Examining the Relationships Between Blood Cadmium, DNA Methylation Biomarker, Telomere Length, and Their Associations with Mortality in U.S. Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement of Blood Cadmium
2.3. Measurement of Telomere Length
2.4. Measurement of Horvath DNAmTL
2.5. Covariates
2.6. Outcomes
2.7. Statistics
3. Results
4. Discussion
4.1. Cadmium and Telomere Length
4.2. Cadmium and Horvath DNAmTL
4.3. The Association Between Blood Cadmium, Horvath DNAmTL, and T/S Ratio
4.4. Blood Cadmium, Telomere Length, DNA Methylation, and Mortality Outcomes
4.5. Modification Effects of T/S Ratio and Horvath DNAmTL on Cadmium Related Mortality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, D.; Wang, P.; Zhao, F.-J. Dietary cadmium exposure, risks to human health and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 939–963. [Google Scholar] [CrossRef]
- Amzal, B.; Julin, B.; Vahter, M.; Wolk, A.; Johanson, G.; Akesson, A. Population toxicokinetic modeling of cadmium for health risk assessment. Environ. Health Perspect. 2009, 117, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Doccioli, C.; Sera, F.; Francavilla, A.; Cupisti, A.; Biggeri, A. Association of cadmium environmental exposure with chronic kidney disease: A systematic review and meta-analysis. Sci. Total Environ. 2024, 906, 167165. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; C. Gobe, G.; A. Vesey, D.; Phelps, K.R. Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Tao, C.; Yan, W.; Huang, Y.; Qian, H.; Xu, Q.; Wan, T.; Chen, Y.; Qin, Y.; et al. Association between exposure to cadmium and risk of all-cause and cause-specific mortality in the general US adults: A prospective cohort study. Chemosphere 2022, 307, 136060. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Navas-Acien, A.; Menke, A.; Crainiceanu, C.M.; Pastor-Barriuso, R.; Guallar, E. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ. Health Perspect. 2012, 120, 1017–1022. [Google Scholar] [CrossRef]
- Yin, H.; Pickering, J.G. Telomere Length: Implications for Atherogenesis. Curr. Atheroscler. Rep. 2023, 25, 95–103. [Google Scholar] [CrossRef]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef]
- Chen, X.; Ren, Q.; Wu, F.; Zhu, K.; Tao, J.; Zhang, A. Exposure to four typical heavy metals induced telomere shortening of peripheral blood mononuclear cells in relevant with declined urinary aMT6s in rats. Ecotoxicol. Environ. Saf. 2024, 283, 116791. [Google Scholar] [CrossRef]
- Wai, K.M.; Umezaki, M.; Kosaka, S.; Mar, O.; Umemura, M.; Fillman, T.; Watanabe, C. Impact of prenatal heavy metal exposure on newborn leucocyte telomere length: A birth-cohort study. Environ. Pollut. 2018, 243, 1414–1421. [Google Scholar] [CrossRef]
- Fillman, T.; Shimizu-Furusawa, H.; Ng, C.F.S.; Parajuli, R.P.; Watanabe, C. Association of cadmium and arsenic exposure with salivary telomere length in adolescents in Terai, Nepal. Environ. Res. 2016, 149, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Ghosh, S. Environmental exposures, epigenetics and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanles, A.; Sayols-Baixeras, S.; Subirana, I.; Degano, I.R.; Elosua, R. Association between DNA methylation and coronary heart disease or other atherosclerotic events: A systematic review. Atherosclerosis 2017, 263, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Ye, S.; Pan, Y.; Bao, Y.; Chen, H.; Shao, C. Long-term cadmium exposure leads to the enhancement of lymphocyte proliferation via down-regulating p16 by DNA hypermethylation. Mutat. Res. 2013, 757, 125–131. [Google Scholar] [CrossRef]
- Castillo, P.; Ibanez, F.; Guajardo, A.; Llanos, M.N.; Ronco, A.M. Impact of cadmium exposure during pregnancy on hepatic glucocorticoid receptor methylation and expression in rat fetus. PLoS ONE 2012, 7, e44139. [Google Scholar] [CrossRef]
- Das, A.; Bhattacharjee, P.; Bhattacharjee, P. Role of arsenic, lead and cadmium on telomere length and the risk of carcinogenesis: A mechanistic insight. Nucleus 2019, 62, 99–107. [Google Scholar] [CrossRef]
- Cowley, M.; Skaar, D.A.; Jima, D.D.; Maguire, R.L.; Hudson, K.M.; Park, S.S.; Sorrow, P.; Hoyo, C. Effects of Cadmium Exposure on DNA Methylation at Imprinting Control Regions and Genome-Wide in Mothers and Newborn Children. Environ. Health Perspect. 2018, 126, 037003. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lee, H.L.; Hwang, Y.T.; Huang, P.C.; Wang, C.; Sung, F.C.; Wu, C.; Su, T.C. Urinary heavy metals, DNA methylation, and subclinical atherosclerosis. Ecotoxicol. Environ. Saf. 2020, 204, 111039. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, X.; Sun, Q.; Shi, W.; Zhang, W.; Gao, X.; Li, Y.; Hao, R.; Dong, X.; Chen, C.; et al. Associations of environmental cadmium exposure with kidney damage: Exploring mediating DNA methylation sites in Chinese adults. Environ. Res. 2024, 251, 118667. [Google Scholar] [CrossRef]
- Joyce, B.T.; Zheng, Y.; Nannini, D.; Zhang, Z.; Liu, L.; Gao, T.; Kocherginsky, M.; Murphy, R.; Yang, H.; Achenbach, C.J.; et al. DNA Methylation of Telomere-Related Genes and Cancer Risk. Cancer Prev. Res. 2018, 11, 511–522. [Google Scholar] [CrossRef]
- Heng, J.; Zhang, F.; Guo, X.; Tang, L.; Peng, L.; Luo, X.; Xu, X.; Wang, S.; Dai, L.; Wang, J. Integrated analysis of promoter methylation and expression of telomere related genes in breast cancer. Oncotarget 2017, 8, 25442–25454. [Google Scholar] [CrossRef]
- Toubiana, S.; Selig, S. Human subtelomeric DNA methylation: Regulation and roles in telomere function. Curr. Opin. Genet. Dev. 2020, 60, 9–16. [Google Scholar] [CrossRef]
- Lu, A.T.; Seeboth, A.; Tsai, P.C.; Sun, D.; Quach, A.; Reiner, A.P.; Kooperberg, C.; Ferrucci, L.; Hou, L.; Baccarelli, A.A.; et al. DNA methylation-based estimator of telomere length. Aging 2019, 11, 5895–5923. [Google Scholar] [CrossRef] [PubMed]
- Khodasevich, D.; Gladish, N.; Daredia, S.; Bozack, A.K.; Shen, H.; Nwanaji-Enwerem, J.C.; Needham, B.L.; Rehkopf, D.H.; Cardenas, A. Exposome-wide association study of environmental chemical exposures and epigenetic aging in the national health and nutrition examination survey. Aging 2025, 17, 408–430. [Google Scholar] [CrossRef] [PubMed]
- CDC. NHANES 1999–2000. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=1999 (accessed on 31 August 2025).
- CDC. 2001–2002 Data Documentation, Codebook, and Frequencies: Cadmium, Lead, Mercury, Cotinine & Nutritional Biochemistries. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2001/DataFiles/L06_B.htm (accessed on 31 August 2025).
- CDC. 2001–2002 Data Documentation, Codebook, and Frequencies: Telomere Mean and Standard Deviation (Surplus). Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2001/DataFiles/TELO_B.htm (accessed on 31 August 2025).
- CDC. NHANES 1999–2002 DNA Methylation Array and Epigenetic Biomarkers. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/DNAm/Default.aspx (accessed on 31 August 2025).
- Lindsay, R.P.; Tsoh, J.Y.; Sung, H.Y.; Max, W. Secondhand smoke exposure and serum cotinine levels among current smokers in the USA. Tob. Control 2016, 25, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.T.; Chao, C.T.; Lin, S.H. Chronic Kidney Disease: Strategies to Retard Progression. Int. J. Mol. Sci. 2021, 22, 10084. [Google Scholar] [CrossRef]
- CDC. National Health and Nutrition Examination Survey: 2001–2002 Data Documentation, Codebook, and Frequencies: Medical Conditions. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2001/DataFiles/MCQ_B.htm (accessed on 31 August 2025).
- NCHS. NCHS Data Linkage: 2019 Public-Use Linked Mortality Files. Available online: https://www.cdc.gov/nchs/linked-data/mortality-files/?CDC_AAref_Val=https://www.cdc.gov/nchs/data-linkage/mortality-public.htm (accessed on 31 August 2025).
- CDC. NHANES Analytic Guidelines—June 2004 Version. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/analyticguidelines/04-analytic-guidelines.pdf (accessed on 31 August 2025).
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, P.C.; Lin, Y.C.; Lin, L.Y. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care 2009, 32, 702–707. [Google Scholar] [CrossRef]
- Zota, A.R.; Needham, B.L.; Blackburn, E.H.; Lin, J.; Park, S.K.; Rehkopf, D.H.; Epel, E.S. Associations of cadmium and lead exposure with leukocyte telomere length: Findings from National Health and Nutrition Examination Survey, 1999–2002. Am. J. Epidemiol. 2015, 181, 127–136. [Google Scholar] [CrossRef]
- Hu, L.t.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Lansdorp, P.M. Sex differences in telomere length, lifespan, and embryonic dyskerin levels. Aging Cell 2022, 21, e13614. [Google Scholar] [CrossRef]
- Needham, B.L.; Salerno, S.; Roberts, E.; Boss, J.; Allgood, K.L.; Mukherjee, B. Do black/white differences in telomere length depend on socioeconomic status? Biodemography Soc. Biol. 2019, 65, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Wróblewski, K.; Wojnicka, J.; Tutka, P.; Szmagara, A.; Błażewicz, A. Measurements of cadmium levels in relation to tobacco dependence and as a function of cytisine administration. Sci. Rep. 2024, 14, 1883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, L.; Liu, B.; Wu, M.; Wang, L.; Zhang, B.; Xiong, C.; Xia, W.; Li, Y.; Cao, Z.; et al. Prenatal cadmium exposure is associated with shorter leukocyte telomere length in Chinese newborns. BMC Med. 2019, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Huo, X.; Zhang, Q.; Fan, X.; Du, L.; Xu, X.; Qiu, S.; Zhang, Y.; Wang, Y.; Gu, J. Short placental telomere was associated with cadmium pollution in an electronic waste recycling town in China. PLoS ONE 2013, 8, e60815. [Google Scholar] [CrossRef]
- Mizuno, Y.; Konishi, S.; Imai, H.; Fujimori, E.; Kojima, N.; Yoshinaga, J. Cadmium Exposure and Blood Telomere Length in Female University Students in Japan. Biol. Trace Elem. Res. 2019, 192, 98–105. [Google Scholar] [CrossRef]
- Virani, S.; Rentschler, K.M.; Nishijo, M.; Ruangyuttikarn, W.; Swaddiwudhipong, W.; Basu, N.; Rozek, L.S. DNA methylation is differentially associated with environmental cadmium exposure based on sex and smoking status. Chemosphere 2016, 145, 284–290. [Google Scholar] [CrossRef]
- Domingo-Relloso, A.; Riffo-Campos, A.L.; Haack, K.; Rentero-Garrido, P.; Ladd-Acosta, C.; Fallin, D.M.; Tang, W.Y.; Herreros-Martinez, M.; Gonzalez, J.R.; Bozack, A.K.; et al. Cadmium, Smoking, and Human Blood DNA Methylation Profiles in Adults from the Strong Heart Study. Environ. Health Perspect. 2020, 128, 67005. [Google Scholar] [CrossRef]
- Needham, B.L.; Rehkopf, D.; Adler, N.; Gregorich, S.; Lin, J.; Blackburn, E.H.; Epel, E.S. Leukocyte Telomere Length and Mortality in the National Health and Nutrition Examination Survey, 1999–2002. Epidemiology 2015, 26, 528–535. [Google Scholar] [CrossRef]
- Schneider, C.V.; Schneider, K.M.; Teumer, A.; Rudolph, K.L.; Hartmann, D.; Rader, D.J.; Strnad, P. Association of Telomere Length with Risk of Disease and Mortality. JAMA Intern. Med. 2022, 182, 291–300. [Google Scholar] [CrossRef]
- Marioni, R.E.; Shah, S.; McRae, A.F.; Chen, B.H.; Colicino, E.; Harris, S.E.; Gibson, J.; Henders, A.K.; Redmond, P.; Cox, S.R.; et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wilson, R.; Heiss, J.; Breitling, L.P.; Saum, K.-U.; Schöttker, B.; Holleczek, B.; Waldenberger, M.; Peters, A.; Brenner, H. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat. Commun. 2017, 8, 14617. [Google Scholar] [CrossRef] [PubMed]
- Föhr, T.; Waller, K.; Viljanen, A.; Rantanen, T.; Kaprio, J.; Ollikainen, M.; Sillanpää, E. Mortality Associations with DNA Methylation-Based Biological Aging and Physical Functioning Measures Across a 20-Year Follow-up Period. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Ruiz, A.B.; González-Herrera, L.; Luna, J.d.D.; Valenzuela, A. DNA methylation levels and telomere length in human teeth: Usefulness for age estimation. Int. J. Leg. Med. 2020, 134, 451–459. [Google Scholar] [CrossRef]
- Zubakov, D.; Liu, F.; Kokmeijer, I.; Choi, Y.; van Meurs, J.B.J.; van Ijcken, W.F.J.; Uitterlinden, A.G.; Hofman, A.; Broer, L.; van Duijn, C.M.; et al. Human age estimation from blood using mRNA, DNA methylation, DNA rearrangement, and telomere length. Forensic Sci. Int. Genet. 2016, 24, 33–43. [Google Scholar] [CrossRef]
- Kippler, M.; Engström, K.; Mlakar, S.J.; Bottai, M.; Ahmed, S.; Hossain, M.B.; Raqib, R.; Vahter, M.; Broberg, K. Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight. Epigenetics 2013, 8, 494–503. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Zhang, C.; Bin, X.; Jiang, J.; Huang, C. Leukocyte telomere length mediates the association between cadmium exposure and cognitive function in US older adults. J. Psychiatr. Res. 2024, 169, 166–173. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, R.; Gao, Y.; Kang, H.; Zhang, Z.; Han, Z.; Zhang, Y.; Li, Y.; Mu, L.; Lei, L. Mediating effect of telomere length in a hypertension population exposed to cadmium: A case–control study. J. Hum. Hypertens. 2023, 37, 386–393. [Google Scholar] [CrossRef]

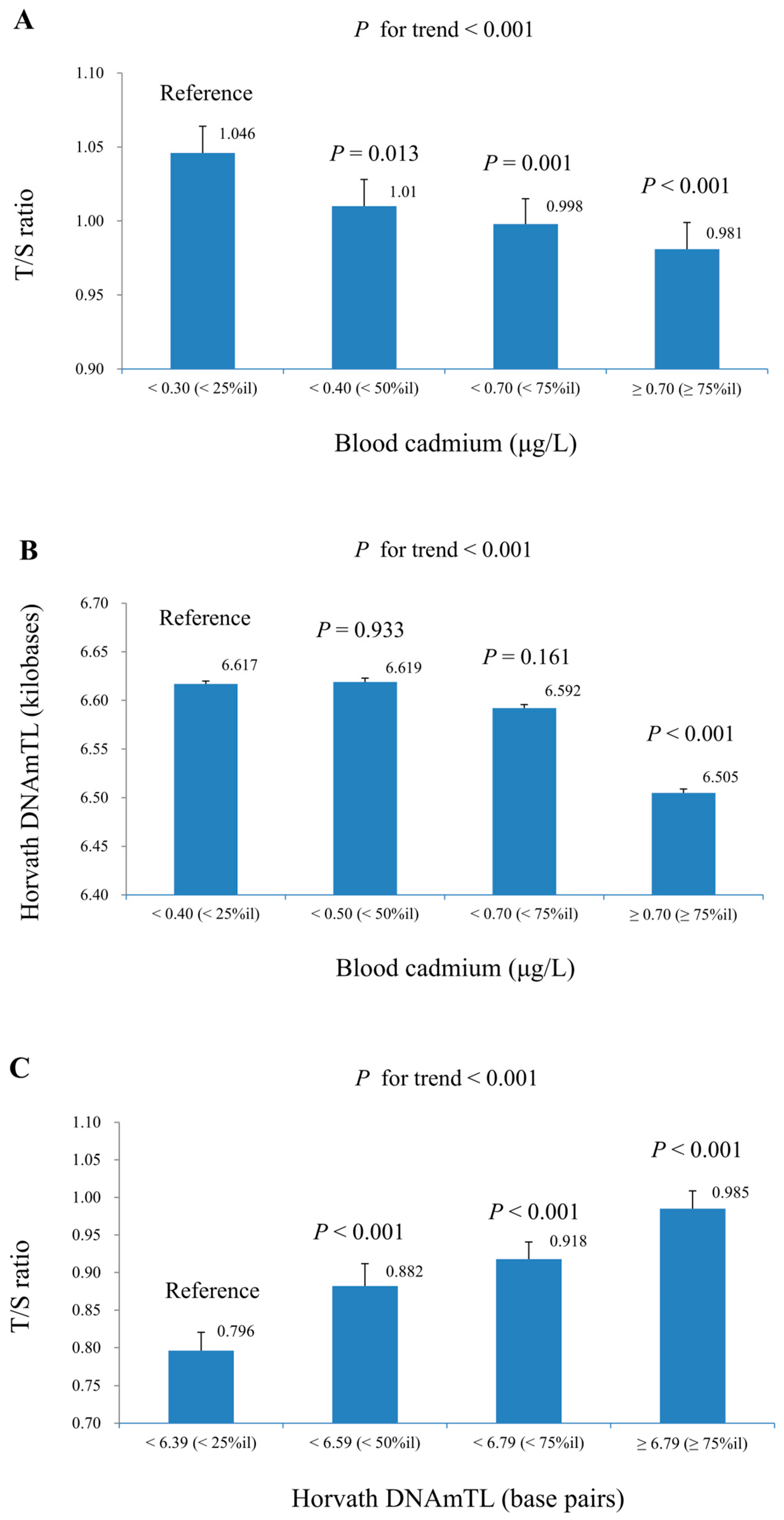
| N | Blood Cadmium (μg/L) | N | T/S Ratio | N | Horvath DNAmTL (Kilobases) | |
|---|---|---|---|---|---|---|
| Total | 8716 | 0.441 (1.007) | 6891 | 0.998 (1.003) | 2126 | 6.568 (1.001) |
| Sex | ||||||
| Men | 4167 | 0.439 (1.010) | 3338 | 0.978 (1.004)‡ | 1082 | 6.512 (1.001) ‡ |
| Women | 4549 | 0.443 (1.009) | 3553 | 1.017 (1.004)‡ | 1044 | 6.626 (1.001) ‡ |
| Age (in years) | ||||||
| 18–39 | 3796 | 0.381 (1.010) ‡ | 2563 | 1.115 (1.004) ‡ | ||
| 40–59 | 2410 | 0.481 (1.014) ‡ | 2113 | 1.005 (1.005) ‡ | 604 | 6.790 (1.001) ‡ |
| ≥60 | 2510 | 0.507 (1.011) ‡ | 2215 | 0.872 (1.005) ‡ | 1522 | 6.482 (1.001) ‡ |
| Ethnicity | ||||||
| Mexican-American | 2222 | 0.419 (1.012) ‡ | 1656 | 0.977 (1.006) ‡ | 604 | 6.541 (1.002) ‡ |
| Other Hispanic | 443 | 0.411 (1.029) ‡ | 353 | 1.032 (1.013) ‡ | 131 | 6.610 (1.004) ‡ |
| Non-Hispanic white | 4124 | 0.449 (1.010) ‡ | 3525 | 0.987 (1.004) ‡ | 882 | 6.500 (1.002) ‡ |
| Non-Hispanic black | 1630 | 0.444 (1.017) ‡ | 1147 | 1.050 (1.008) ‡ | 439 | 6.731 (1.002) ‡ |
| Other ethnicity | 297 | 0.529 (1.036) ‡ | 210 | 1.021 (1.016) ‡ | 70 | 6.569 (1.004) ‡ |
| Family poverty income ratio | ||||||
| <1 | 1739 | 0.498 (1.017) ‡ | 1217 | 1.011 (1.007) ‡ | 360 | 6.557 (1.002) ‡ |
| 1–3 | 3648 | 0.455 (1.011) ‡ | 2876 | 0.980 (1.004) ‡ | 958 | 6.526 (1.002) ‡ |
| >3 | 3330 | 0.400 (1.010) ‡ | 2798 | 1.011 (1.005) ‡ | 808 | 6.622 (1.002) ‡ |
| Body mass index (kg/m2) | ||||||
| <25 | 3025 | 0.461 (1.013) ‡ | 2167 | 1.022 (1.006) ‡ | 563 | 6.518 (1.002) ‡ |
| 25–30 | 3047 | 0.437 (1.011) ‡ | 2524 | 0.989 (1.005) ‡ | 841 | 6.561 (1.002) ‡ |
| >30 | 2644 | 0.423 (1.012) ‡ | 2200 | 0.986 (1.005) ‡ | 722 | 6.615 (1.002) ‡ |
| Smoking status | ||||||
| Non-smoker | 4469 | 0.361 (1.007) ‡ | 3633 | 0.979 (1.004) ‡ | 1273 | 6.563 (1.001) |
| ETS | 1794 | 0.348 (1.011) ‡ | 1370 | 1.011 (1.007) ‡ | 429 | 6.580 (1.002) |
| Active smoker | 2453 | 0.753 (1.014) ‡ | 1888 | 1.026 (1.006) ‡ | 424 | 6.569 (1.002) |
| Alcohol consumption (drinks/year) | ||||||
| <12 | 3666 | 0.404 (1.010) ‡ | 2386 | 0.992 (1.005) | 826 | 6.576 (1.002) |
| ≥12 | 5050 | 0.469 (1.009) ‡ | 4505 | 1.001 (1.004) | 1300 | 6.563 (1.001) |
| Hypertension | ||||||
| Yes | 2772 | 0.485 (1.011) ‡ | 2398 | 0.932(1.005) ‡ | 1286 | 6.543 (1.001) ‡ |
| No | 5944 | 0.422 (1.009) ‡ | 4493 | 1.036 (1.004) ‡ | 840 | 6.605 (1.002) ‡ |
| Diabetes Mellitus | ||||||
| Yes | 977 | 0.449 (1.019) | 829 | 0.921 (1.008) ‡ | 504 | 6.546 (1.002) |
| No | 7739 | 0.440 (1.007) | 6062 | 1.009 (1.003) ‡ | 1622 | 6.574 (1.001) |
| Chronic kidney disease | ||||||
| Yes | 551 | 0.529 (1.025) ‡ | 498 | 0.861 (1.011) ‡ | 283 | 6.421 (1.003) ‡ |
| No | 8165 | 0.435 (1.007) ‡ | 6393 | 1.010 (1.003) ‡ | 1843 | 6.590 (1.001) ‡ |
| Hypercholesterolemia | ||||||
| Yes | 2080 | 0.461 (1.014) ‡ | 1816 | 0.957 (1.006) ‡ | 751 | 6.569 (1.002) |
| No | 6636 | 0.435 (1.008) ‡ | 5075 | 1.013 (1.004) ‡ | 1375 | 6.567 (1.001) |
| History of CVD | ||||||
| Yes | 777 | 0.536 (1.022) ‡ | 674 | 0.875 (1.009) ‡ | 403 | 6.462 (1.002) ‡ |
| No | 7939 | 0.432 (1.007) ‡ | 6217 | 1.013 (1.003) ‡ | 1723 | 6.593 (1.001) ‡ |
| History of cancer | ||||||
| Yes | 616 | 0.516 (1.023) ‡ | 551 | 0.902 (1.011) ‡ | 284 | 6.445 (1.003) ‡ |
| No | 8100 | 0.436 (1.007) ‡ | 6340 | 1.007 (1.003) ‡ | 1842 | 6.587 (1.001) ‡ |
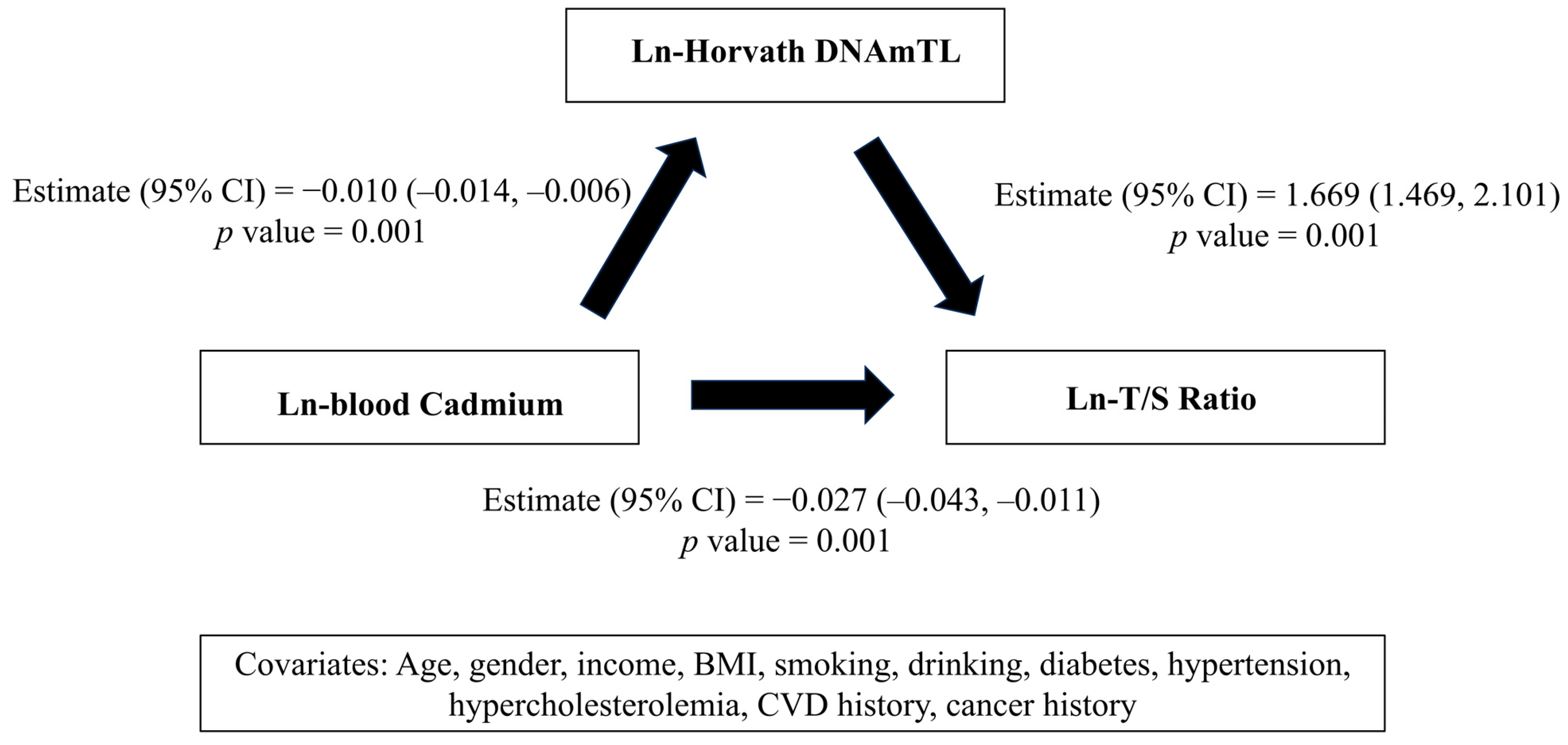
| Ln-blood Cadmium (μg/L) | Ln-Horvath DNAmTL (Kilobases) | |||||
|---|---|---|---|---|---|---|
| Unweighted No./Population Size | Adjusted β (95% CI) | p Value | Unweighted No./Population Size | Adjusted β (95% CI) | p Value | |
| Ln-T/S ratio | 6891/150,739,685 | 2126/65,502,614 | ||||
| Model 1 | −0.043 (−0.059, −0.027) | <0.001 | 1.782 (1.462, 2.102) | <0.001 | ||
| Model 2 | −0.043 (−0.059, −0.027) | <0.001 | 1.784 (1.464, 2.104) | <0.001 | ||
| Model 3 | −0.043 (−0.059, −0.027) | <0.001 | 1.785 (1.465, 2.105) | <0.001 | ||
| Ln-Horvath DNAmTL (kilobases) | 2126/32,034,886 | |||||
| Model 1 | −0.010 (−0.014, −0.006) | <0.001 | ||||
| Model 2 | −0.010 (−0.014, −0.006) | <0.001 | ||||
| Model 3 | −0.010 (−0.014, −0.006) | <0.001 | ||||
| Ln-T/S Ratio | Ln-Horvath DNAmTL (Kilobases) | |||||
|---|---|---|---|---|---|---|
| Adjusted β (95% CI) | p Value | p or Interaction | Adjusted β (95% CI) | p Value | p for Interaction | |
| Gender | 0.102 | 0.744 | ||||
| Men | −0.046 (−0.064, −0.028) | <0.001 | −0.008 (−0.014, −0.002) | 0.005 | ||
| Women | −0.037 (−0.059, −0.015) | 0.002 | −0.012 (−0.018, −0.006) | <0.001 | ||
| Age, years | 0.035 | 0.525 | ||||
| 18–59 | −0.043 (−0.061, −0.025) | <0.001 | −0.007 (−0.013, −0.001) | 0.017 | ||
| ≥60 | −0.047 (−0.073, −0.021) | 0.001 | −0.012 (−0.016, −0.008) | <0.001 | ||
| Ethnicity | 0.591 | 0.058 | ||||
| Non-Hispanic white | −0.045 (−0.065, −0.025) | <0.001 | −0.009 (−0.015, −0.003) | 0.001 | ||
| Other | −0.029 (−0.045, −0.013) | 0.001 | −0.010 (−0.016, −0.004) | 0.001 | ||
| BMI (kg/m2) | 0.629 | 0.158 | ||||
| <30 | −0.038 (−0.058, −0.018) | 0.001 | −0.011 (−0.017, −0.005) | <0.001 | ||
| ≥30 | −0.051 (−0.073, −0.029) | <0.001 | −0.007 (−0.013, −0.001) | 0.038 | ||
| Smoking status | 0.074 | 0.142 | ||||
| Active smoker and ETS | −0.078 (−0.110, −0.046) | <0.001 | −0.010 (−0.018, −0.002) | 0.024 | ||
| Non-smoker | −0.050 (−0.082, −0.018) | 0.003 | −0.006 (−0.010, −0.002) | 0.014 | ||
| Alcohol consumption (drinks/year) | 0.067 | 0.891 | ||||
| <12 | −0.049 (−0.081, −0.017) | 0.004 | −0.010 (−0.016, −0.004) | 0.002 | ||
| ≥12 | −0.041 (−0.057, −0.025) | <0.001 | −0.009 (−0.013, −0.005) | 0.001 | ||
| HR (95% CI) | p Value | |
| All-cause mortality | ||
| Ln-blood cadmium (μg/L) | 1.449 (1.274–1.649) | <0.001 |
| Ln-T/S ratio | 0.675 (0.510–0.892) | 0.007 |
| Ln-Horvath DNAmTL (kilobases) | 0.002 (0.001–0.028) | <0.001 |
| Cardiovascular mortality * | ||
| Ln-blood cadmium (μg/L) | 1.385 (1.130–1.698) | 0.003 |
| Ln-T/S ratio | 0.582 (0.354–0.958) | 0.034 |
| Ln-Horvath DNAmTL (kilobases) | 0.007 (2.702 × 10−5–2.080) | 0.086 |
| Cancer-related mortality | ||
| Ln-blood cadmium (μg/L) | 1.633 (1.341–1.989) | <0.001 |
| Ln-T/S ratio | 0.589 (0.314–1.108) | 0.097 |
| Ln-Horvath DNAmTL (kilobases) | 0.001 (2.264 × 10−6–0.112) | 0.008 |
| Unweighted No./Population Size | HR | 95% CI | p Value | p for Interaction | |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Total | 6819/149,403,806 | 1.445 | 1.251–1.670 | 0.001 | 0.255 |
| T/S ratio < 50%ile | 3390/68,074,798 | 1.468 | 1.241–1.736 | <0.001 | |
| T/S ratio ≥ 50%ile | 3429/81,329,008 | 1.413 | 1.163–1.716 | 0.001 | |
| Total | 2031/31,051,368 | 1.313 | 1.135–1.519 | 0.001 | 0.493 |
| Horvath DNAmTL < 50%ile | 1004/13,570,298 | 1.355 | 1.056–1.738 | 0.019 | |
| Horvath DNAmTL ≥ 50%ile | 1027/17,481,070 | 1.307 | 1.048–1.631 | 0.019 | |
| Cardiovascular mortality * | |||||
| Total | 6819/149,403,806 | 1.407 | 1.106–1.790 | 0.007 | 0.508 |
| T/S ratio < 50%ile | 3390/68,074,798 | 1.683 | 1.052–2.693 | 0.031 | |
| T/S ratio ≥ 50%ile | 3429/81,329,008 | 1.296 | 1.018–1.650 | 0.036 | |
| Total | 2031/31,051,368 | 1.213 | 0.950–1.548 | 0.117 | 0.605 |
| Horvath DNAmTL < 50%ile | 1004/13,570,298 | 1.175 | 0.908–1.520 | 0.210 | |
| Horvath DNAmTL ≥ 50%ile | 1027/17,481,070 | 1.257 | 0.808–1.958 | 0.298 | |
| Cancer-related mortality | |||||
| Total | 6819/149,403,806 | 1.643 | 1.314–2.054 | <0.001 | 0.157 |
| T/S ratio < 50%ile | 3390/68,074,798 | 1.751 | 1.267–2.422 | 0.001 | |
| T/S ratio ≥ 50%ile | 3429/81,329,008 | 1.422 | 1.129–1.791 | 0.004 | |
| Total | 2031/31,051,368 | 1.378 | 0.928–2.047 | 0.108 | 0.477 |
| Horvath DNAmTL < 50%ile | 1004/13,570,298 | 1.241 | 0.741–2.079 | 0.399 | |
| Horvath DNAmTL ≥ 50%ile | 1027/17,481,070 | 1.412 | 0.836–2.386 | 0.189 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Chen, C.-W.; Chu, P.-L. Examining the Relationships Between Blood Cadmium, DNA Methylation Biomarker, Telomere Length, and Their Associations with Mortality in U.S. Adults. Life 2025, 15, 1467. https://doi.org/10.3390/life15091467
Lin C-Y, Chen C-W, Chu P-L. Examining the Relationships Between Blood Cadmium, DNA Methylation Biomarker, Telomere Length, and Their Associations with Mortality in U.S. Adults. Life. 2025; 15(9):1467. https://doi.org/10.3390/life15091467
Chicago/Turabian StyleLin, Chien-Yu, Ching-Way Chen, and Pei-Lun Chu. 2025. "Examining the Relationships Between Blood Cadmium, DNA Methylation Biomarker, Telomere Length, and Their Associations with Mortality in U.S. Adults" Life 15, no. 9: 1467. https://doi.org/10.3390/life15091467
APA StyleLin, C.-Y., Chen, C.-W., & Chu, P.-L. (2025). Examining the Relationships Between Blood Cadmium, DNA Methylation Biomarker, Telomere Length, and Their Associations with Mortality in U.S. Adults. Life, 15(9), 1467. https://doi.org/10.3390/life15091467







