Rootstock Genotype Dictates Phosphorus Deficiency Tolerance and Transcriptional Plasticity in Grafted Camellia oleifera Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Determination of the Morphology
2.3. Determination of Biomass and P Concentration
2.4. RNA Extraction, Illumina Sequencing, and Data Analysis
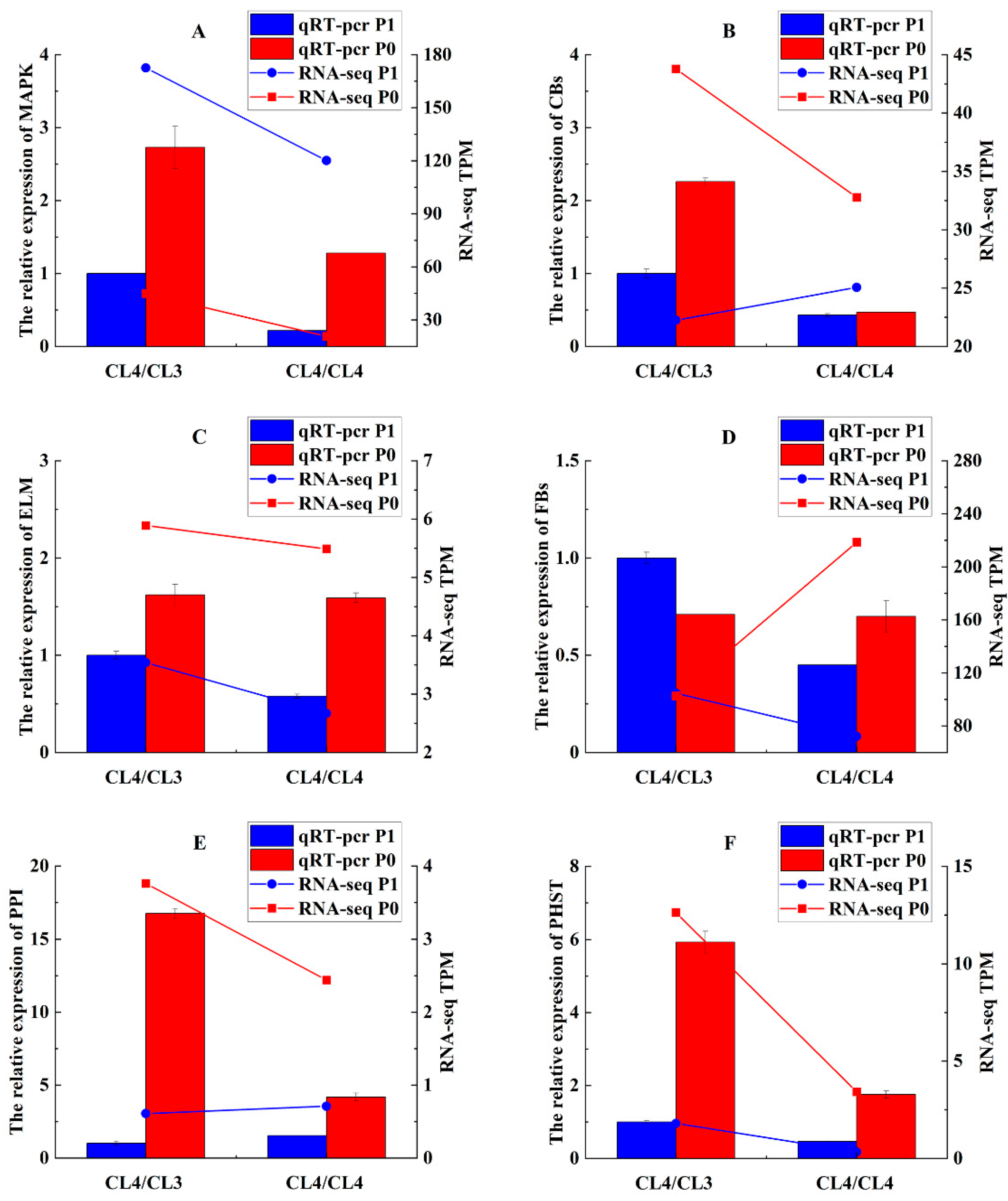
2.5. Quantitative Real-Time PCR Analysis
2.6. Statistical Analysis
3. Results
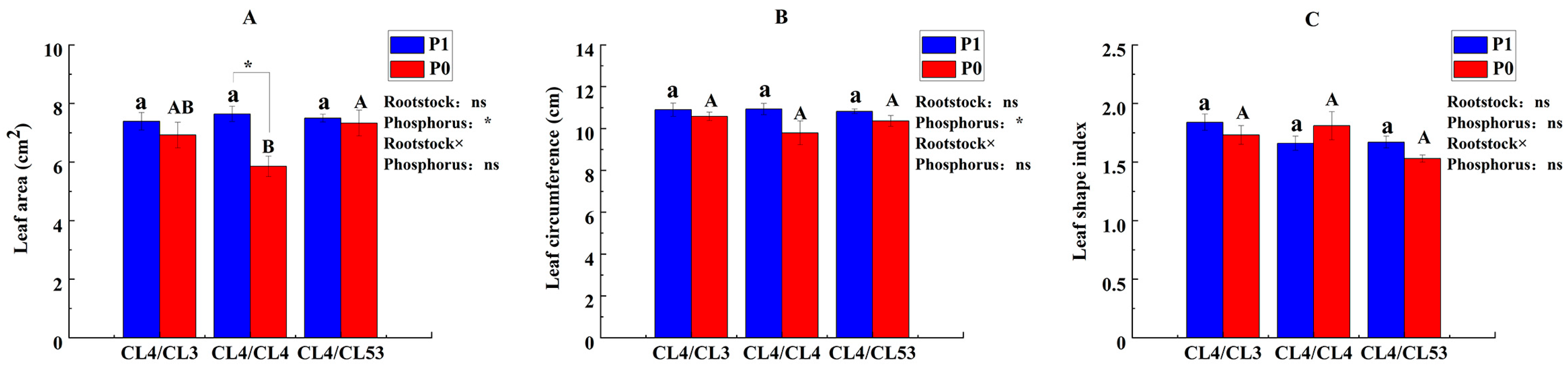
3.1. Effects of Different Rootstocks on the Morphology of C. oleifera Grafted Plants
3.2. Effect of the Rootstock on the Biomass of Grafted C. oleifera
3.3. Effects of the Rootstock on P Content, P Accumulation, and PUE in the Grafted C. oleifera
3.4. Transcriptomic Analysis for Different Grafted Plants Under Two Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, P.; Meng, Y.; Li, B.; Ma, X.; Si, E.; Lai, Y.; Wang, J.; Yao, L.; Yang, K.; Shang, X.; et al. Molecular mechanisms of acclimatization to phosphorus starvation and recovery underlying full-length transcriptome profiling in barley (Hordeum vulgare L.). Front. Plant Sci. 2018, 9, 500. [Google Scholar] [CrossRef]
- Navarro, M.; Munné-Bosch, S. Reduced phosphate availability improves tomato quality through hormonal modulation in developing fruits. J. Plant Growth Regul. 2022, 41, 153–162. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Lu, H.; Liu, Y.; Mao, C. Phosphate uptake and transport in plants: An elaborate regulatory system. Plant Cell Physiol. 2021, 62, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kuo, H.-F.; Chiou, T.J. Intracellular phosphate sensing and regulation of phosphate transport systems in plants. Plant Physiol. 2021, 187, 2043–2055. [Google Scholar] [CrossRef]
- Kuchenbuch, R.O.; Buczko, U. Re-visiting potassium- and phosphate-fertilizer responses in field experiments and soil-test interpretations by means of data mining. J. Plant Nutr. Soil Sci. 2011, 174, 171–185. [Google Scholar] [CrossRef]
- Andersson, H.; Bergström, L.; Djodjic, F.; Ulén, B.; Kirchmann, H. Topsoil and subsoil properties influence phosphorus leaching from four agricultural soils. J. Environ. Qual. 2013, 42, 455–463. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Lott, J.N.A.; Kolasa, J.; Batten, G.D.; Campbell, L.C. The critical role of phosphorus in world production of cereal grains and legume seeds. Food. Secur. 2011, 3, 451–462. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Lambers, H.; Finnegan, P.M.; Laliberté, E.; Pearse, S.J.; Ryan, M.H.; Shane, M.W.; Veneklaas, E.J. Phosphorus nutrition of proteaceae in severely phosphorus-impoverished soils: Are there lessons to be learned for future crops? Plant Physiol. 2011, 156, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Nielsen, N.E. Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant Soil 2004, 262, 55–62. [Google Scholar] [CrossRef]
- Haling, R.E.; Brown, L.K.; Bengough, A.G.; Young, I.M.; Hallett, P.D.; White, P.J.; George, T.S. Root hairs improve root penetration, root–soil contact, and phosphorus acquisition in soils of different strength. J. Exp. Bot. 2013, 64, 3711–3721. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Li, D.; Wang, N.; Sun, H.; Zhang, Y.; Zhang, K.; Li, A.; Bai, Z.; Li, C.; et al. In situ root phenotypes of cotton seedlings under phosphorus stress revealed through RhizoPot. Front. Plant Sci. 2021, 12, 716691. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, H.; Lucas, W.J. Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. J. Integr. Plant Biol. 2014, 56, 192–220. [Google Scholar] [CrossRef]
- Su, W.; Zhou, Z.; Zeng, J.; Cao, R.; Zhang, Y.; Hu, D.; Liu, J. Genome-wide identification of the WRKY gene family in Camellia oleifera and expression analysis under phosphorus deficiency. Front. Plant Sci. 2023, 14, 1082496. [Google Scholar] [CrossRef]
- Jaganath, B.; Subramanyam, K.; Mayavan, S.; Karthik, S.; Elayaraja, D.; Udayakumar, R.; Manickavasagam, M.; Ganapathi, A. An efficient in planta transformation of Jatropha curcas (L.) and multiplication of transformed plants through in vivo grafting. Protoplasma 2014, 251, 591–601. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; Von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.B.; Ulas, A. Vigorous rootstocks improve nitrogen efficiency of tomato by inducing morphological, physiological and biochemical responses. Gesunde Pflanz. 2023, 75, 565–575. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Primo-Millo, E.; Forner, B.J.; Forner-Giner, M.A. Citrus rootstock responses to water stress. Sci. Hortic. 2010, 126, 95–102. [Google Scholar] [CrossRef]
- Feng, J.L.; Yang, Z.J.; Chen, S.P.; El-Kassaby, Y.A.; Chen, H. Signaling pathway in development of Camellia oleifera nurse seedling grafting union. Trees 2017, 31, 1543–1558. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, J.; Lian, L.; Xu, A.; Guo, X.; Zhang, L.; Zhang, W.; Hu, D. Effects of scion variety on the phosphorus efficiency of grafted Camellia oleifera seedlings. Forests 2022, 13, 203. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, L.; Liu, J.; Duan, Y.K.; Wang, S.Y.; Wang, Z.L.; Gai, T.T.; Ren, Z.H.; Guo, X.M.; Hu, D.N. Screen of Camellia oleifera rootstock genotypes tolerant to lo phosphorus and identification of index tolerant to phosphorus deficiency. For. Res. 2019, 34, 166–173. [Google Scholar] [CrossRef]
- Chen, C.; Chu, Y.; Huang, Q.; Ding, C.; Zhang, W.; Li, B.; Zhang, J.; Su, X. Morphological and physiological plasticity response to low nitrogen stress in black cottonwood (Populus deltoides Marsh.). J. For. Res. 2022, 33, 51–62. [Google Scholar] [CrossRef]
- Chai, X.; Wang, X.; Li, H.; Xu, X.; Wu, T.; Zhang, X.; Wang, Y.; Han, Z. Apple scion cultivars regulate the rhizosphere microbiota of scion/rootstock combinations. Appl. Soil Ecol. 2022, 170, 104305. [Google Scholar] [CrossRef]
- Hou, S.; Zhu, Y.; Wu, X.; Xin, Y.; Guo, J.; Wu, F.; Yu, H.; Sun, Z.; Xu, C. Scion-to-rootstock mobile transcription factor CmHY5 positively modulates the nitrate uptake capacity of melon scion grafted on squash rootstock. Int. J. Mol. Sci. 2023, 24, 162. [Google Scholar] [CrossRef]
- Xie, B.; An, X.H.; Chen, Y.H.; Cheng, C.G.; Kang, G.D.; Zhou, J.T.; Zhao, D.Y.; Li, Z.; Zhang, Y.Z.; Yang, A. Response and adaptability evaluation of different apple rootstocks to continuous phosphorus deficiency. Sci. Agric. Sin. 2022, 55, 2598–2612. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Guo, J.; Zhao, J.; Wang, S.; Zheng, Z.; Jiang, Q.; Ren, F. Reponse of root endophytes to phosphorus avaibility in peach rootstocks with constrasting phosphorus-use efficiencies. Front. Plant Sci. 2021, 12, 2021. [Google Scholar] [CrossRef]
- Ibacache, G.A.; Sierra, B.C. Influence of rootstocks on nitrogen, phosphorus and potassium content in petioles of four table grape varieties. Chil. J. Agric. Res. 2009, 69, 503–508. [Google Scholar] [CrossRef]
- Chu, S.; Li, H.; Zhang, X.; Yu, K.; Chao, M.; Han, S.; Zhang, D. Physiological and proteomics analyses reveal low-phosphorus stress affected the regulation of photosynthesis in soybean. Int. J. Mol. Sci. 2018, 19, 1688. [Google Scholar] [CrossRef]
- Kayoumu, M.; Iqbal, A.; Muhammad, N.; Li, X.; Li, L.; Wang, X.; Gui, H.; Qi, Q.; Ruan, S.; Guo, R.; et al. Phosphorus availability affects the photosynthesis and antioxidant system of contrasting low-P-tolerant cotton genotypes. Antioxidants 2023, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, J.; Chen, Y.; Zhang, L.; Zhang, Y.; Wang, S.; Shi, X.; Li, L.; Liang, J. Effects of phosphate solubilizing bacteria on the growth, photosynthesis, and nutrient uptake of Camellia oleifera Abel. Forests 2019, 10, 348. [Google Scholar] [CrossRef]
- Neocleous, D.; Savvas, D. The effects of phosphorus supply limitation on photosynthesis, biomass production, nutritional quality, and mineral nutrition in lettuce grown in a recirculating nutrient solution. Sci. Hortic. 2019, 252, 379–387. [Google Scholar] [CrossRef]
- Hayat, F.; Asghar, S.; Yanmin, Z.; Xue, T.; Nawaz, M.A.; Xu, X.F.; Wang, Y.; Wu, T.; Zhang, X.Z.; Qiu, C.P.; et al. Rootstock induced vigour is associated with physiological, biochemical and molecular changes in “Red Fuji” apple. Int. J. Agric. Biol. 2020, 24, 1823–1834. [Google Scholar]
- Zhou, Y.; Underhill, S.J.R. Expression of gibberellin metabolism genes and signalling component in dwarf phenotype pf breadfruit (Artocarpus alilis) plants growing on marang (Artocarpus odoratissimus) rootstocks. Plants 2020, 9, 634. [Google Scholar] [CrossRef]
- Tombesi, S.; Johnson, R.S.; Day, K.R.; DeJong, T.M. Interactions between rootstock, inter-stem and scion xylem vessel characteristics of peach trees growing on rootstocks with contrasting size-controlling characteristics. AoB Plants 2010, 2010, plq013. [Google Scholar] [CrossRef]
- Chen, B.; Wang, C.; Tian, Y.; Chu, Q.; Hu, C. Anatomical characteristics of young stems and mature leaves of dwarf pear. Sci. Hortic. 2015, 186, 172–179. [Google Scholar] [CrossRef]
- Adams, S.; Lordan, J.; Fazio, G.; Bugbee, B.; Francescatto, P.; Robinson, T.L.; Black, B. Effect of scion and graft type on transpiration, hydraulic resistance and xylem hormone profile of apples grafted on Geneva®41 and M.9-NICTM29 rootstocks. Sci. Hortic. 2018, 227, 213–222. [Google Scholar] [CrossRef]
- Cichy, K.A.; Snapp, S.S.; Kirk, W.W. Fusarium root rot incidence and root system architecture in grafted common bean lines. Plant Soil 2007, 300, 233–244. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Hu, H.; Li, Z.; Xu, X.; Wang, Y.; Han, Z. Drought tolerance and impacts of four rootstock genotypes on the morphology, yield and fruit quality of Fuji scion apple under drought conditions. Hortic. Environ. Biotechnol. 2024, 65, 491–500. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, D. Transcriptome analysis of scions grafted to potato rootstock for improving late blight resistance. BMC Plant Biol. 2021, 21, 272. [Google Scholar] [CrossRef]
- Rebolledo-Martínez, A.; Peralta-Antonio, N.; Rebolledo-Martínez, L.; Becerril-Román, E.A.; Rebolledo-García, R.L. Effect of rootstock in tree growth, dry matter, flowering, yield and quality of ‘Manila’ mango. Sci. Hortic. 2019, 251, 155–161. [Google Scholar] [CrossRef]
- Ookawa, T.; Tomita, N.; Hirasawa, T. Interaction of scion and stock on leaf senescence of soybean plants grafted at mid-stem during ripening. Plant Prod. Sci. 2005, 8, 32–37. [Google Scholar] [CrossRef]
- López-Serrano, L.; Canet-Sanchis, G.; Vuletin Selak, G.; Penella, C.; San Bautista, A.; López-Galarza, S.; Calatayud, Á. Pepper rootstock and scion physiological responses under drought stress. Front. Plant Sci. 2019, 10, 38. [Google Scholar] [CrossRef]
- Zebro, M.; Heo, J.Y. Rootstock type differentially affects pollen germination characteristics and chilling stress response at flowering time in ‘Fuji’ spple. Appl. Fruit Sci. 2023, 66, 333–340. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, Y.; Wang, K.; Wang, H.; Huang, J.; Yu, H.; Cui, X. Rootstock-scion interactions affect fruit flavor in grafted tomato. Hortic. Plant J. 2022, 8, 499–510. [Google Scholar] [CrossRef]
- Chen, J.; Han, X.; Ye, S.; Liu, L.; Yang, B.; Cao, Y.; Zhuo, R.; Yao, X. Integration of small RNA, degradome, and transcriptome sequencing data illustrates the mechanism of low phosphorus adaptation in Camellia oleifera. Front. Plant Sci. 2022, 13, 932926. [Google Scholar] [CrossRef]
- Moustafa, K.; AbuQamar, S.; Jarrar, M.; Al-Rajab, A.J.; Trémouillaux-Guiller, J. MAPK cascades and major abiotic stresses. Plant Cell Rep. 2014, 33, 1217–1225. [Google Scholar] [CrossRef]
- Hao, L.; Wen, Y.; Zhao, Y.; Lu, W.; Xiao, K. Wheat mitogen-activated protein kinase gene TaMPK4 improves plant tolerance to multiple stresses through modifying root growth, ROS metabolism, and nutrient acquisitions. Plant Cell Rep. 2015, 34, 2081–2097. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xie, Y.; Shi, M.; Yao, S.; Lu, W.; Xiao, K. TaMPK2B, a member of the MAPK family in T. aestivum, enhances plant low-Pi stress tolerance through modulating physiological processes associated with phosphorus starvation defensiveness. Plant Sci. 2022, 323, 111375. [Google Scholar] [CrossRef] [PubMed]





| Name | Gene_id | Description | Primer Sequences |
|---|---|---|---|
| MAPK | augustus_masked-HiC_scaffold_ 13-processed-gene-1462.3 | MAPK signaling pathway | F: CACTCGCCACAAACAATCCC |
| R: TACGCTTGTGGGTTGTCGAA | |||
| CBs | snap_masked-HiC_scaffold_ 6-processed-gene-823.4 | Carotenoid biosynthesis | F: CTCAGAGCGTGATGGGGATC |
| R: ATTCTTGTTGAGCCGAGGCA | |||
| ELM | maker-HiC_scaffold_ 14-snap-gene-732.64 | Ether lipid metabolism | F: TACCCATGGTGGTTGCCTTC |
| R: ACGGGCCTGACTAATTGCAT | |||
| FBs | augustus_masked-HiC_scaffold _10-processed-gene-1746.20 | Fatty acid biosynthesis | F: GTCCCACTCCGACATTCTCC |
| R: TGGTTAAGTCGGTTGGAGGC | |||
| PPI | augustus_masked-HiC_scaffold _1-processed-gene-131.29 | Plant–pathogen interaction | F: CCAGTGGCGGAGTCCAAATA |
| R: GGGCGGGTCATGATGTAGAG | |||
| PHST | snap_masked-HiC_scaffold _14-processed-gene-167.29 | Plant hormone signal transduction | F: GTGCCCTCTCAAATGGTGGA |
| R: GGCCTAAGCTAGCACTACCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Liu, J.; Zeng, J.; Cheng, L.; Liu, H.; Zhang, Y.; Cheng, Q.; Su, W.; Wu, H.; Hu, D. Rootstock Genotype Dictates Phosphorus Deficiency Tolerance and Transcriptional Plasticity in Grafted Camellia oleifera Plants. Life 2025, 15, 1489. https://doi.org/10.3390/life15091489
Ren Z, Liu J, Zeng J, Cheng L, Liu H, Zhang Y, Cheng Q, Su W, Wu H, Hu D. Rootstock Genotype Dictates Phosphorus Deficiency Tolerance and Transcriptional Plasticity in Grafted Camellia oleifera Plants. Life. 2025; 15(9):1489. https://doi.org/10.3390/life15091489
Chicago/Turabian StyleRen, Zhihua, Juan Liu, Jin Zeng, Li Cheng, Huiyun Liu, Yunyu Zhang, Qinhua Cheng, Wenjuan Su, Huaiyuan Wu, and Dongnan Hu. 2025. "Rootstock Genotype Dictates Phosphorus Deficiency Tolerance and Transcriptional Plasticity in Grafted Camellia oleifera Plants" Life 15, no. 9: 1489. https://doi.org/10.3390/life15091489
APA StyleRen, Z., Liu, J., Zeng, J., Cheng, L., Liu, H., Zhang, Y., Cheng, Q., Su, W., Wu, H., & Hu, D. (2025). Rootstock Genotype Dictates Phosphorus Deficiency Tolerance and Transcriptional Plasticity in Grafted Camellia oleifera Plants. Life, 15(9), 1489. https://doi.org/10.3390/life15091489





