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Elmslie, J.L.; Mann, J.I.; Silverstone, J.T.; Williams, S.M.; Romans, S.E. Determinants of overweight and obesity in patients with bipolar disorder. J. Clin. Psychiatry 2001, 62, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Noaghiul, S.; Hibbeln, J.R. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am. J. Psychiatry 2003, 160, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, A.M.; Rofey, D.L.; McCarthy, J.F.; Post, E.P.; Welsh, D.; Blow, F.C. Nutrition and exercise behavior among patients with bipolar disorder. Bipolar Disord. 2007, 9, 443–452. [Google Scholar] [CrossRef]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Diet quality in bipolar disorder in a population-based sample of women. J. Affect. Disord. 2011, 129, 332–337. [Google Scholar] [CrossRef]
- Fagiolini, A.; Chengappa, K.N. Weight gain and metabolic issues of medicines used for bipolar disorder. Curr. Psychiatry Rep. 2007, 9, 521–528. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Lackner, N.; Fellendorf, F.T.; Bengesser, S.; Birner, A.; Reininghaus, B.; Unterweger, R.; Platzer, M.; Wallner-Liebmann, S.J.; Zelzer, S.; et al. Weight cycling in bipolar disorder. J. Affect. Disord. 2015, 171, 33–38. [Google Scholar] [CrossRef]
- Calkin, C.V.; Ruzickova, M.; Uher, R.; Hajek, T.; Slaney, C.M.; Garnham, J.S.; O’Donovan, M.C.; Alda, M. Insulin resistance and outcome in bipolar disorder. Br. J. Psychiatry 2015, 206, 52–57. [Google Scholar] [CrossRef]
- Whiteford, H.A.; Ferrari, A.J.; Degenhardt, L.; Feigin, V.; Vos, T. The global burden of mental, neurological and substance use disorders: An analysis from the Global Burden of Disease Study 2010. PLoS ONE 2015, 10, e0116820. [Google Scholar] [CrossRef]
- Chisholm, D.; Sweeny, K.; Sheehan, P.; Rasmussen, B.; Smit, F.; Cuijpers, P.; Saxena, S. Scaling-up treatment of depression and anxiety: A global return on investment analysis. Lancet Psychiatry 2016, 3, 415–424. [Google Scholar] [CrossRef]
- Casacalenda, N.; Perry, J.C.; Looper, K. Remission in major depressive disorder: A comparison of pharmacotherapy, psychotherapy, and control conditions. Am. J. Psychiatry 2002, 159, 1354–1360. [Google Scholar] [CrossRef]
- Olfson, M.; Druss, B.G.; Marcus, S.C. Trends in mental health care among children and adolescents. N. Engl. J. Med. 2015, 372, 2029–2038. [Google Scholar] [CrossRef]
- Jorm, A.F.; Patten, S.B.; Brugha, T.S.; Mojtabai, R. Has increased provision of treatment reduced the prevalence of common mental disorders? Review of the evidence from four countries. World Psychiatry 2017, 16, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C.; Jacka, F.N. Nutritional psychiatry research: An emerging discipline and its intersection with global urbanization, environmental challenges and the evolutionary mismatch. J. Physiol. Anthropol. 2014, 33, 22. [Google Scholar] [CrossRef]
- O’Neil, A.; Berk, M.; Itsiopoulos, C.; Castle, D.; Opie, R.; Pizzinga, J.; Brazionis, L.; Hodge, A.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised, controlled trial of a dietary intervention for adults with major depression (the “SMILES” trial): Study protocol. BMC Psychiatry 2013, 13, 114. [Google Scholar] [CrossRef]
- White, B. Dietary fatty acids. Am. Fam. Physician 2009, 80, 345–350. [Google Scholar] [PubMed]
- Bonaccio, M.; Di Castelnuovo, A.; Pounis, G.; De Curtis, A.; Costanzo, S.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; et al. A score of low-grade inflammation and risk of mortality: Prospective findings from the Moli-sani study. Haematologica 2016, 101, 1434–1441. [Google Scholar] [CrossRef]
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-related biomarkers in major psychiatric disorders: A cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 2019, 9, 233. [Google Scholar] [CrossRef]
- Horsdal, H.T.; Köhler-Forsberg, O.; Benros, M.E.; Gasse, C. C-reactive protein and white blood cell levels in schizophrenia, bipolar disorders and depression: Associations with mortality and psychiatric outcomes: A population-based study. Eur. Psychiatry 2017, 44, 164–172. [Google Scholar] [CrossRef]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nielsen, S.F.; Nordestgaard, B.G. Elevated C-reactive protein levels, psychological distress, and depression in 73,131 individuals. JAMA Psychiatry 2013, 70, 176–184. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; De Curtis, A.; Costanzo, S.; Persichillo, M.; Donati, M.B.; Cerletti, C.; Iacoviello, L.; de Gaetano, G.; Moli-sani Project Investigators. Adherence to the Mediterranean diet is associated with lower platelet and leukocyte counts: Results from the Moli-sani study. Blood 2014, 123, 3037–3044. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Egea Rodrigues, C.; Aleksandrova, K. Effects of dietary patterns on biomarkers of inflammation and immune responses: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Shao, R.; Wang, R.; Lu, W.; Zou, W.; Chen, K.; Gao, Y.; Brietzke, E.; McIntyre, R.S.; Mansur, R.B.; et al. Inflammation, brain structure and cognition interrelations among individuals with differential risks for bipolar disorder. Brain Behav. Immun. 2020, 83, 192–199. [Google Scholar] [CrossRef]
- Sagduyu, K.; Dokucu, M.E.; Eddy, B.A.; Craigen, G.; Baldassano, C.F.; Yildiz, A. Omega-3 fatty acids decreased irritability of patients with bipolar disorder in an add-on, open-label study. Nutr. J. 2005, 4, 6. [Google Scholar] [CrossRef]
- Cheng, W.W.; Zhu, Q.; Zhang, H.Y. Mineral nutrition and the risk of chronic diseases: A Mendelian randomization study. Nutrients 2019, 11, 378. [Google Scholar] [CrossRef]
- Lyu, N.; Xing, G.; Yang, J.; Zhu, X.; Zhao, X.; Zhang, L.; Wang, G. Comparison of inflammatory, nutrient, and neurohormonal indicators in patients with schizophrenia, bipolar disorder and major depressive disorder. J. Psychiatr. Res. 2021, 137, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Williams, L.J.; Andreazza, A.C.; Pasco, J.A.; Dodd, S.; Jacka, F.N.; Moylan, S.; Reiner, E.J.; Magalhaes, P.V. POP, heavy metal and the blues: Secondary analysis of persistent organic pollutants, heavy metals and depressive symptoms in the NHANES National Epidemiological Survey. BMJ Open 2014, 4, e005142. [Google Scholar] [CrossRef]
- González-Estecha, M.; Trasobares, E.M.; Tajima, K.; Cano, S.; Fernández, C.; López, J.L.; Unzeta, B.; Arroyo, M.; Fuentenebro, F. Trace elements in bipolar disorder. J. Trace Elem. Med. Biol. 2011, 25, S78–S83. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Chilicka, K.; Dzieńdziora-Urbińska, I.; Szyguła, R.; Asanova, B.; Nowicka, D. Microbiome and probiotics in acne vulgaris—A narrative review. Life 2022, 12, 422. [Google Scholar] [CrossRef]
- Nowicka, D.; Chilicka, K.; Dzieńdziora-Urbińska, I. Host–microbe interaction on the skin and its role in the pathogenesis and treatment of atopic dermatitis. Pathogens 2022, 11, 71. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The human gut microbiome—A potential controller of wellness and disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Zogg, H.; Wei, L.; Bartlett, A.; Ghoshal, U.C.; Rajender, S.; Ro, S. Gut microbial dysbiosis in the pathogenesis of gastrointestinal dysmotility and metabolic disorders. J. Neurogastroenterol. Motil. 2021, 27, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Enaud, R.; Vandenborght, L.E.; Coron, N.; Bazin, T.; Prevel, R.; Schaeverbeke, T.; Berger, P.; Fayon, M.; Lamireau, T.; Delhaes, L. The mycobiome: A neglected component in the microbiota–gut–brain axis. Microorganisms 2018, 6, 22. [Google Scholar] [CrossRef]
- Vemuri, R.; Shankar, E.M.; Chieppa, M.; Eri, R.; Kavanagh, K. Beyond just bacteria: Functional biomes in the gut ecosystem including virome, mycobiome, archaeome and helminths. Microorganisms 2020, 8, 483. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Mazza, M.; Pomponi, M.; Janiri, L.; Bria, P.; Mazza, S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: An overview. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 12–26. [Google Scholar] [CrossRef]
- Marano, G.; Traversi, G.; Nannarelli, C.; Mazza, S.; Mazza, M. Omega-3 fatty acids and schizophrenia: Evidences and recommendations. Clin. Ter. 2013, 164, e529–e537. [Google Scholar] [CrossRef] [PubMed]
- Marano, G.; Boggio, G.; Abate, F.; Caroppo, E.; Traversi, G.; Mazza, O.; Capristo, E.; Gaetani, E.; Mazza, M. From food to mood: Psychological and psychiatric impact of diet in bipolar disorder. Nutrients 2025, 17, 3728. [Google Scholar] [CrossRef]
- Marano, G.; Mazza, M.; Lisci, F.M.; Ciliberto, M.; Traversi, G.; Kotzalidis, G.D.; De Berardis, D.; Laterza, L.; Sani, G.; Gasbarrini, A.; et al. The microbiota–gut–brain axis: Psychoneuroimmunological insights. Nutrients 2023, 15, 1496. [Google Scholar] [CrossRef] [PubMed]
- Marano, G.; Rossi, S.; Sfratta, G.; Traversi, G.; Lisci, F.M.; Anesini, M.B.; Pola, R.; Gasbarrini, A.; Gaetani, E.; Mazza, M. Gut microbiota: A new challenge in mood disorder research. Life 2025, 15, 593. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- Jain, R.; Larsuphrom, P.; Degremont, A.; Latunde-Dada, G.O.; Philippou, E. Association between vegetarian and vegan diets and depression: A systematic review. Nutr. Bull. 2022, 47, 27–49. [Google Scholar] [CrossRef]
- Evans, S.J.; Prossin, A.R.; Harrington, G.J.; Kamali, M.; Ellingrod, V.L.; Burant, C.F.; McInnis, M.G. Fats and factors: Lipid profiles associate with personality factors and suicidal history in bipolar subjects. PLoS ONE 2012, 7, e29297. [Google Scholar] [CrossRef]
- Mazza, M.; Marano, G.; Traversi, G.; Di Nicola, M.; Catalano, V.; Janiri, L. The complex interplay of depression, inflammation and omega-3: State of the art and progresses in research. Clin. Ter. 2015, 166, e242–e247. [Google Scholar] [CrossRef]
- Hirashima, F.; Parow, A.M.; Stoll, A.L.; Demopulos, C.M.; Damico, K.E.; Rohan, M.L.; Eskesen, J.G.; Zuo, C.S.; Cohen, B.M.; Renshaw, P.F. Omega-3 fatty acid treatment and T2 whole brain relaxation times in bipolar disorder. Am. J. Psychiatry 2004, 161, 1922–1924. [Google Scholar] [CrossRef]
- Osher, Y.; Bersudsky, Y.; Belmaker, R.H. Omega-3 eicosapentaenoic acid in bipolar depression: Report of a small open-label study. J. Clin. Psychiatry 2005, 66, 726–729. [Google Scholar] [CrossRef]
- Frangou, S.; Lewis, M.; Wollard, J.; Simmons, A. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentaenoic acid treatment in patients with bipolar disorder. J. Psychopharmacol. 2007, 21, 435–439. [Google Scholar] [CrossRef]
- Keck, P.E., Jr.; Mintz, J.; McElroy, S.L.; Freeman, M.P.; Suppes, T.; Frye, M.A.; Altshuler, L.L.; Kupka, R.; Nolen, W.A.; Leverich, G.S.; et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentaenoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol. Psychiatry 2006, 60, 1020–1022. [Google Scholar] [CrossRef]
- Murphy, B.L.; Stoll, A.L.; Harris, P.Q.; Ravichandran, C.; Babb, S.M.; Carlezon, W.A., Jr.; Cohen, B.M. Omega-3 fatty acid treatment, with or without cytidine, fails to show therapeutic properties in bipolar disorder: A double-blind, randomized add-on clinical trial. J. Clin. Psychopharmacol. 2012, 32, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Jandacek, R.; Tso, P.; Blom, T.J.; Welge, J.A.; Strawn, J.R.; Adler, C.M.; DelBello, M.P.; Strakowski, S.M. First-episode bipolar disorder is associated with erythrocyte membrane docosahexaenoic acid deficits: Dissociation from clinical response to lithium or quetiapine. Psychiatry Res. 2015, 230, 447–453. [Google Scholar] [CrossRef]
- Ciappolino, V.; DelVecchio, G.; Prunas, C.; Andreella, A.; Finos, L.; Caletti, E.; Siri, F.; Mazzocchi, A.; Botturi, A.; Turolo, S.; et al. The effect of DHA supplementation on cognition in patients with bipolar disorder: An exploratory randomized control trial. Nutrients 2020, 12, 708. [Google Scholar] [CrossRef] [PubMed]
- McPhilemy, G.; Byrne, F.; Waldron, M.; Hibbeln, J.R.; Davis, J.; McDonald, C.; Hallahan, B. A 52-week prophylactic randomised control trial of omega-3 polyunsaturated fatty acids in bipolar disorder. Bipolar Disord. 2021, 23, 697–706. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, F.; Ma, H.; Chen, X.; Yang, J.; Yang, Y.; Yang, X.; Tian, X.; Yu, Q.; Zhou, X. Effects of different types and doses of whey protein on the physiological and intestinal flora in D-galactose-induced aging mice. PLoS ONE 2021, 16, e0248329. [Google Scholar] [CrossRef]
- Pradeep, A.S.; Naga Raju, G.J.; Sattar, S.A.; Sarita, P.; Prasada Rao, A.D.; Ray, D.K.; Reddy, B.S.; Reddy, S.B. Trace elemental distribution in the scalp hair of bipolars using PIXE technique. Med. Hypotheses 2014, 82, 470–477. [Google Scholar] [CrossRef]
- Hernández-Galiot, A.; Goñi, I. Adherence to the Mediterranean diet pattern, cognitive status and depressive symptoms in an elderly non-institutionalized population. Nutr. Hosp. 2017, 34, 338–344. [Google Scholar] [CrossRef]
- Mantzorou, M.; Vadikolias, K.; Pavlidou, E.; Tryfonos, C.; Vasios, G.; Serdari, A.; Giaginis, C. Mediterranean diet adherence is associated with better cognitive status and less depressive symptoms in a Greek elderly population. Aging Clin. Exp. Res. 2021, 33, 1033–1040. [Google Scholar] [CrossRef]
- Açik, M.; Altan, M.; Çakiroğlu, F.P. A cross-sectional analysis of two dietary quality indices and the mental health profile in female adults. Curr. Psychol. 2022, 41, 5514–5523. [Google Scholar] [CrossRef]
- Recchia, D.; Baghdadli, A.; Lassale, C.; Brunner, E.; Verdier, J.M.; Kivimäki, M.; Akbaraly, T. Associations between long-term adherence to healthy diet and recurrent depressive symptoms in Whitehall II Study. Eur. J. Nutr. 2020, 59, 1031–1041. [Google Scholar] [CrossRef]
- Vall Castelló, J.; Tubianosa, C. Linking Mediterranean diet and lifestyle with cardiometabolic disease and depressive symptoms: A study on the elderly in Europe. Int. J. Environ. Res. Public Health 2020, 17, 7053. [Google Scholar] [CrossRef]
- Gibson-Smith, D.; Bot, M.; Brouwer, I.A.; Visser, M.; Giltay, E.J.; Penninx, B.W.J.H. Association of food groups with depression and anxiety disorders. Eur. J. Nutr. 2020, 59, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R. Importance of functional foods in the Mediterranean diet. Public Health Nutr. 2006, 9, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos, C.; Panagiotakos, D.B.; Tzima, N.; Chrysohoou, C.; Economou, M.; Zampelas, A.; Stefanadis, C. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2005, 82, 694–699. [Google Scholar] [CrossRef]
- Rajizadeh, A.; Mozaffari-Khosravi, H.; Yassini-Ardakani, M.; Dehghani, A. Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: A randomized, double-blind, placebo-controlled trial. Nutrition 2017, 35, 56–60. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Klabnik, J.J.; O’Donnell, J.M. Novel therapeutic targets in depression and anxiety: Antioxidants as a candidate treatment. Curr. Neuropharmacol. 2014, 12, 108–119. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Holt, E.M.; Steffen, L.M.; Moran, A.; Basu, S.; Steinberger, J.; Ross, J.A.; Hong, C.P.; Sinaiko, A.R. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J. Am. Diet Assoc. 2009, 109, 414–421. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Steffen, L.M.; Mayer-Davis, E.J.; Jenny, N.S.; Jiang, R.; Herrington, D.M.; Jacobs, D.R., Jr. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2006, 83, 1369–1379. [Google Scholar] [CrossRef]
- Numakawa, T.; Richards, M.; Nakajima, S.; Adachi, N.; Furuta, M.; Odaka, H.; Kunugi, H. The role of brain-derived neurotrophic factor in comorbid depression: Possible linkage with steroid hormones, cytokines, and nutrition. Front. Psychiatry 2014, 5, 136. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Galbete, C.; Martinez-González, M.A.; Martinez, J.A.; Razquin, C.; Salas-Salvadó, J.; Estruch, R.; Buil-Cosiales, P.; Martí, A. The effect of the Mediterranean diet on plasma brain-derived neurotrophic factor (BDNF) levels: The PREDIMED-NAVARRA randomized trial. Nutr. Neurosci. 2011, 14, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Sachdev, P.; Butterworth, P. Western diet is associated with a smaller hippocampus: A longitudinal investigation. BMC Med. 2015, 13, 215. [Google Scholar] [CrossRef]
- Kishi, T.; Hirooka, Y.; Nagayama, T.; Isegawa, K.; Katsuki, M.; Takesue, K.; Sunagawa, K. Calorie restriction improves cognitive decline via up-regulation of brain-derived neurotrophic factor: Tropomyosin-related kinase B in hippocampus of obesity-induced hypertensive rats. Int. Heart J. 2015, 56, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, Z.; Kalyani, M.; Janik, J.M.; Shi, H. Effects of energy status and diet on Bdnf expression in the ventromedial hypothalamus of male and female rats. Physiol. Behav. 2014, 130, 99–107. [Google Scholar] [CrossRef]
- Beyer, J.L.; Payne, M.E. Nutrition and bipolar depression. Psychiatr. Clin. N. Am. 2016, 39, 75–86. [Google Scholar] [CrossRef]
- Łojko, D.; Stelmach-Mardas, M.; Suwalska, A. Is diet important in bipolar disorder? Psychiatr. Pol. 2018, 52, 783–795. [Google Scholar] [CrossRef]
- Napoleão, A.; Fernandes, L.; Miranda, C.; Marum, A.P. Effects of calorie restriction on health span and insulin resistance: Classic calorie restriction diet vs. ketosis-inducing diet. Nutrients 2021, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Tahreem, A.; Rakha, A.; Rabail, R.; Nazir, A.; Socol, C.T.; Maerescu, C.M.; Aadil, R.M. Fad diets: Facts and fiction. Front. Nutr. 2022, 9, 960922. [Google Scholar] [CrossRef]
- Woodyatt, R.T. Objects and method of diet adjustment in diabetes. Arch. Intern. Med. 1921, 28, 125. [Google Scholar] [CrossRef]
- Chen, S.; Su, X.; Feng, Y.; Li, R.; Liao, M.; Fan, L.; Liu, J.; Chen, S.; Zhang, S.; Cai, J.; et al. Ketogenic diet and multiple health outcomes: An umbrella review of meta-analysis. Nutrients 2023, 15, 4161. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Sun, J.X.; Yang, J.Q.; Li, Y.S.; Bi, K.; Zhang, Z.Y.; Wang, K.H.; Luo, H.Y.; Zhu, M.; Xu, Y. Ketogenic diet: A potential adjunctive treatment for substance use disorders. Front. Nutr. 2023, 10, 1191903. [Google Scholar] [CrossRef]
- Miranda, M.J.; Turner, Z.; Magrath, G. Alternative diets to the classical ketogenic diet—Can we be more liberal? Epilepsy Res. 2012, 100, 278–285. [Google Scholar] [CrossRef]
- Needham, N.; Campbell, I.H.; Grossi, H.; Kamenska, I.; Rigby, B.P.; Simpson, S.A.; McIntosh, E.; Bahuguna, P.; Meadowcroft, B.; Creasy, F.; et al. Pilot study of a ketogenic diet in bipolar disorder. BJPsych Open 2023, 9, e176. [Google Scholar] [CrossRef]
- Phelps, J.R.; Siemers, S.V.; El-Mallakh, R.S. The ketogenic diet for type II bipolar disorder. Neurocase 2013, 19, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, I. Ketogenic diet in therapy of bipolar affective disorder—Case report and literature review. Psychiatr. Pol. 2022, 56, 1345–1363. [Google Scholar] [CrossRef]
- Yaroslavsky, Y.; Stahl, Z.; Belmaker, R.H. Ketogenic diet in bipolar illness. Bipolar Disord. 2002, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.H.; Campbell, H. Ketosis and bipolar disorder: Controlled analytic study of online reports. BJPsych Open 2019, 5, e58. [Google Scholar] [CrossRef]
- Sethi, S.; Wakeham, D.; Ketter, T.; Hooshmand, F.; Bjornstad, J.; Richards, B.; Westman, E.; Krauss, R.M.; Saslow, L. Ketogenic diet intervention on metabolic and psychiatric health in bipolar and schizophrenia: A pilot trial. Psychiatry Res. 2024, 335, 115866. [Google Scholar] [CrossRef]
- Hemmings, B.A.; Restuccia, D.F. The PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2015, 7, a026609. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Beezhold, B.L.; Johnston, C.S. Restriction of meat, fish, and poultry in omnivores improves mood: A pilot randomized controlled trial. Nutr. J. 2012, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Sánchez-Villegas, A. Food patterns and the prevention of depression. Proc. Nutr. Soc. 2016, 75, 139–146. [Google Scholar] [CrossRef]
- Dreher, M.L. Dietary Patterns and Whole Plant Foods in Aging and Disease; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Fazelian, S.; Sadeghi, E.; Firouzi, S.; Haghighatdoost, F. Adherence to the vegetarian diet may increase the risk of depression: A systematic review and meta-analysis of observational studies. Nutr. Rev. 2022, 80, 242–254. [Google Scholar] [CrossRef]
- Michalak, J.; Zhang, X.C.; Jacobi, F. Vegetarian diet and mental disorders: Results from a representative community survey. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 67. [Google Scholar] [CrossRef]
- Jin, Y.; Kandula, N.R.; Kanaya, A.M.; Talegawkar, S.A. Vegetarian diet is inversely associated with prevalence of depression in middle-older aged South Asians in the United States. Ethn. Health 2021, 26, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.F.; Eather, R.; Best, T. Plant-based dietary quality and depressive symptoms in Australian vegans and vegetarians: A cross-sectional study. BMJ Nutr. Prev. Health 2021, 4, 479–486. [Google Scholar] [CrossRef]
- Askari, M.; Daneshzad, E.; Darooghegi Mofrad, M.; Bellissimo, N.; Suitor, K.; Azadbakht, L. Vegetarian diet and the risk of depression, anxiety, and stress symptoms: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 261–271. [Google Scholar] [CrossRef]
- Iguacel, I.; Huybrechts, I.; Moreno, L.A.; Michels, N. Vegetarianism and veganism compared with mental health and cognitive outcomes: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 361–381. [Google Scholar] [CrossRef]
- Matta, J.; Czernichow, S.; Kesse-Guyot, E.; Hoertel, N.; Limosin, F.; Goldberg, M.; Zins, M.; Lemogne, C. Depressive symptoms and vegetarian diets: Results from the Constances cohort. Nutrients 2018, 10, 1695. [Google Scholar] [CrossRef]
- Walsh, H.; Lee, M.; Best, T. The association between vegan, vegetarian, and omnivore diet quality and depressive symptoms in adults: A cross-sectional study. Int. J. Environ. Res. Public Health 2023, 20, 3258. [Google Scholar] [CrossRef]
- Bozzatello, P.; Brignolo, E.; De Grandi, E.; Bellino, S. Supplementation with omega-3 fatty acids in psychiatric disorders: A review of literature data. J. Clin. Med. 2016, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J.; Kamali, M.; Prossin, A.R.; Harrington, G.J.; Ellingrod, V.L.; McInnis, M.G.; Burant, C.F. Association of plasma ω-3 and ω-6 lipids with burden of disease measures in bipolar subjects. J. Psychiatr. Res. 2012, 46, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Scola, G.; Versace, A.; Metherel, A.H.; Monsalve-Castro, L.A.; Phillips, M.L.; Bazinet, R.P.; Andreazza, A.C. Alterations in peripheral fatty acid composition in bipolar and unipolar depression. J. Affect. Disord. 2018, 233, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J.; Assari, S.; Harrington, G.J.; Chang, Y.W.; Burant, C.F.; McInnis, M.G. Plasma linoleic acid partially mediates the association of bipolar disorder on self-reported mental health scales. J. Psychiatr. Res. 2015, 68, 61–67. [Google Scholar] [CrossRef]
- Lee-Okada, H.C.; Xue, C.; Yokomizo, T. Recent advances on the physiological and pathophysiological roles of polyunsaturated fatty acids and their biosynthetic pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2025, 1870, 159564. [Google Scholar] [CrossRef]
- Malhi, G.S.; Bassett, D.; Boyce, P.; Bryant, R.; Fitzgerald, P.B.; Fritz, K.; Hopwood, M.; Lyndon, B.; Mulder, R.; Murray, G.; et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust. N. Z. J. Psychiatry 2015, 49, 1087–1106. [Google Scholar] [CrossRef]
- Mischoulon, D. Popular herbal and natural remedies used in psychiatry. Focus 2018, 16, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Saunders, E.F.; Ramsden, C.E.; Sherazy, M.S.; Gelenberg, A.J.; Davis, J.M.; Rapoport, S.I. Omega-3 and omega-6 polyunsaturated fatty acids in bipolar disorder: A review of biomarker and treatment studies. J. Clin. Psychiatry 2016, 77, e1301–e1308. [Google Scholar] [CrossRef] [PubMed]
- Rog, J.; Łobejko, Ł.; Hordejuk, M.; Marciniak, W.; Derkacz, R.; Kiljańczyk, A.; Matuszczak, M.; Lubiński, J.; Nesterowicz, M.; Żendzian-Piotrowska, M.; et al. Pro/antioxidant status and selenium, zinc and arsenic concentration in patients with bipolar disorder treated with lithium and valproic acid. Front. Mol. Neurosci. 2024, 17, 1441575. [Google Scholar] [CrossRef]
- Psara, E.; Papadopoulou, S.K.; Mentzelou, M.; Voulgaridou, G.; Vorvolakos, T.; Apostolou, T.; Giaginis, C. Omega-3 Fatty Acids for the Treatment of Bipolar Disorder Symptoms: A Narrative Review of the Current Clinical Evidence. Mar. Drugs 2025, 23, 84. [Google Scholar] [CrossRef]
- Antao, H.S.; Sacadura-Leite, E.; Bandarra, N.M.; Figueira, M.L. Omega-3 Index as Risk Factor in Psychiatric Diseases: A Narrative Review. Front. Psychiatry 2023, 14, 1200403. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D Receptor and 1 Alpha-Hydroxylase in Human Brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Prentice, A. Vitamin D Deficiency: A Global Perspective. Nutr. Rev. 2008, 66, S153–S164. [Google Scholar] [CrossRef]
- Brown, J.; Bianco, J.I.; McGrath, J.J.; Eyles, D.W. 1,25-Dihydroxyvitamin D3 Induces Nerve Growth Factor, Promotes Neurite Outgrowth and Inhibits Mitosis in Embryonic Rat Hippocampal Neurons. Neurosci. Lett. 2003, 343, 139–143. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A Helpful Immuno-Modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef]
- Groves, N.J.; McGrath, J.J.; Burne, T.H. Vitamin D as a Neurosteroid Affecting the Developing and Adult Brain. Annu. Rev. Nutr. 2014, 34, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Tuohimaa, P. Neurosteroid Hormone Vitamin D and Its Utility in Clinical Nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 12–19. [Google Scholar] [CrossRef]
- Obradovic, D.; Gronemeyer, H.; Lutz, B.; Rein, T. Cross-Talk of Vitamin D and Glucocorticoids in Hippocampal Cells. J. Neurochem. 2006, 96, 500–509. [Google Scholar] [CrossRef]
- Sabir, M.S.; Haussler, M.R.; Mallick, S.; Kaneko, I.; Lucas, D.A.; Haussler, C.A.; Whitfield, G.K.; Jurutka, P.W. Optimal Vitamin D Spurs Serotonin: 1,25-Dihydroxyvitamin D Represses Serotonin Reuptake Transport (SERT) and Degradation (MAO-A) Gene Expression in Cultured Rat Serotonergic Neuronal Cell Lines. Genes Nutr. 2018, 13, 19. [Google Scholar] [CrossRef]
- Sedaghat, K.; Yousefian, Z.; Vafaei, A.A.; Rashidy-Pour, A.; Parsaei, H.; Khaleghian, A.; Choobdar, S. Mesolimbic Dopamine System and Its Modulation by Vitamin D in a Chronic Mild Stress Model of Depression in the Rat. Behav. Brain Res. 2019, 356, 156–169. [Google Scholar] [CrossRef]
- Anglin, R.E.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D Deficiency and Depression in Adults: Systematic Review and Meta-Analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, N.; Sun, W.; Chen, D.; Zhao, J.; Zhang, W. Association Between Vitamin D Deficiency and Antepartum and Postpartum Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies. Arch. Gynecol. Obstet. 2018, 298, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Mikola, T.; Marx, W.; Lane, M.M.; Hockey, M.; Loughman, A.; Rajapolvi, S.; Rocks, T.; O’Neil, A.; Mischoulon, D.; Valkonen-Korhonen, M.; et al. The Effect of Vitamin D Supplementation on Depressive Symptoms in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 11784–11801. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Huang, Y.C.; Huang, W.L. The Effect of Vitamin D Supplement on Negative Emotions: A Systematic Review and Meta-Analysis. Depress. Anxiety 2020, 37, 549–564. [Google Scholar] [CrossRef]
- Vellekkatt, F.; Menon, V. Efficacy of Vitamin D Supplementation in Major Depression: A Meta-Analysis of Randomized Controlled Trials. J. Postgrad. Med. 2019, 65, 74–80. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of Optimal Serum Concentrations of 25-Hydroxyvitamin D for Multiple Health Outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28. [Google Scholar] [CrossRef]
- Cereda, G.; Enrico, P.; Ciappolino, V.; Delvecchio, G.; Brambilla, P. The Role of Vitamin D in Bipolar Disorder: Epidemiology and Influence on Disease Activity. J. Affect. Disord. 2021, 278, 209–217. [Google Scholar] [CrossRef]
- Altunsoy, N.; Yüksel, R.N.; Cingi Yirun, M.; Kılıçarslan, A.; Aydemir, Ç. Exploring the Relationship Between Vitamin D and Mania: Correlations Between Serum Vitamin D Levels and Disease Activity. Nord. J. Psychiatry 2018, 72, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Belzeaux, R.; Boyer, L.; Ibrahim, E.C.; Féron, F.; Leboyer, M.; Fond, G. Mood Disorders Are Associated with a More Severe Hypovitaminosis D than Schizophrenia. Psychiatry Res. 2015, 229, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Grønli, O.; Kvamme, J.M.; Jorde, R.; Wynn, R. Vitamin D deficiency is common in psycho-geriatric patients, independent of diagnosis. BMC Psychiatry 2014, 14, 134. [Google Scholar] [CrossRef]
- Menkes, D.B.; Lancaster, K.; Grant, M.; Marsh, R.W.; Dean, P.; du Toit, S.A. Vitamin D status of psychiatric inpatients in New Zealand’s Waikato region. BMC Psychiatry 2012, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Marsh, W.K.; Penny, J.L.; Rothschild, A.J. Vitamin D supplementation in bipolar depression: A double blind placebo controlled trial. J. Psychiatr. Res. 2017, 95, 48–53. [Google Scholar] [CrossRef]
- Sikoglu, E.M.; Navarro, A.A.; Starr, D.; Dvir, Y.; Nwosu, B.U.; Czerniak, S.M.; Rogan, R.C.; Castro, M.C.; Edden, R.A.; Frazier, J.A.; et al. Vitamin D3 Supplemental Treatment for Mania in Youth with Bipolar Spectrum Disorders. J. Child Adolesc. Psychopharmacol. 2015, 25, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Brietzke, E.; Stertz, L.; Fernandes, B.S.; Kauer-Sant’anna, M.; Mascarenhas, M.; Escosteguy Vargas, A.; Chies, J.A.; Kapczinski, F. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J. Affect. Disord. 2009, 116, 214–217. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Gama, C.S.; Ceresér, K.M.; Yatham, L.N.; Fries, G.R.; Colpo, G.; de Lucena, D.; Kunz, M.; Gomes, F.A.; Kapczinski, F. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: A systematic review and meta-regression analysis. J. Psychiatr. Res. 2011, 45, 995–1004. [Google Scholar] [CrossRef]
- Ortiz-Domínguez, A.; Hernández, M.E.; Berlanga, C.; Gutiérrez-Mora, D.; Moreno, J.; Heinze, G.; Pavón, L. Immune variations in bipolar disorder: Phasic differences. Bipolar Disord. 2007, 9, 596–602. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Bergink, V.; Gibney, S.M.; Drexhage, H.A. Autoimmunity, inflammation, and psychosis: A search for peripheral markers. Biol. Psychiatry 2014, 75, 324–331. [Google Scholar] [CrossRef]
- Faugere, M.; Maakaron, É.; Achour, V.; Verney, P.; Andrieu-Haller, C.; Obadia, J.; Fond, G.; Lançon, C.; Korchia, T. Vitamin D, B9, and B12 Deficiencies as Key Drivers of Clinical Severity and Metabolic Comorbidities in Major Psychiatric Disorders. Nutrients 2025, 17, 1167. [Google Scholar] [CrossRef]
- Lam, N.S.K.; Long, X.X.; Li, X.; Saad, M.; Lim, F.; Doery, J.C.; Griffin, R.C.; Galletly, C. The potential use of folate and its derivatives in treating psychiatric disorders: A systematic review. Biomed. Pharmacother. 2022, 146, 112541. [Google Scholar] [CrossRef]
- Ryan, M.F. The role of magnesium in clinical biochemistry: An overview. Ann. Clin. Biochem. 1991, 28, 19–26. [Google Scholar] [CrossRef]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef] [PubMed]
- Pochwat, B.; Szewczyk, B.; Sowa-Kucma, M.; Siwek, A.; Doboszewska, U.; Piekoszewski, W.; Gruca, P.; Papp, M.; Nowak, G. Antidepressant-like activity of magnesium in the chronic mild stress model in rats: Alterations in the NMDA receptor subunits. Int. J. Neuropsychopharmacol. 2014, 17, 393–405. [Google Scholar] [CrossRef]
- Poleszak, E. Modulation of antidepressant-like activity of magnesium by serotonergic system. J. Neural. Transm. 2007, 114, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Murck, H. Ketamine, magnesium and major depression—From pharmacology to pathophysiology and back. J. Psychiatr. Res. 2013, 47, 955–965. [Google Scholar] [CrossRef]
- Serefko, A.; Szopa, A.; Wlaź, P.; Nowak, G.; Radziwoń-Zaleska, M.; Skalski, M.; Poleszak, E. Magnesium in depression. Pharmacol. Rep. 2013, 65, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Botturi, A.; Ciappolino, V.; Delvecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients 2020, 12, 1661. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Nozaki, C.; Vergnano, A.M.; Filliol, D.; Ouagazzal, A.M.; Le Goff, A.; Carvalho, S.; Reiss, D.; Gaveriaux-Ruff, C.; Neyton, J.; Paoletti, P.; et al. Zinc alleviates pain through high-affinity binding to the NMDA receptor NR2A subunit. Nat. Neurosci. 2011, 14, 1017–1022. [Google Scholar] [CrossRef]
- Paoletti, P.; Ascher, P.; Neyton, J. High-affinity zinc inhibition of NMDA NR1–NR2A receptors. J. Neurosci. 1997, 17, 5711–5725. [Google Scholar] [CrossRef] [PubMed]
- Swardfager, W.; Herrmann, N.; Mazereeuw, G.; Goldberger, K.; Harimoto, T.; Lanctôt, K.L. Zinc in depression: A meta-analysis. Biol. Psychiatry 2013, 74, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Millett, C.E.; Mukherjee, D.; Reider, A.; Can, A.; Groer, M.; Fuchs, D.; Postolache, T.T.; Kelleher, S.L.; Saunders, E.F.H. Peripheral zinc and neopterin concentrations are associated with mood severity in bipolar disorder in a gender-specific manner. Psychiatry Res. 2017, 255, 52–58. [Google Scholar] [CrossRef]
- Santa Cruz, E.C.; Madrid, K.C.; Arruda, M.A.Z.; Sussulini, A. Association between trace elements in serum from bipolar disorder and schizophrenia patients considering treatment effects. J. Trace Elem. Med. Biol. 2020, 59, 126467. [Google Scholar] [CrossRef] [PubMed]
- Siwek, M.; Sowa-Kućma, M.; Styczeń, K.; Szewczyk, B.; Reczyński, W.; Misztak, P.; Topór-Mądry, R.; Nowak, G.; Dudek, D.; Rybakowski, J.K. Decreased serum zinc concentration during depressive episode in patients with bipolar disorder. J. Affect. Disord. 2016, 190, 272–277. [Google Scholar] [CrossRef]
- Jonsson, B.H.; Orhan, F.; Bruno, S.; Oliveira, A.O.; Sparding, T.; Landén, M.; Sellgren, C.M. Serum concentration of zinc is elevated in clinically stable bipolar disorder patients. Brain Behav. 2022, 12, e2472. [Google Scholar] [CrossRef]
- Coodin, S. Body mass index in persons with schizophrenia. Can. J. Psychiatry 2001, 46, 549–555. [Google Scholar] [CrossRef]
- Osby, U.; Brandt, L.; Correia, N.; Ekbom, A.; Sparén, P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch. Gen. Psychiatry 2001, 58, 844–850. [Google Scholar] [CrossRef]
- Jones, D.R.; Macias, C.; Barreira, P.J.; Fisher, W.H.; Hargreaves, W.A.; Harding, C.M. Prevalence, severity, and co-occurrence of chronic physical health problems of persons with serious mental illness. Psychiatr. Serv. 2004, 55, 1250–1257. [Google Scholar] [CrossRef]
- Bradley, T.; Campbell, E.; Dray, J.; Bartlem, K.; Wye, P.; Hanly, G.; Gibson, L.; Fehily, C.; Bailey, J.; Wynne, O.; et al. Systematic review of lifestyle interventions to improve weight, physical activity and diet among people with a mental health condition. Syst. Rev. 2022, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Bruins, J.; Jörg, F.; Bruggeman, R.; Slooff, C.; Corpeleijn, E.; Pijnenborg, M. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: A meta-analysis. PLoS ONE 2014, 9, e112276. [Google Scholar] [CrossRef]
- Højlund, M.; Andersen, K.; Ernst, M.T.; Correll, C.U.; Hallas, J. Use of low-dose quetiapine increases the risk of major adverse cardiovascular events: Results from a nationwide active comparator-controlled cohort study. World Psychiatry 2022, 21, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.J.; McNeil, J.; Tremblay, M.S.; Saunders, T.J. Sit less, stand more: A randomized point-of-decision prompt intervention to reduce sedentary time. Prev. Med. 2015, 73, 67–69. [Google Scholar] [CrossRef]
- De Hert, M.; Dekker, J.M.; Wood, D.; Kahl, K.G.; Holt, R.I.; Möller, H.J. Cardiovascular disease and diabetes in people with severe mental illness: Position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur. Psychiatry 2009, 24, 412–424. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. Available online: http://www.ncbi.nlm.nih.gov/books/NBK305057/ (accessed on 26 December 2025).
- Meyer, J.M.; Nasrallah, H.A.; McEvoy, J.P.; Goff, D.C.; Davis, S.M.; Chakos, M.; Patel, J.K.; Keefe, R.S.; Stroup, T.S.; Lieberman, J.A. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Trial: Clinical comparison of subgroups with and without the metabolic syndrome. Schizophr. Res. 2005, 80, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ziegelstein, R.C.; Fauerbach, J.A.; Stevens, S.S.; Romanelli, J.; Richter, D.P.; Bush, D.E. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch. Intern. Med. 2000, 160, 1818–1823. [Google Scholar] [CrossRef]
- Fiorillo, A.; Del Vecchio, V.; Luciano, M.; Sampogna, G.; De Rosa, C.; Malangone, C.; Volpe, U.; Bardicchia, F.; Ciampini, G.; Crocamo, C.; et al. Efficacy of psychoeducational family intervention for bipolar I disorder: A controlled, multicentric, real-world study. J. Affect. Disord. 2015, 172, 291–299. [Google Scholar] [CrossRef]
- Speyer, H.; Nørgaard, H.C.; Hjorthøj, C.; Madsen, T.A.; Drivsholm, S.; Pisinger, C.; Gluud, C.; Mors, O.; Krogh, J.; Nordentoft, M. Protocol for CHANGE: A randomized clinical trial assessing lifestyle coaching plus care coordination versus care coordination alone versus treatment as usual to reduce risks of cardiovascular disease in adults with schizophrenia and abdominal obesity. BMC Psychiatry 2015, 15, 119. [Google Scholar] [CrossRef]
- Ritunnano, R.; Stanghellini, G.; Broome, M.R. Self-interpretation and meaning-making processes: Re-humanizing research on early psychosis. World Psychiatry 2021, 20, 304–306. [Google Scholar] [CrossRef]
- Torous, J.; Bucci, S.; Bell, I.H.; Kessing, L.V.; Faurholt-Jepsen, M.; Whelan, P.; Carvalho, A.F.; Keshavan, M.; Linardon, J.; Firth, J. The growing field of digital psychiatry: Current evidence and the future of apps, social media, chatbots, and virtual reality. World Psychiatry 2021, 20, 318–335. [Google Scholar] [CrossRef]
- Luciano, M.; Sampogna, G.; D’Ambrosio, E.; Rampino, A.; Amore, M.; Calcagno, P.; Rossi, A.; Rossi, R.; Carmassi, C.; Dell’Osso, L.; et al. One-year efficacy of a lifestyle behavioural intervention on physical and mental health in people with severe mental disorders: Results from a randomized controlled trial. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 903–915. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H. Psychiatric symptoms and cognitive impairment in “Long COVID”: The relevance of immunopsychiatry. World Psychiatry 2021, 20, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Schuch, H.S.; Peres, K.G.; Haag, D.G.; Boing, A.F.; Peres, M.A. The independent and joint contribution of objective and subjective socioeconomic status on oral health indicators. Community Dent. Oral Epidemiol. 2022, 50, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Luciano, M.; Sampogna, G.; Amore, M.; Andriola, I.; Calcagno, P.; Carmassi, C.; Del Vecchio, V.; Dell’Osso, L.; Di Lorenzo, G.; Gelao, B.; et al. How to improve the physical health of people with severe mental illness? A multicentric randomized controlled trial on the efficacy of a lifestyle group intervention. Eur. Psychiatry 2021, 64, e72. [Google Scholar] [CrossRef] [PubMed]
- Jahrami, H.; Saif, Z.; Ammar, A.; Husain, W.; Trabelsi, K.; Ghazzawi, H.; Pandi-Perumal, S.R.; Seeman, M.V. Development and Validation of a Food Frequency Questionnaire for Evaluating the Nutritional Status of Patients with Serious Mental Illnesses (DIETQ-SMI) in Bahrain. Brain Sci. 2024, 14, 312. [Google Scholar] [CrossRef]

| Nutrient/Component | Proposed Mechanism | Evidence Type | Relevance to BD | References |
|---|---|---|---|---|
| Omega-3 fatty acids (EPA, DHA) | Anti-inflammatory, membrane stabilization | Multiple RCTs (depression, some in BD) | Adjunctive benefit, esp. for depression | Sarris et al. 2012 [1] |
| Folate/B vitamins | Homocysteine regulation, neurotransmitter synthesis | Observational, small trials | Mixed findings, possible stabilization | Lam et al. 2022 [155] |
| Vitamin D | Neuroimmunomodulation, neurotrophins | Observational | Deficiency common; unclear supplementation benefit | Lin et al. 2020 [37] |
| Magnesium | NMDA receptor modulation, neuroprotection | Case reports, small trials | Possible mood stabilization | Botturi et al. 2020 [162] |
| Zinc, selenium | Antioxidant, enzymatic regulation | Limited data | Deficiency linked to mood symptoms | Siwek et al. 2016 [170] |
| Recommendation | Evidence Support | Clinical Applicability | Research Gaps |
|---|---|---|---|
| Encourage Mediterranean-style diet | Strong indirect evidence | Safe, broadly applicable | BD-specific RCTs needed |
| Consider ketogenic diet in refractory cases | Weak (case reports, pilots) | Limited by adherence | Larger controlled studies required |
| Ensure adequate omega-3 intake | Moderate evidence | Safe, feasible | More BD-focused RCTs needed |
| Monitor deficiencies in restrictive diets | Clinical consensus | Important in long-term care | Prospective BD-specific trials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Marano, G.; Marzo, E.M.; Sfratta, G.; Traversi, G.; Capristo, E.; Gaetani, E.; Mazza, M. Dietetic Prescriptions in Bipolar Disorder: Nutritional Strategies to Support Mood Stability and Reduce Relapse Risk—A Narrative Review. Life 2026, 16, 146. https://doi.org/10.3390/life16010146
Marano G, Marzo EM, Sfratta G, Traversi G, Capristo E, Gaetani E, Mazza M. Dietetic Prescriptions in Bipolar Disorder: Nutritional Strategies to Support Mood Stability and Reduce Relapse Risk—A Narrative Review. Life. 2026; 16(1):146. https://doi.org/10.3390/life16010146
Chicago/Turabian StyleMarano, Giuseppe, Ester Maria Marzo, Greta Sfratta, Gianandrea Traversi, Esmeralda Capristo, Eleonora Gaetani, and Marianna Mazza. 2026. "Dietetic Prescriptions in Bipolar Disorder: Nutritional Strategies to Support Mood Stability and Reduce Relapse Risk—A Narrative Review" Life 16, no. 1: 146. https://doi.org/10.3390/life16010146
APA StyleMarano, G., Marzo, E. M., Sfratta, G., Traversi, G., Capristo, E., Gaetani, E., & Mazza, M. (2026). Dietetic Prescriptions in Bipolar Disorder: Nutritional Strategies to Support Mood Stability and Reduce Relapse Risk—A Narrative Review. Life, 16(1), 146. https://doi.org/10.3390/life16010146









