Intensive Management of a Patient with HIV, Active Tuberculosis, and COVID-19: A Multidisciplinary Approach in the Intensive Care Unit
Abstract
1. Introduction
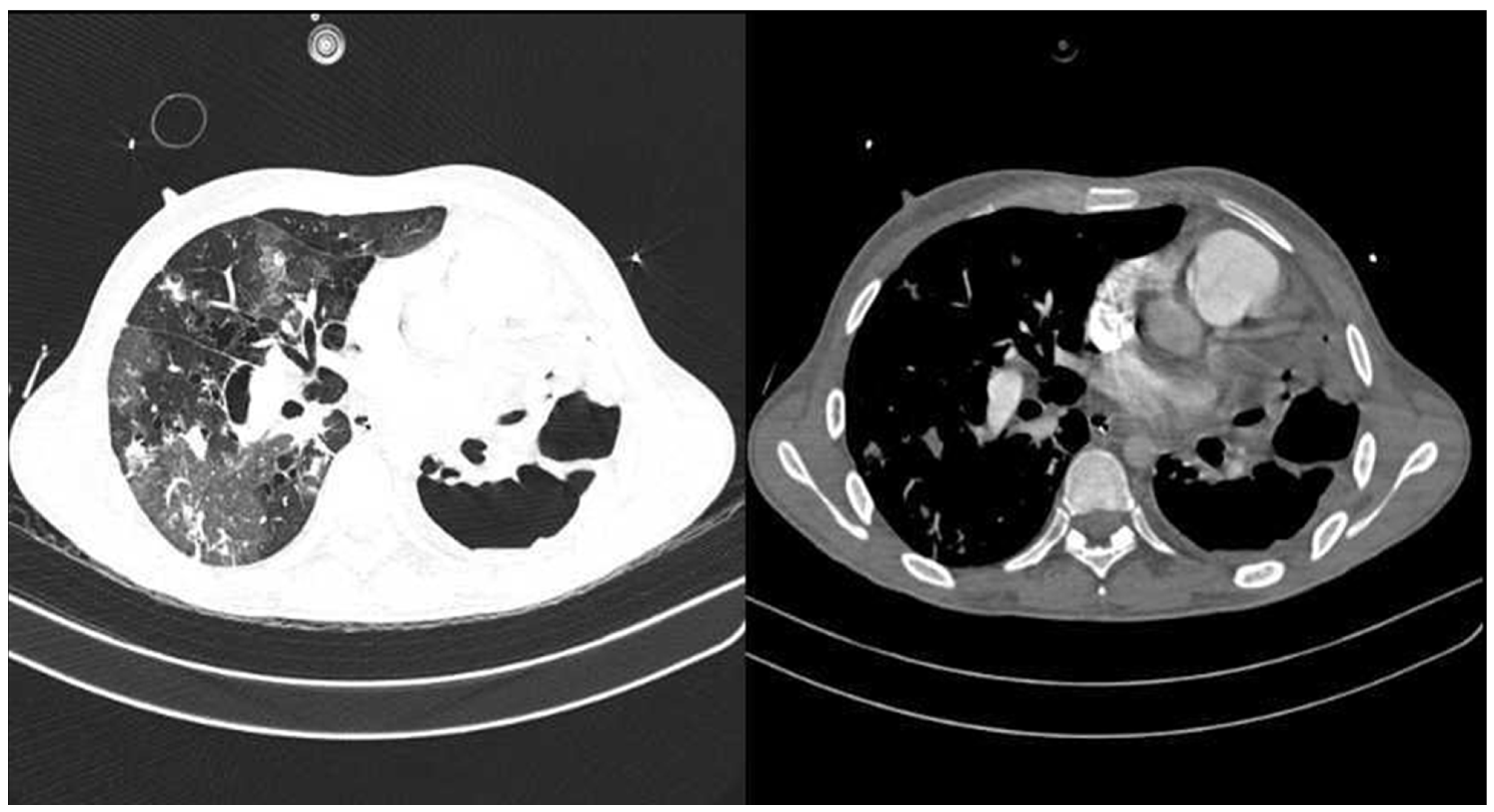
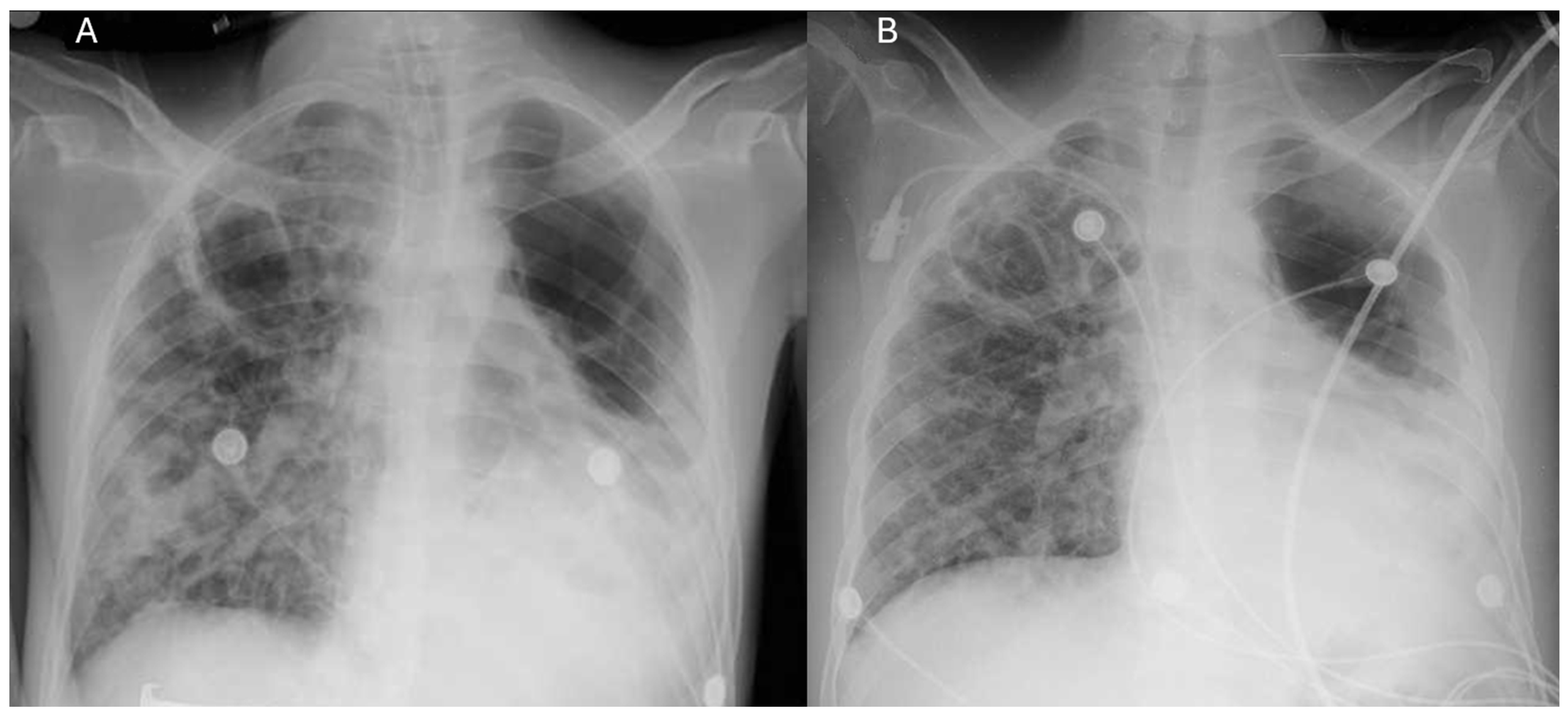
2. Case Report
3. Discussion
New Insights for Clinical Practice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amegashie, E.A.; Asamoah, P.; Ativi, L.E.A.; Adusei-Poku, M.; Bonney, E.Y.; Tagoe, E.A.; Paintsil, E.; Torpey, K.; Quaye, O. Clinical outcomes and immunological response to SARS-CoV-2 infection among people living with HIV. Exp. Biol. Med. 2024, 249, 10059. [Google Scholar] [CrossRef]
- Hanson, H.A.; Kim, E.; Badowski, M.E. A Systematic Review: Impact of SARS-CoV-2 Infection on Morbidity, Mortality, and Viral Suppression in Patients Living With HIV. SN Compr. Clin. Med. 2023, 5, 144. [Google Scholar] [CrossRef]
- François Anicet, O.A.; Kouanfack, C.; Dama, U.; Nkfusai, C.N.; Abanda, J.N.; Tchoffo, D.; Mbu, P.N.; Yoniene, P.Y. Epidemiological, and Clinical Profile of Patients Coinfected with Human Immunodeficiency Virus and Tuberculosis in the Coronavirus Disease 2019 Context in Health Facilities in the East Region, Cameroon. Int. J. Matern. Child Health AIDS 2024, 13, e006. [Google Scholar] [CrossRef]
- du Bruyn, E.; Stek, C.; Daroowala, R.; Said-Hartley, Q.; Hsiao, M.; Schafer, G.; Goliath, R.T.; Abrahams, F.; Jackson, A.; Wasserman, S.; et al. Effects of tuberculosis and/or HIV-1 infection on COVID-19 presentation and immune response in Africa. Nat. Commun. 2023, 14, 188. [Google Scholar] [CrossRef]
- Aiello, A.; Najafi-Fard, S.; Goletti, D. Initial immune response after exposure to Mycobacterium tuberculosis or to SARS-CoV-2: Similarities and differences. Front. Immunol. 2023, 14, 1244556. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolio, S.; Thwin, S.S.; Silva, R.; Nagarajan, S.; Jassat, W.; Fowler, R.; Haniffa, R.; Reveiz, L.; Ford, N.; Doherty, M.; et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: Analysis of data from the WHO Global Clinical Platform of COVID-19. Lancet HIV 2022, 9, e486–e495. [Google Scholar] [CrossRef]
- Visca, D.; Ong, C.W.M.; Tiberi, S.; Centis, R.; D’Ambrosio, L.; Chen, B.; Mueller, J.; Mueller, P.; Duarte, R.; Dalcolmo, M.; et al. Tuberculosis and COVID-19 interaction: A review of biological, clinical and public health effects. Pulmonology 2021, 27, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, P. Tuberculosis and HIV responses threatened by COVID-19. Lancet HIV 2020, 7, e319–e320. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, M.; Trunfio, M.; Sasset, L.; Leoni, D.; Castelli, E.; Lo Menzo, S.; Gardin, S.; Putaggio, C.; Brundu, M.; Garzotto, P.; et al. Factors Associated with Severe COVID-19 and Post-Acute COVID-19 Syndrome in a Cohort of People Living with HIV on Antiretroviral Treatment and with Undetectable HIV RNA. Viruses 2022, 14, 493. [Google Scholar] [CrossRef]
- Grifoni, A.; Alonzi, T.; Alter, G.; Noonan, D.M.; Landay, A.L.; Albini, A.; Goletti, D. Impact of aging on immunity in the context of COVID-19, HIV, and tuberculosis. Front. Immunol. 2023, 14, 1146704. [Google Scholar] [CrossRef]
- Sohrabi, C.; Mathew, G.; Maria, N.; Kerwan, A.; Franchi, T.; Agha, R.A. The SCARE 2023 guideline: Updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2023, 109, 1136. [Google Scholar] [CrossRef]
- Harvey, S.; Matthai, S.; King, D.A. How to use the Bristol Stool Chart in childhood constipation. Arch. Dis. Child.-Educ. Pract. 2023, 108, 335–339. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Vu, P.H.; Tran, V.D.; Duong, M.C.; Cong, Q.T.; Nguyen, T. Predictive value of the negative inspiratory force index as a predictor of weaning success: A crosssectional study. Acute Crit. Care 2020, 35, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Udoakang, A.J.; Djomkam Zune, A.L.; Tapela, K.; Nganyewo, N.N.; Olisaka, F.N.; Anyigba, C.A.; Tawiah-Eshun, S.; Owusu, I.A.; Paemka, L.; Awandare, G.A.; et al. The COVID-19, tuberculosis and HIV/AIDS: Ménage à Trois. Front. Immunol. 2023, 14, 1104828. [Google Scholar] [CrossRef]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Baden, L.R.; Cheng, V.C.C.; Edwards, K.M.; Gallagher, J.C.; Gandhi, R.T.; Muller, W.J.; Nakamura, M.M.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients With COVID-19 (September 2022). Clin. Infect. Dis. 2024, 78, e250–e349. [Google Scholar] [CrossRef]
- Tamuzi, J.L.; Ayele, B.T.; Shumba, C.S.; Adetokunboh, O.O.; Uwimana-Nicol, J.; Haile, Z.T.; Inugu, J.; Nyasulu, P.S. Implications of COVID-19 in high burden countries for HIV/TB: A systematic review of evidence. BMC Infect. Dis. 2020, 20, 744. [Google Scholar] [CrossRef]
- Le, X.; Shen, Y. Advances in Antiretroviral Therapy for Patients with Human Immunodeficiency Virus-Associated Tuberculosis. Viruses 2024, 16, 494. [Google Scholar] [CrossRef] [PubMed]
- Hogan, A.B.; Jewell, B.L.; Sherrard-Smith, E.; Vesga, J.F.; Watson, O.J.; Whittaker, C.; Hamlet, A.; Smith, J.A.; Winskill, P.; Verity, R.; et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: A modelling study. Lancet Glob. Health 2020, 8, e1132–e1141. [Google Scholar] [CrossRef]
- Tolossa, T.; Tsegaye, R.; Shiferaw, S.; Wakuma, B.; Ayala, D.; Bekele, B.; Shibiru, T. Survival from a Triple Co-Infection of COVID-19, HIV, and Tuberculosis: A Case Report. Int. Med. Case Rep. J. 2021, 14, 611–615. [Google Scholar] [CrossRef]
- Payán Salcedo, H.A.; Cabrera Barandica, M.C.; Estela Zape, J.L. Estrategias de destete y desmonte de cánula nasal de alto flujo en adultos. Respirar 2024, 16, 151–159. [Google Scholar] [CrossRef]
| Laboratory Parameters | Patient’s Values | ||||||
|---|---|---|---|---|---|---|---|
| Emergencies Date of Entry | ICU | Hospitalization | |||||
| 15 December 24 | 15 December 24 | 17 December 24 | 21 December 24 | 22 December 24 | 26 December 24 | 4 January 25 | |
| Hematology | |||||||
| Hemoglobin (13.5–17.5 g/dL) | 10.5 | 11.3 | 10.3 | 9.2 | 8.9 | 9.4 | 11.9 |
| Hematocrit (40–50%) | 35% | 39% | 36% | 31% | 29% | 32% | 40% |
| Platelets (150–450 × 103/µL) | 237 | 372 | 248 | 242 | 210 | 234 | 307 |
| Leukocytes (4.5–11.0 × 103/µL) | 22.0 | 22.0 | 12.0 | 4.2 | 3.2 | 3.6 | 5.6 |
| C-reactive protein (less than 2 mg/L) | 39.5 | ||||||
| Lactate (0.5–2.2 mmol/L) | 1.4 | 1.3 | 2.9 | 1.8 | 1.2 | ||
| Renal Function | |||||||
| Creatinine (0.7–1.3 mg/dL) | 1.1 | 0.43 | 0.45 | 0.52 | 0.50 | 0.69 | |
| Blood urea nitrogen (7–20 mg/dL) | 13.5 | 5.9 | 4.2 | 6.8 | 12.1 | 13.4 | |
| Coagulation | |||||||
| Prothrombin time (11.7–15.5 s) | 12.6 | ||||||
| Partial thromboplastin time (24–45 s) | 36.1 | ||||||
| Biochemistry | |||||||
| Electrolytes | |||||||
| Sodium (135–145 mmol/L) | 137 | 136 | 126 | 141 | 142 | 138 | |
| Potassium (3.5–4.5 mmol/L) | 5.1 | 5.4 | 4,9 | 3.4 | 3.4 | 3.6 | 3.9 |
| Chloride (95–105 mmol/L) | 105.8 | 102.0 | 99.9 | 106.2 | 108.7 | 100.9 | |
| Arterial blood gases, mmHg | |||||||
| Supplemental oxygen support | Venturi mask | Invasive mechanical ventilation | High-flow nasal cannula | Simple nasal cannula | Ambient breathing | ||
| FiO2 | 50% | 70% | 40% | 50% | 50% | 28% | 21% |
| Flow per minute | 50 L/min | ||||||
| pH (7.35–7.45) | 6.93 (pre-intubation) | 7.10 | 7.14 | 7.39 | 7.40 | 7.41 | 7.30 |
| pCO2 (35–45 mmHg) | 113.0 | 72 | 68 | 44 | 44.0 | 46 | 46 |
| PaO2 (75–100 mmHg) | 128.0 | 89 | 97 | 93 | 158 | 87 | 66 |
| HCO3− (22–26 mmol/L) | 15.1 | 22.4 | 23.1 | 26.6 | 27 | 29.2 | 22 |
| BE (–2 to +2 mmol/L) | −12.5 | −7.4 | −6.6 | 1.5 | 2.2 | 4.0 | −3.8 |
| PaO2/FiO2 > 400 (ratio) | 256 | 127 | 242 | 186 | 316 | 310 | 314 |
| ROX Index (SpO2/FiO2)/RR | 15 (indicative of high probability of HFNC success, threshold > 5) | ||||||
| Test | Sample | Result | Interpretation |
|---|---|---|---|
| Respiratory panel | Nasopharyngeal swab | Negative | No respiratory viral or bacterial pathogens detected |
| SARS-CoV-2 RT-PCR | Nasopharyngeal swab | Positive | Confirmed COVID-19 infection |
| TB drug susceptibility (DST) | Sputum (SOT) | Sensitive pattern | Sensitive pattern |
| Blood culture 1 | Blood | Negative | No bacterial growth |
| Blood culture 2 | Blood | Negative | No bacterial growth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosquera-Arias, B.R.; Sanclemente-Cardoza, V.; Estela-Zape, J.L. Intensive Management of a Patient with HIV, Active Tuberculosis, and COVID-19: A Multidisciplinary Approach in the Intensive Care Unit. Life 2025, 15, 1435. https://doi.org/10.3390/life15091435
Mosquera-Arias BR, Sanclemente-Cardoza V, Estela-Zape JL. Intensive Management of a Patient with HIV, Active Tuberculosis, and COVID-19: A Multidisciplinary Approach in the Intensive Care Unit. Life. 2025; 15(9):1435. https://doi.org/10.3390/life15091435
Chicago/Turabian StyleMosquera-Arias, Brayan Ricardo, Valeria Sanclemente-Cardoza, and Jose Luis Estela-Zape. 2025. "Intensive Management of a Patient with HIV, Active Tuberculosis, and COVID-19: A Multidisciplinary Approach in the Intensive Care Unit" Life 15, no. 9: 1435. https://doi.org/10.3390/life15091435
APA StyleMosquera-Arias, B. R., Sanclemente-Cardoza, V., & Estela-Zape, J. L. (2025). Intensive Management of a Patient with HIV, Active Tuberculosis, and COVID-19: A Multidisciplinary Approach in the Intensive Care Unit. Life, 15(9), 1435. https://doi.org/10.3390/life15091435






