C-Reactive Protein for Early Diagnosis and Severity Monitoring in Melioidosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Search Strategy
2.3. Eligibility Criteria
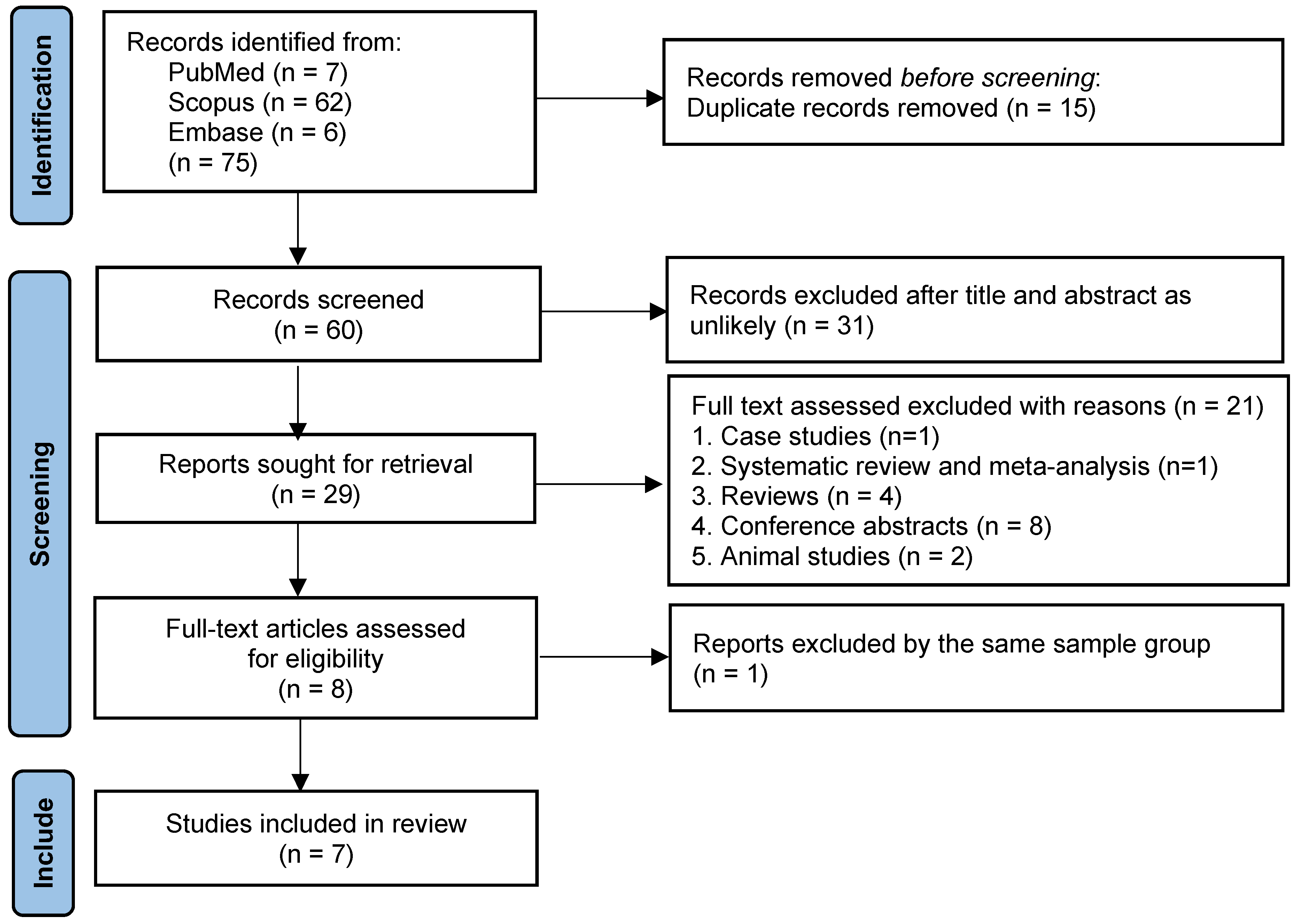
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Data Synthesis and Statistical Analysis
2.7.1. Subgroup Analysis
2.7.2. Sensitivity Analysis
2.7.3. Publication Bias
3. Results
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Quality of Included Studies
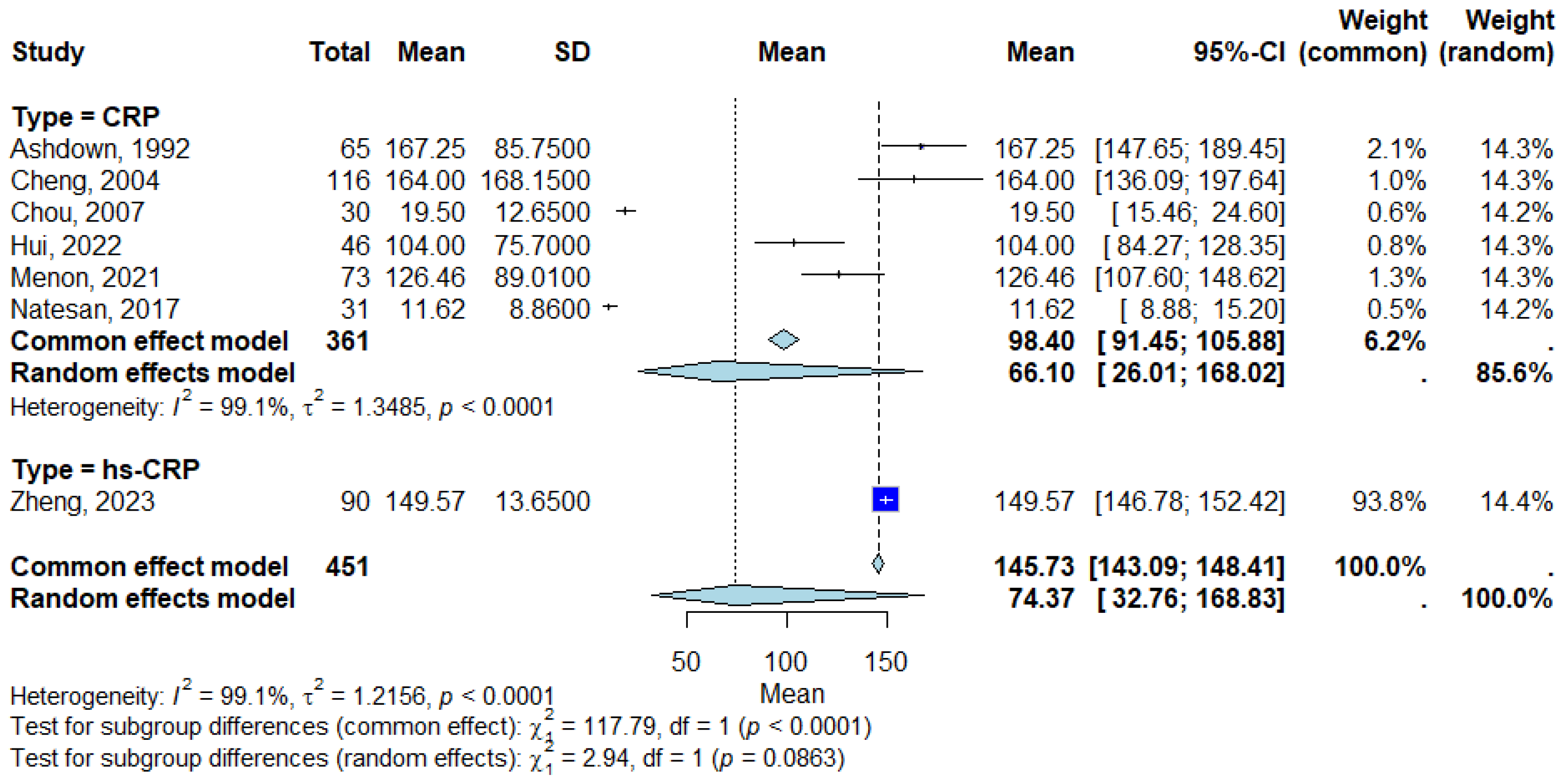
3.4. Pooled CRP Levels in Melioidosis Patients
3.5. Subgroup Analysis
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRP | C-reactive protein |
| ELISA | enzyme-linked immunosorbent assay |
| hs-CRP | High-sensitivity C-reactive protein |
| ICU | intensive care unit |
| IL-6 | interleukin-6 |
| IU | international unit |
| mg | milligram |
| mL | milliliter |
| NOS | Newcastle–Ottawa Scale |
| µg | microgram |
References
- Wiersinga, W.J.; Currie, B.J.; Peacock, S.J. Melioidosis. N. Engl. J. Med. 2012, 367, 1035–1044. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.B.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef]
- Cheng, A.C.; Currie, B.J. Melioidosis: Epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 2005, 18, 383–416. [Google Scholar] [CrossRef]
- Dance, D. Treatment and prophylaxis of melioidosis. Int. J. Antimicrob. Agents 2014, 43, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Limmathurotsakul, D.; Chaowagul, W.; Chierakul, W.; Stepniewska, K.; Maharjan, B.; Wuthiekanun, V.; White, N.J.; Day, N.P.J.; Peacock, S.J. Risk factors for recurrent melioidosis in northeast Thailand. Clin. Infect. Dis. 2006, 43, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Songsri, J.; Chatatikun, M.; Wisessombat, S.; Mala, W.; Phothaworn, P.; Senghoi, W.; Palachum, W.; Chanmol, W.; Intakhan, N.; Chuaijit, S.; et al. Diagnostic accuracy of automation and non-automation techniques for identifying Burkholderia pseudomallei: A systematic review and meta-analysis. J. Infect. Public Health 2024, 17, 102438. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Ashdown, L.R. Serial serum C-reactive protein levels as an aid to the management of melioidosis. Am. J. Trop. Med. Hyg. 1992, 46, 151–157. [Google Scholar] [CrossRef]
- Cheng, A.C.; Obrien, M.; Jacups, S.P.; Anstey, N.M.; Currie, B.J. C-reactive protein in the diagnosis of melioidosis. Am. J. Trop. Med. Hyg. 2004, 70, 580–582. [Google Scholar] [CrossRef]
- Cheng, A.C.; Currie, B.J. Serum C-reactive protein and liver disease in patients with melioidosis. Intensive Care Med. 2007, 33, 562. [Google Scholar] [CrossRef]
- Natesan, M.; Corea, E.; Krishnananthasivam, S.; Sathkumara, H.D.; Dankmeyer, J.L.; Dyas, B.K.; Amemiya, K.; De Silva, A.D.; Ulrich, R.G. Calprotectin as a Biomarker for Melioidosis Disease Progression and Management. J. Clin. Microbiol. 2017, 55, 1205–1210. [Google Scholar] [CrossRef]
- Hui, H.; Sheng, Y.; Han, X.; Wang, S.; Zhang, G.; Wei, X. A clinical study on clinical features, manifestations and drug resistance of melioidosis. Pak. J. Med. Sci. 2022, 38, 2301–2306. [Google Scholar] [CrossRef]
- Menon, R.; Baby, P.; Kumar V, A.; Surendran, S.; Pradeep, M.; Rajendran, A.; Suju, G.; Ashok, A. Risk Factors for Mortality in Melioidosis: A Single-Centre, 10-Year Retrospective Cohort Study. Sci. World J. 2021, 2021, 8154810. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Kuang, S.; Zhong, C.; Zhou, J.; Long, W.; Xiao, S.; Wu, B. Risk Factors for Melioidosis Mortality and Epidemics: A Multicentre, 10-Year Retrospective Cohort Study in Northern Hainan. Infect. Dis. Ther. 2023, 12, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.W.; Chung, K.M.; Chen, C.H.; Cheung, B.M. Bacteremic melioidosis in southern Taiwan: Clinical characteristics and outcome. J. Formos. Med. Assoc. 2007, 106, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Mouliou, D.S. C-Reactive Protein: Pathophysiology, Diagnosis, False Test Results and a Novel Diagnostic Algorithm for Clinicians. Diseases 2023, 11, 132. [Google Scholar] [CrossRef]
- Le Turnier, P.; Bonifay, T.; Mosnier, E.; Schaub, R.; Jolivet, A.; Demar, M.; Bourhy, P.; Nacher, M.; Djossou, F.; Epelboin, L. Usefulness of C-Reactive Protein in Differentiating Acute Leptospirosis and Dengue Fever in French Guiana. Open Forum Infect. Dis. 2019, 6, ofz323. [Google Scholar] [CrossRef]
- Maillard, O.; Hirschinger, D.; Bénéteau, S.; Koumar, Y.; Vague, A.; Girerd, R.; DiAscia, L.; Jabot, J.; Cousty, J.; Randrianjohany, A.; et al. C-reactive protein: An easy marker for early differentiation between leptospirosis and dengue fever in endemic area. PLoS ONE 2023, 18, e0285900. [Google Scholar] [CrossRef]
- Lin, I.F.; Lin, J.N.; Tsai, C.T.; Wu, Y.Y.; Chen, Y.H.; Lai, C.H. Serum C-reactive protein and procalcitonin values in acute Q fever, scrub typhus, and murine typhus. BMC Infect. Dis. 2020, 20, 334. [Google Scholar] [CrossRef]
- Kılınç Toker, A.; Çelik, İ.; Özdemir, A.T.; Sağlam, H.; Koçer, D.; Eşlik, M.; Toker, İ. The value of C-reactive protein velocity (CRPv) on mortality in sepsis patients who are emergently hospitalized in the ICU: A retrospective single-center study. Heliyon 2024, 10, e38797. [Google Scholar] [CrossRef]
- Song, C.; Hu, Z.; Zhang, J. The value of lymphocyte-to-C-reactive protein ratio for predicting clinical outcomes in patients with sepsis in intensive care unit: A retrospective single-center study. Front. Mol. Biosci. 2024, 11, 1429372. [Google Scholar] [CrossRef]
- Abakar, M.A.A.; Hamad, D.H.A.; Faisal, E.; Omer, H.M.F.; Faki, M.T.M.; Idris, A.E.M.; Omer, R.; Osman, Z.; Elhassan, E.A.G.; Abrahim-Holie, M.A.; et al. Comparative analysis of immunological biomarkers in COVID-19 and bacterial pneumonia. J. Med. Life 2023, 16, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Peisajovich, A.; Marnell, L.; Mold, C.; Du Clos, T.W. C-reactive protein at the interface between innate immunity and inflammation. Expert Rev. Clin. Immunol. 2008, 4, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Wilairatana, P.; Mahannop, P.; Tussato, T.; Hayeedoloh, I.M.; Boonhok, R.; Klangbud, W.K.; Mala, W.; Kotepui, K.U.; Kotepui, M. C-reactive protein as an early biomarker for malaria infection and monitoring of malaria severity: A meta-analysis. Sci. Rep. 2021, 11, 22033. [Google Scholar] [CrossRef]
- Bassuk, S.S.; Rifai, N.; Ridker, P.M. High-sensitivity C-reactive protein: Clinical importance. Curr. Probl. Cardiol. 2004, 29, 439–493. [Google Scholar]
- Weiss, N.; Vierbaum, L.; Kremser, M.; Kaufmann-Stoeck, A.; Kappler, S.; Ballert, S.; Kabrodt, K.; Hunfeld, K.P.; Schellenberg, I. Longitudinal evaluation of manufacturer-specific differences for high-sensitive CRP EQA results. Front. Mol. Biosci. 2024, 11, 1401405. [Google Scholar] [CrossRef]

| First Author (Ref.) | Year of Publication | Study Site | Study Design | Year Conducted | Participant Description | Number of Cases | % Male | Age (Mean ± SD or Range) Year | CRP Level (Mean ± SD or Median (IQR)) | CRP Type | Notes: Basis for Classification and Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ashdown [8] | 1992 | Darwin, Australia | Prospective observational | 1989–1990 | Patients admitted with culture-confirmed melioidosis | 65 | NR | NR | Median = 154.0 mg/L (range 9.0–352.0) | Standard CRP | Inferred from era and high-range reporting. Author reported Abbott® TDx® automate |
| Cheng [9] | 2004 | Darwin, Australia | Retrospective cohort | 1995–2002 | Patients with confirmed melioidosis and other febrile illnesses | 116 | NR | NR | Median 164.0 mg/L(IQR) of 59.0–286.0 mg/L | Standard CRP | Author-reported immunoturbidimetric assay |
| Chou [15] | 2007 | Taiwan | Case series | 2002–2006 | Melioidosis cases from ICU | 30 | 80% | Mean 63.7 years | Mean ± SD = 19.55 ± 12.65 mg/L | Standard CRP | Inferred from clinical context and range. Severe cases, small sample |
| Hui [12] | 2022 | Malaysia | Retrospective cohort | 2015–2019 | Culture-confirmed melioidosis patients | 46 | 67.4% | 54.5 ± 14.3 years | Median = 104.0 mg/L (IQR 52.9–155.1) | Standard CRP | Assumed (no hs-CRP label; high median values). Reported CRP trends with severity |
| Menon [13] | 2021 | Kochi, India | Retrospective cohort | 2011–2020 | Hospitalized melioidosis cases | 73 | NR | NR | # Mean ± SD = 126.46 ± 89.01 mg/L | Standard CRP | Assumed from clinical context; manufacturer not specified. Logistic regression showed CRP as mortality predictor |
| Natesan [11] | 2017 | Sri Lanka | Prospective observational | 2014–2015 | Confirmed case of melioidosis with diabetes (74%), alcoholism (10%), kidney disease (10%), and other comorbidities (20%). Blood serum samples were collected during the acute and eradication phases of the antibiotic treatment | 31 | 74% | Mean 50 (range 32–74) | ## Acute phase median = 9.7 mg/L (IQR 6.6–18.6); eradication median = 2.9 mg/L (IQR 6.7–8.6) | Standard CRP | Assumed from clinical context. Author-reported “enzyme-linked immunosorbent assay (ELISA)”. Also evaluated calprotectin |
| Zheng [14] | 2023 | Hainan, China | Retrospective cohort | 2010–2020 | Multicenter hospitalized cases | 90 | 87.8% | Range 0–81 | hs-CRP: mean ± SD = 149.57 ± 13.65 mg/L | hs-CRP | Author-reported “hs-CRP”. Explored rainfall correlations. Procalcitonin level of 1.31 (0.39, 6.21) ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phongphithakchai, A.; Chatatikun, M.; Tangpong, J.; Laklaeng, S.-n.; Huang, J.C.; Wongyikul, P.; Phinyo, P.; Thanasai, J.; Khemla, S.; Chanthot, C.; et al. C-Reactive Protein for Early Diagnosis and Severity Monitoring in Melioidosis: A Systematic Review and Meta-Analysis. Life 2025, 15, 1360. https://doi.org/10.3390/life15091360
Phongphithakchai A, Chatatikun M, Tangpong J, Laklaeng S-n, Huang JC, Wongyikul P, Phinyo P, Thanasai J, Khemla S, Chanthot C, et al. C-Reactive Protein for Early Diagnosis and Severity Monitoring in Melioidosis: A Systematic Review and Meta-Analysis. Life. 2025; 15(9):1360. https://doi.org/10.3390/life15091360
Chicago/Turabian StylePhongphithakchai, Atthaphong, Moragot Chatatikun, Jitabanjong Tangpong, Sa-ngob Laklaeng, Jason C. Huang, Pakpoom Wongyikul, Phichayut Phinyo, Jongkonnee Thanasai, Supphachoke Khemla, Chaimongkhon Chanthot, and et al. 2025. "C-Reactive Protein for Early Diagnosis and Severity Monitoring in Melioidosis: A Systematic Review and Meta-Analysis" Life 15, no. 9: 1360. https://doi.org/10.3390/life15091360
APA StylePhongphithakchai, A., Chatatikun, M., Tangpong, J., Laklaeng, S.-n., Huang, J. C., Wongyikul, P., Phinyo, P., Thanasai, J., Khemla, S., Chanthot, C., Chittamma, A., & Klangbud, W. K. (2025). C-Reactive Protein for Early Diagnosis and Severity Monitoring in Melioidosis: A Systematic Review and Meta-Analysis. Life, 15(9), 1360. https://doi.org/10.3390/life15091360








