Cytotoxic Effects of Thymus serpyllum L. and Mentha × piperita L. Essential Oils on Basal Cell Carcinoma—An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Essential Oil Extraction
2.2. Gas Chromatography–Mass Spectroscopic (GC-MS) Analysis
2.3. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.4. Cell Cultures
2.5. MTT Cytotoxicity Assay
2.6. Neutral Red Assay
2.7. Colony Forming Assay
2.8. Spheroid Formation Assay
2.9. Scratch Wound Healing ASSAY
2.10. RNA Extraction
2.11. Gene Expression Analysis of Signaling Markers
Statistical Analysis
3. Results
3.1. Gas Chromatography–Mass Spectroscopic (GC-MS) Analysis
3.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
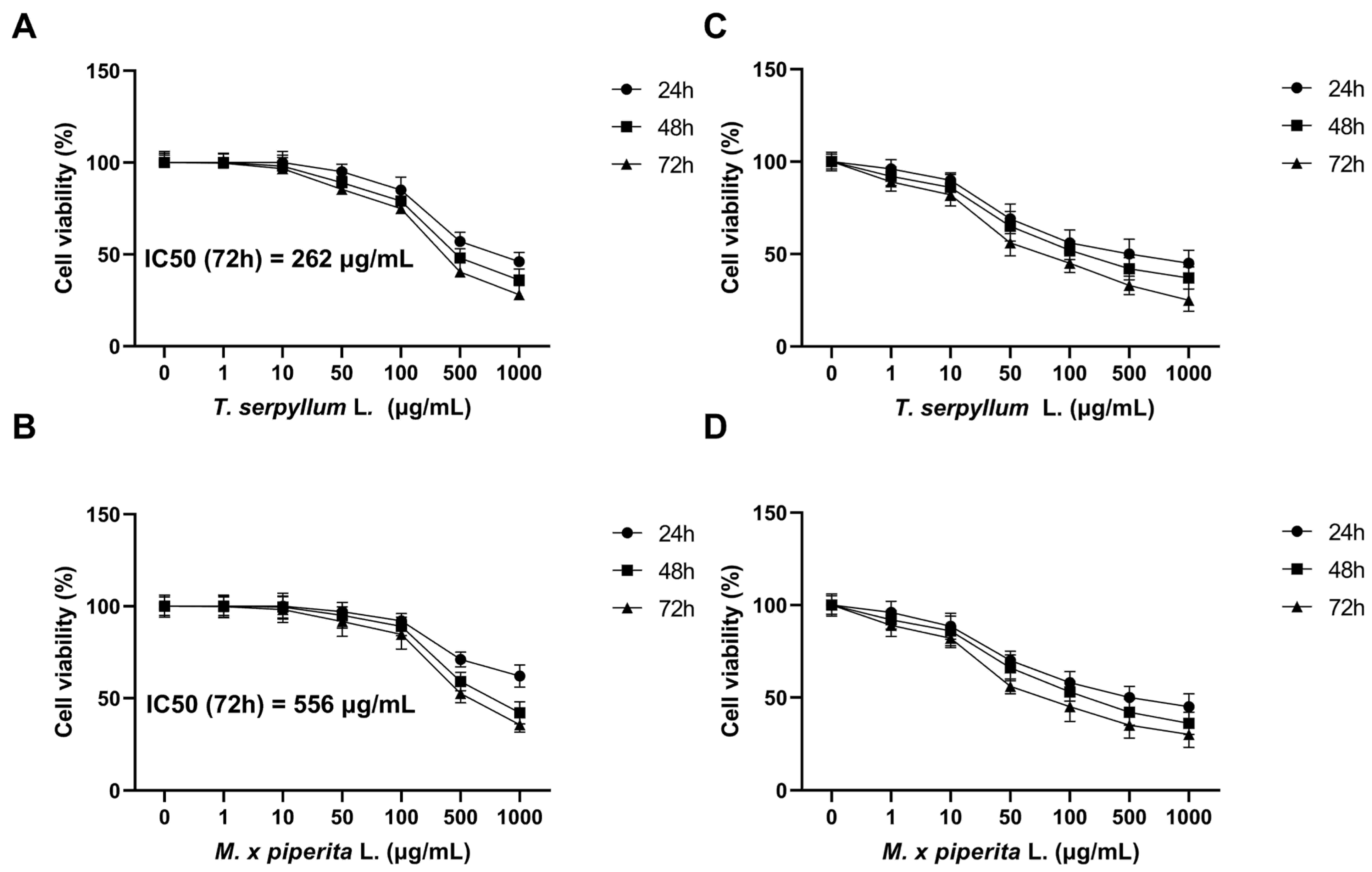
3.3. MTT Assay
3.4. Neutral Red (NR) Assay
3.5. Colony Forming Assay
3.6. Spheroid Formation Assay
3.7. Scratch Wound Healing Assay
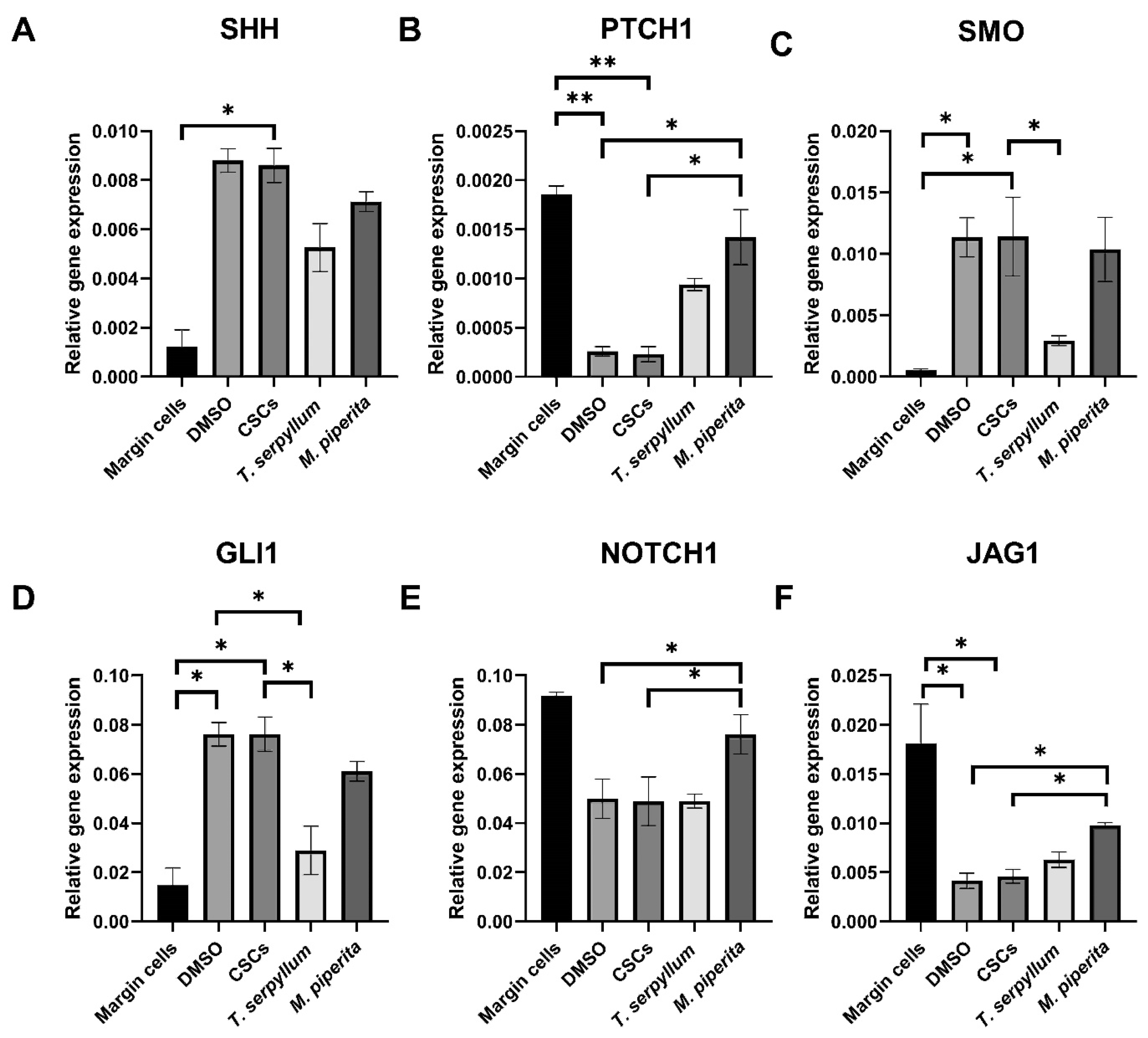
3.8. Gene Expression Analysis: Sonic Hedgehog and Notch Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Burden of Disease 2019 Cancer Collaboration; Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Lai, V.; Cranwell, W.; Sinclair, R. Epidemiology of Skin Cancer in the Mature Patient. Clin. Dermatol. 2018, 36, 167–176. [Google Scholar] [CrossRef]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal Cell Carcinoma: Epidemiology; Pathophysiology; Clinical and Histological Subtypes; and Disease Associations. J. Am. Acad. Dermatol. 2019, 80, 303–317. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Savarese, I.; Gori, A.; Scarfi, F.; Topa, A.; Trane, L.; Portelli, F.; Innocenti, A.; Covarelli, P. Advanced Basal Cell Carcinoma: When a Good Drug Is Not Enough. J. Dermatolog. Treat. 2020, 31, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Basset-Seguin, N.; Herms, F. Update in the Management of Basal Cell Carcinoma. Acta Derm. Venereol. 2020, 100, adv00140. [Google Scholar] [CrossRef] [PubMed]
- Milenković, A.D.; Milenković, V.; Petrovic, M.; Tomic, A.; Matejic, A.; Brkic, N.; Jovanović, M. Infiltrative Basal Cell Carcinoma of the Head: Factors Influencing Bone Invasion and Surgical Outcomes. Life 2025, 15, 551. [Google Scholar] [CrossRef]
- Kappelin, J.; Green, A.C.; Ingvar, Å.; Ahnlide, I.; Nielsen, K. Incidence and Trends of Basal Cell Carcinoma in Sweden: A Population-Based Registry Study. Br. J. Dermatol. 2022, 186, 963–969. [Google Scholar] [CrossRef]
- Schreuder, K.; Hollestein, L.; Nijsten, T.E.C.; Wakkee, M.; Louwman, M.W.J. A Nationwide Study of the Incidence and Trends of First and Multiple Basal Cell Carcinomas in the Netherlands and Prediction of Future Incidence. Br. J. Dermatol. 2022, 186, 476–484. [Google Scholar] [CrossRef]
- Castrisos, G.; Lewandowski, R. Narrative Review of the Epidemiology/Biology of Basal Cell Carcinoma: A Need for Public Health Consensus. ANZ J. Surg. 2021, 91, 1098–1103. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer Stem Cells Revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Dimitrijevic, M.; Brasanac, D.; Todorovic, N.; Petrovic, M.; Dimitrijevic, A. Basal Cell Carcinoma—Principles of Treatment. Srp. Arh. Celok. Lek. 2023, 151, 98–105. [Google Scholar] [CrossRef]
- Epstein, E.H. Basal Cell Carcinomas: Attack of the Hedgehog. Nat. Rev. Cancer 2008, 8, 743–754. [Google Scholar] [CrossRef]
- Cortes, J.E.; Gutzmer, R.; Kieran, M.W.; Solomon, J.A. Hedgehog Signaling Inhibitors in Solid and Hematological Cancers. Cancer Treat. Rev. 2019, 76, 41–50. [Google Scholar] [CrossRef]
- Ng, J.M.Y.; Curran, T. The Hedgehog’s Tale: Developing Strategies for Targeting Cancer. Nat. Rev. Cancer 2011, 11, 493–501. [Google Scholar] [CrossRef]
- Milosevic, M.; Lazarevic, M.; Toljic, B.; Petrovic, M.; Vukadinovic, M.; Jezdic, Z.; Anicic, B.; Jelovac, D.; Jovanovic, S.; Milasin, J. Basal Cell Carcinoma Stem Cells Exhibit Osteogenic and Chondrogenic Differentiation Potential. Biocell 2021, 45, 1543–1550. [Google Scholar] [CrossRef]
- Jain, R.; Dubey, S.K.; Singhvi, G. The Hedgehog Pathway and Its Inhibitors: Emerging Therapeutic Approaches for Basal Cell Carcinoma. Drug Discov. Today 2022, 27, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Armbruster, H.; Pardo, G.; Archambeau, B.; Kim, N.H.; Jeter, J.; Wu, R.; Kendra, K.; Contreras, C.M.; Spaccarelli, N.; et al. Hedgehog Pathway Inhibitors for Locally Advanced and Metastatic Basal Cell Carcinoma: A Real-World Single-Center Retrospective Review. PLoS ONE 2024, 19, e0297531. [Google Scholar] [CrossRef] [PubMed]
- Eberl, M.; Mangelberger, D.; Swanson, J.B.; Verhaegen, M.E.; Harms, P.W.; Frohm, M.L.; Dlugosz, A.A.; Wong, S.Y. Tumor Architecture and Notch Signaling Modulate Drug Response in Basal Cell Carcinoma. Cancer Cell 2018, 33, 229–243.e4. [Google Scholar] [CrossRef]
- Nickoloff, B.J.; Qin, J.-Z.; Chaturvedi, V.; Denning, M.F.; Bonish, B.; Miele, L. Jagged-1 Mediated Activation of Notch Signaling Induces Complete Maturation of Human Keratinocytes through NF-κB and PPARγ. Cell Death Differ. 2002, 9, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Weaver, K.L.; Capobianco, A.J. Notch Signalling in Solid Tumours: A Little Bit of Everything but Not All the Time. Nat. Rev. Cancer 2011, 11, 338–351. [Google Scholar] [CrossRef]
- Nicolas, M.; Wolfer, A.; Raj, K.; Kummer, J.A.; Mill, P.; van Noort, M.; Hui, C.; Clevers, H.; Dotto, G.P.; Radtke, F. Notch1 Functions as a Tumor Suppressor in Mouse Skin. Nat. Genet. 2003, 33, 416–421. [Google Scholar] [CrossRef]
- Tampa, M.; Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Scheau, C.; Constantin, C.; Neagu, M. Recent Advances in Signaling Pathways Comprehension as Carcinogenesis Triggers in Basal Cell Carcinoma. J. Clin. Med. 2020, 9, 3010. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, Anti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential Oils as Anticancer Agents: Potential Role in Malignancies, Drug Delivery Mechanisms, and Immune System Enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid. Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential Oils and Their Constituents as Anticancer Agents: A Mechanistic View. Biomed. Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. Evid Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef]
- Jalil, B.; Pischel, I.; Feistel, B.; Suarez, C.; Blainski, A.; Spreemann, R.; Roth-Ehrang, R.; Heinrich, M. Wild thyme (Thymus serpyllum L.): A Review of the Current Evidence of Nutritional and Preventive Health Benefits. Front. Nutr. 2024, 11, 1380962. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint Essential Oil: Its Phytochemistry, Biological Activity, Pharmacological Effect and Application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Dolghi, A.; Coricovac, D.; Dinu, S.; Pinzaru, I.; Dehelean, C.A.; Grosu, C.; Chioran, D.; Merghes, P.E.; Sarau, C.A. Chemical and Antimicrobial Characterization of Mentha piperita L. and Rosmarinus Officinalis L. Essential Oils and In Vitro Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells. Molecules 2022, 27, 6106. [Google Scholar] [CrossRef]
- Ateeq-ur-Rehman; Mannan, A.; Inayatullah, S.; Akhtar, M.Z.; Qayyum, M.; Mirza, B. Biological Evaluation of Wild thyme (Thymus serpyllum). Pharm. Biol. 2009, 47, 628–633. [Google Scholar] [CrossRef]
- Sfaei-Ghomi, J.; Meshkatalsadat, M.H.; Shamai, S.; Hasheminejad, M.; Hassani, A. Chemical characterization of bioactive volatile molecules of four thymus species using nanoscale injection method. Dig. J. Nanomater. Biostruct. 2009, 4, 835–841. [Google Scholar]
- Jaksic Karisik, M.; Lazarevic, M.; Mitic, D.; Milosevic Markovic, M.; Riberti, N.; Jelovac, D.; Milasin, J. MicroRNA-21 as a Regulator of Cancer Stem Cell Properties in Oral Cancer. Cells 2025, 14, 91. [Google Scholar] [CrossRef]
- Raal, A.; Paaver, U.; Arak, E.; Orav, A. Content and Composition of the Essential Oil of Thymus serpyllum L. Growing Wild in Estonia. Medicina 2004, 40, 795–800. [Google Scholar] [PubMed]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Exploitation of Cytotoxicity of Some Essential Oils for Translation in Cancer Therapy. Evid. Based Complement. Altern. Med. 2015, 2015, 397821. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical Composition, Antimicrobial, Antioxidant and Antitumor Activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. Essential Oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Preljević, K.; Pašić, I.; Vlaović, M.; Matić, I.Z.; Krivokapić, S.; Petrović, N.; Stanojković, T.; Živković, V.; Perović, S. Comparative Analysis of Chemical Profiles, Antioxidant, Antibacterial, and Anticancer Effects of Essential Oils of Two Thymus Species from Montenegro. Fitoterapia 2024, 174, 105871. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential Oils of Aromatic Plants with Antibacterial, Antifungal, Antiviral, and Cytotoxic Properties—An Overview. Forsch. Komplementmed. 2009, 16, 79–90. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A.; Prajapati, K.S.; Patel, S.; Kumar, S.; Jaitak, V. Chemical Composition, In Vitro and In Silico Evaluation of Essential Oil Extracted from Mentha piperita L. for Lung Cancer. Lett. Drug Des. Discov. 2023, 21, 3018–3029. [Google Scholar] [CrossRef]
- Abedinpour, N.; Ghanbariasad, A.; Taghinezhad, A.; Osanloo, M. Preparation of Nanoemulsions of Mentha piperita Essential Oil and Investigation of Their Cytotoxic Effect on Human Breast Cancer Lines. BioNanoScience 2021, 11, 428–436. [Google Scholar] [CrossRef]
- Valderrama, A.C.S.; De, G.C.R. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. Am. J. Anal. Chem. 2017, 8, 726–741. [Google Scholar] [CrossRef]
- Skala, D.; Žizović, I.; Petrović, S.S. Etarska ulja—Destilacija, ekstrakcija, izbor tehnologije i kvalitet. Hem. Ind. 1999, 53, 123–138. [Google Scholar]
- Lazarević, M.; Milošević, M.; Petrović, N.; Petrović, S.; Damante, G.; Milašin, J.; Milovanović, B. Cytotoxic Effects of Different Aromatic Plants Essential Oils on Oral Squamous Cell Carcinoma: An In Vitro Study. Balk. J. Dent. Med. 2019, 23, 73–79. [Google Scholar] [CrossRef]
- Milosevic, M.; Lazarevic, M.; Toljic, B.; Simonovic, J.; Trisic, D.; Nikolic, N.; Petrovic, M.; Milasin, J. Characterization of Stem-like Cancer Cells in Basal Cell Carcinoma and Its Surgical Margins. Exp. Dermatol. 2018, 27, 1160–1165. [Google Scholar] [CrossRef]
- Grando, S.A.; Schofield, O.M.; Skubitz, A.P.; Kist, D.A.; Zelickson, B.D.; Zachary, C.B. Nodular Basal Cell Carcinoma in Vivo vs In Vitro. Establishment of Pure Cell Cultures, Cytomorphologic Characteristics, Ultrastructure, Immunophenotype, Biosynthetic Activities, and Generation of Antisera. Arch. Dermatol. 1996, 132, 1185–1193. [Google Scholar] [CrossRef]
- Bozkurt, E.; Atmaca, H.; Kisim, A.; Uzunoglu, S.; Uslu, R.; Karaca, B. Effects of Thymus Serpyllum Extract on Cell Proliferation, Apoptosis and Epigenetic Events in Human Breast Cancer Cells. Nutr. Cancer 2012, 64, 1245–1250. [Google Scholar] [CrossRef]
- Mancic, L.; Djukic-Vukovic, A.; Dinic, I.; Nikolic, M.G.; Rabasovic, M.D.; Krmpot, A.J.; Costa, A.M.L.M.; Trisic, D.; Lazarevic, M.; Mojovic, L.; et al. NIR Photo-Driven Upconversion in NaYF4:Yb,Er/PLGA Particles for In Vitro Bioimaging of Cancer Cells. Mater. Sci. Eng. C 2018, 91, 597–605. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Shaheen, S.; Ahmed, M.; Lorenzi, F.; Nateri, A.S. Spheroid-Formation (Colonosphere) Assay for In Vitro Assessment and Expansion of Stem Cells in Colon Cancer. Stem Cell Rev. Rep. 2016, 12, 492–499. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In Vitro Cell Migration and Invasion Assays. J. Vis. Exp. 2014, 88, 51046. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Tranquillo, E.; Sapio, L.; Illiano, M.; Caiafa, I.; Naviglio, S. Chemical Analysis and Anti-Proliferative Activity of Campania Thymus Vulgaris Essential Oil. J. Essent. Oil Res. 2017, 29, 461–470. [Google Scholar] [CrossRef]
- Boukhira, S.; Amrati, F.E.-Z.; Chebaibi, M.; Grafov, A.; Mothana, R.A.; Al-Yousef, H.M.; Bousta, D. The Chemical Composition and the Preservative, Antimicrobial, and Antioxidant Effects of Thymus Broussonetii Boiss. Essential Oil: An In Vitro and in Silico Approach. Front. Chem. 2024, 12, 1402310. [Google Scholar] [CrossRef] [PubMed]
- Jayasekher, A.; Panchariya, P.C.; Maurelli, F.; Prajapati, D.; Palit, A.K. Authentication of Mentha Arvensis Essential Oil Using Attenuated Total Reflectance-Fourier Transform Infrared Spectrophotometry Coupled with Chemometrics. J. Food Compos. Anal. 2024, 135, 106576. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative Approaches for Cancer Treatment: Current Perspectives and New Challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Baig, S.; Ahmad, B.A.; Azizan, A.H.S.; Ali, H.M.; Rouhollahi, E. Hexane Extract of Thymus serpyllum L.: GC-MS Profile, Antioxidant Potential and Anticancer Impact on HepG2 (Liver Carcinoma) Cell Line. Int. J. Pharmacol. Pharm. Sci. 2014, 8, 90765. [Google Scholar]
- Baig, S.; Azizan, A.H.S.; Raghavendran, H.R.B.; Natarajan, E.; Naveen, S.; Murali, M.R.; Nam, H.Y.; Kamarul, T. Effect of Chitosan Nanoparticle-Loaded Thymus serpyllum on Hydrogen Peroxide-Induced Bone Marrow Stromal Cell Damage. Stem Cells Int. 2019, 2019, 5142518. [Google Scholar] [CrossRef]
- Kumar, V.; Bhattarai, S. Isolation and Characterization of Thymus serpyllum L. Essential Oil and Mass Fragmentation Analysis of Major Constituents. J. Essent. Oil Res. 2024, 36, 441–459. [Google Scholar] [CrossRef]
- Shanaida, M.; Hudz, N.; Białoń, M.; Kryvtsowa, M.; Svydenko, L.; Filipska, A.; Paweł Wieczorek, P. Chromatographic Profiles and Antimicrobial Activity of the Essential Oils Obtained from Some Species and Cultivars of the Mentheae tribe (Lamiaceae). Saudi. J. Biol. Sci. 2021, 28, 6145–6152. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Aćimović, M.; Cvetković, M.; Stanković, J.; Igić, R.; Todosijević, M.; Vuković, D.; Brašanac, D. Essential oil composition of the Thymus serpyllum L. from Kopaonik mountain. J. Agron. Technol. Eng. Manag. 2019, 2, 241–247. [Google Scholar]
- Stanisavljević, D.; Zlatković, B.; Ristić, M.; Veličković, D.; Đorđević, S.; Lazić, M. The chemical composition of the essential oil of (Thymus serpyllum L.) from Kopaonik Mountain. Adv. Technol. 2012, 1, 25–29. [Google Scholar]
- Ghasemi Pirbalouti, A.; Hashemi, M.; Ghahfarokhi, F.T. Essential Oil and Chemical Compositions of Wild and Cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind. Crops Prod. 2013, 48, 43–48. [Google Scholar] [CrossRef]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef] [PubMed]
- Marwa, C.; Fikri-Benbrahim, K.; Ou-Yahia, D.; Farah, A. African Peppermint (Mentha piperita) from Morocco: Chemical Composition and Antimicrobial Properties of Essential Oil. J. Adv. Pharm. Technol. Res. 2017, 8, 86. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical Composition and Anti-Inflammatory, Cytotoxic and Antioxidant Activities of Essential Oil from Leaves of Mentha piperita Grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef]
- Lim, H.-W.; Kim, H.; Kim, J.; Bae, D.; Song, K.-Y.; Chon, J.-W.; Lee, J.-M.; Kim, S.-H.; Kim, D.-H.; Seo, K.-H. Antimicrobial Effect of Mentha piperita (Peppermint) Oil against Bacillus Cereus, Staphylococcus Aureus, Cronobacter Sakazakii, and Salmonella Enteritidis in Various Dairy Foods: Preliminary Study. J. Dairy Sci. Biotechnol. 2018, 36, 146–154. [Google Scholar] [CrossRef]
- Ilić, D.P.; Stanojević, J.S.; Cvetković, D.J.; Ristić, I.S.; Nikolić, V.D. Grinding of Serbian Peppermint (Mentha × piperita L.) leaves: Variations regarding yield, composition and antimicrobial activity of isolated essential oil. Adv. Technol. 2022, 11, 5–12. [Google Scholar] [CrossRef]
- Nilo, M.C.S.; Riachi, L.G.; Simas, D.L.R.; Coleho, G.C.; Da Silva, A.J.R.; Costa, D.C.M.; Alviano, D.S.; Alviano, C.S.; De Maria, C.A.B. Chemical Composition and Antioxidant and Antifungal Properties of Mentha × piperita L. (Peppermint) and Mentha arvensis L. (Cornmint) Samples. Food Res. 2017, 1, 147–156. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Liu, W.; Liu, E.; Pang, Y.; Gao, H.; He, Q.; Liao, W.; Yao, Y.; Zeng, J.; et al. Menthol: An Underestimated Anticancer Agent. Front. Pharmacol. 2023, 14, 1148790. [Google Scholar] [CrossRef]
- Fatima, K.; Masood, N.; Ahmad Wani, Z.; Meena, A.; Luqman, S. Neomenthol Prevents the Proliferation of Skin Cancer Cells by Restraining Tubulin Polymerization and Hyaluronidase Activity. J. Adv. Res. 2021, 34, 93–107. [Google Scholar] [CrossRef]
- Nowak, A.; Kalemba, D.; Krala, L.; Piotrowska, M.; Czyzowska, A. The Effects of Thyme (Thymus vulgaris) and Rosemary (Rosmarinus officinalis) Essential Oils on Brochothrix Thermosphacta and on the Shelf Life of Beef Packaged in High-Oxygen Modified Atmosphere. Food Microbiol. 2012, 32, 212–216. [Google Scholar] [CrossRef]
- Sadowska, U.; Matwijczuk, A.; Niemczynowicz, A.; Dróżdż, T.; Żabiński, A. Spectroscopic Examination and Chemometric Analysis of Essential Oils Obtained from Peppermint Herb (Mentha piperita L.) and Caraway Fruit (Carum carvi L.) Subjected to Pulsed Electric Fields. Processes 2019, 7, 466. [Google Scholar] [CrossRef]
- Surapaneni, A.; Surapaneni, A.; Wu, J.; Bajaj, A.; Reyes, K.; Adwankar, R.; Vittaladevuni, A.; Njoo, E. Kinetic Monitoring and Fourier-Transform Infrared (FTIR) Spectroscopy of the Green Oxidation of (−)-Menthol to (−)-Menthone. J. Emerg. Investig. 2020, 3, 1. [Google Scholar] [CrossRef]
- Taylan, O.; Cebi, N.; Sagdic, O. Rapid Screening of Mentha Spicata Essential Oil and L-Menthol in Mentha piperita Essential Oil by ATR-FTIR Spectroscopy Coupled with Multivariate Analyses. Foods 2021, 10, 202. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Gegechkori, V.; Morton, D.W. Anxiolytic Terpenoids and Aromatherapy for Anxiety and Depression. Adv. Exp. Med. Biol. 2020, 1260, 283–296. [Google Scholar] [CrossRef]
- Elmastaş, M.; Dermirtas, I.; Isildak, O.; Aboul-Enein, H.Y. Antioxidant activity of S-carvone isolated from spearmint (Mentha spicata L. Fam Lamiaceae). J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1465–1475. [Google Scholar] [CrossRef]
- Al-Bayati, F.A. Isolation and Identification of Antimicrobial Compound from Mentha Longifolia L. Leaves Grown Wild in Iraq. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Natesan, R.; Samiappan, S.C.; Ramalingam, S. Anti-Oxidant, Anti-Bacterial and Anti-Cancer Activity of Mentha piperita Against Mcf-7 Cells. Biomed. Pharmacol. J. 2021, 14, 1685–1693. [Google Scholar] [CrossRef]
- Alexa, E.; Danciu, C.; Radulov, I.; Obistioiu, D.; Sumalan, R.M.; Morar, A.; Dehelean, C.A. Phytochemical Screening and Biological Activity of Mentha × piperita L. and Lavandula angustifolia Mill. Extracts. Anal. Cell Pathol. 2018, 2018, 2678924. [Google Scholar] [CrossRef]
- Aldoghachi, F.E.H.; Almousawei, U.M.N.; Shari, F.H. In Vitro Anticancer Activity of RA Extracts of Peppermint Leaves against Human Cancer Breast and Cervical Cancer Cells. In Vitro 2022, 45, 3467–3479. [Google Scholar]
- Safinejad, K.; Mohebifar, A.; Tolouei, H.; Monfared, P.; Razmjou, A. Comparative study on the toxicity of Mentha piperita L. And Artemisia dracunculus L. Hydroalcoholic extracts on human breast cancer cell lines. Int. J. Biol. Biotechnol. 2021, 18, 253–261. [Google Scholar]
- Jain, D.; Pathak, N.; Khan, S.; Raghuram, G.V.; Bhargava, A.; Samarth, R.; Mishra, P.K. Evaluation of Cytotoxicity and Anticarcinogenic Potential of Mentha Leaf Extracts. Int. J. Toxicol. 2011, 30, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Umar, H.; Aliyu, M.R.; Ozsahin, D.U. Iron Oxide Nanoparticles Synthesized using Mentha Spicata extract and Evaluation of Its Antibacterial, Cytotoxicity and Antimigratory Potential on Highly Metastatic Human Breast Cells. Biomed. Phys. Eng. Express 2024, 10, 035019. [Google Scholar] [CrossRef] [PubMed]
- Brinkhuizen, T.; Reinders, M.G.; van Geel, M.; Hendriksen, A.J.L.; Paulussen, A.D.C.; Winnepenninckx, V.J.; Keymeulen, K.B.; Soetekouw, P.M.M.B.; van Steensel, M.A.M.; Mosterd, K. Acquired Resistance to the Hedgehog Pathway Inhibitor Vismodegib Due to Smoothened Mutations in Treatment of Locally Advanced Basal Cell Carcinoma. J. Am. Acad. Dermatol. 2014, 71, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Gailani, M.R.; Ståhle-Bäckdahl, M.; Leffell, D.J.; Glyn, M.; Zaphiropoulos, P.G.; Undén, A.B.; Dean, M.; Brash, D.E.; Bale, A.E.; Toftgård, R. The Role of the Human Homologue of Drosophila Patched in Sporadic Basal Cell Carcinomas. Nat. Genet. 1996, 14, 78–81. [Google Scholar] [CrossRef]
- Xie, J.; Murone, M.; Luoh, S.M.; Ryan, A.; Gu, Q.; Zhang, C.; Bonifas, J.M.; Lam, C.W.; Hynes, M.; Goddard, A.; et al. Activating Smoothened Mutations in Sporadic Basal-Cell Carcinoma. Nature 1998, 391, 90–92. [Google Scholar] [CrossRef]
- Pietrobono, S.; Gagliardi, S.; Stecca, B. Non-Canonical Hedgehog Signaling Pathway in Cancer: Activation of GLI Transcription Factors Beyond Smoothened. Front. Genet. 2019, 10, 556. [Google Scholar] [CrossRef]
- Gambini, D.; Passoni, E.; Nazzaro, G.; Beltramini, G.; Tomasello, G.; Ghidini, M.; Kuhn, E.; Garrone, O. Basal Cell Carcinoma and Hedgehog Pathway Inhibitors: Focus on Immune Response. Front. Med. 2022, 9, 893063. [Google Scholar] [CrossRef]
- Shi, F.-T.; Yu, M.; Zloty, D.; Bell, R.H.; Wang, E.; Akhoundsadegh, N.; Leung, G.; Haegert, A.; Carr, N.; Shapiro, J.; et al. Notch Signaling Is Significantly Suppressed in Basal Cell Carcinomas and Activation Induces Basal Cell Carcinoma Cell Apoptosis. Mol. Med. Rep. 2017, 15, 1441–1454. [Google Scholar] [CrossRef]
- Rampal, R.; Arboleda-Velasquez, J.F.; Nita-Lazar, A.; Kosik, K.S.; Haltiwanger, R.S. Highly Conserved O-Fucose Sites Have Distinct Effects on Notch1 Function. J. Biol. Chem. 2005, 280, 32133–32140. [Google Scholar] [CrossRef]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. Cytotoxicity of Thymus Vulgaris Essential Oil towards Human Oral Cavity Squamous Cell Carcinoma. Anticancer Res. 2011, 31, 81–87. [Google Scholar]
- Zeng, Q.; Che, Y.; Zhang, Y.; Chen, M.; Guo, Q.; Zhang, W. Thymol Isolated from Thymus Vulgaris L. Inhibits Colorectal Cancer Cell Growth and Metastasis by Suppressing the Wnt/β-Catenin Pathway. Drug Des. Dev. Ther. 2020, 14, 2535–2547. [Google Scholar] [CrossRef] [PubMed]
- Pietrobono, S.; Santini, R.; Gagliardi, S.; Dapporto, F.; Colecchia, D.; Chiariello, M.; Leone, C.; Valoti, M.; Manetti, F.; Petricci, E.; et al. Targeted Inhibition of Hedgehog-GLI Signaling by Novel Acylguanidine Derivatives Inhibits Melanoma Cell Growth by Inducing Replication Stress and Mitotic Catastrophe. Cell Death Dis. 2018, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Carrera, C.; Mariscal, A.; Malvehy, J.; Puig, S. Long-Term Complete Remission of Cutaneous Melanoma Metastases in Association with a Folk Remedy. J. Am. Acad. Dermatol. 2005, 52, 713–715. [Google Scholar] [CrossRef]
- Mikheil, D.; Prabhakar, K.; Ng, T.L.; Teertam, S.; Longley, B.J.; Newton, M.A.; Setaluri, V. Notch Signaling Suppresses Melanoma Tumor Development in BRAF/Pten Mice. Cancers 2023, 15, 519. [Google Scholar] [CrossRef]
- Kumar, A.; Samarth, R.M.; Yasmeen, S.; Sharma, A.; Sugahara, T.; Terado, T.; Kimura, H. Anticancer and Radioprotective Potentials of Mentha piperita. BioFactors 2004, 22, 87–91. [Google Scholar] [CrossRef]
- Wulandari, M.A.M.; Wiraguna, A.A.G.P.; Pangkahila, W. Peppermint Leaf Extract Cream Increased Transforming Growth Factor β (TGF-β) Expression and Collagen Amount In The Male Wistar Rat’s Skin Exposed to UVB. J. Ilm. Permas. J. Ilm. STIKES Kendal 2023, 13, 849–856. [Google Scholar] [CrossRef]
- Athar, M.; Tang, X.; Lee, J.L.; Kopelovich, L.; Kim, A.L. Hedgehog Signalling in Skin Development and Cancer. Exp. Dermatol. 2006, 15, 667–677. [Google Scholar] [CrossRef]
- Fujita, Y.; Biswas, K.B.; Kawai, Y.; Takayama, S.; Masutani, T.; Iddamalgoda, A.; Sakamoto, K. Mentha piperita leaf extract suppresses the release of ATP from epidermal keratinocytes and reduces dermal thinning as well as wrinkle formation. Int. J. Cosmet. Sci. 2024, 46, 972–981. [Google Scholar] [CrossRef]



| Product Name | Sequences (5′→3′) | |
|---|---|---|
| SHH | Forward | GAAAGCAGAGAACTCGGTGG |
| Reverse | GGAAAGTGAGGAAGTCGCTG | |
| PTCH1 | Forward | GGGTGGCACAGTCAAGAACAG |
| Reverse | TACCCCTTGAAGTGCTCGTACA | |
| SMO | Forward | GGGAGGCTACTTCCTCATCC |
| Reverse | GGCAGCTGAAGGTAATGAGC | |
| GLI1 | Forward | GAAGACCTCTCCAGCTTGGA |
| Reverse | GGCTGACAGTATAGGCAGAG | |
| Notch 1 | Forward | AGCCTCAACATCCCCTACAA |
| Reverse | CCACGAAGAACAGAAGCACA | |
| JAG1 | Forward | CGGGATTTGGTTAATGGTTATC |
| Reverse | ATAGTCACTGGCACGGTTGTAGCAC | |
| GAPDH | Forward | TCATGACCACAGTCCATGCCATCA |
| Reverse | CCCTGTTGCTGTAGCCAAATTCGT |
| No. | Compound | RI | R.T. (min) | Relative Percentage (%) |
|---|---|---|---|---|
| 1 | Tricyclene | 921 | 5.569 | 0.15 |
| 2 | α-Pinene | 931 | 5.859 | 1.13 |
| 3 | β-Fenchene | 935 | 5.978 | 0.02 |
| 4 | α-Fenchene | 944 | 6.228 | 0.04 |
| 5 | Camphene | 945 | 6.269 | 2.33 |
| 6 | trans-p-Menthane | 972 | 7.007 | 0.13 |
| 7 | β-Pinene | 974 | 7.074 | 0.27 |
| 8 | Myrcene | 988 | 7.459 | 0.94 |
| 9 | p-Mentha-1(7),8-diene | 1003 | 7.917 | 0.06 |
| 10 | 1,4-Cineole | 1013 | 8.283 | 0.07 |
| 11 | p-Cymene | 1023 | 8.670 | 23.56 |
| 12 | Limonene | 1026 | 8.776 | 0.62 |
| 13 | 1,8-Cineole | 1029 | 8.866 | 4.98 |
| 14 | γ-Terpinene | 1057 | 9.885 | 0.02 |
| 15 | cis-Linalool oxide (furanoid) | 1069 | 10.37 | 0.21 |
| 16 | trans-Linalool oxide (furanoid) | 1086 | 10.996 | 0.20 |
| 17 | Linalool | 1099 | 11.469 | 5.84 |
| 18 | endo-Fenchol | 1112 | 12.009 | 0.01 |
| 19 | cis-β-Terpineol | 1143 | 13.315 | 0.06 |
| 20 | Isoborneol | 1153 | 13.821 | 0.39 |
| 21 | Borneol | 1162 | 14.206 | 0.66 |
| 22 | Terpinen-4-ol | 1174 | 14.727 | 0.73 |
| 23 | α-Terpineol | 1189 | 15.334 | 0.61 |
| 24 | Linalyl acetate | 1257 | 18.336 | 0.07 |
| 25 | Thymol | 1295 | 20.056 | 50.47 |
| 26 | Carvacrol | 1301 | 20.327 | 3.18 |
| 27 | Neryl acetate | 1365 | 23.058 | 0.02 |
| 28 | α-Copaene | 1375 | 23.542 | 0.06 |
| 29 | Geranyl acetate | 1383 | 23.876 | 0.43 |
| 30 | Longifolene | 1405 | 24.807 | 0.01 |
| 31 | (E)-Caryophyllene | 1419 | 25.414 | 0.08 |
| 32 | α-Humulene | 1454 | 26.858 | 0.01 |
| 33 | δ-Cadinene | 1524 | 29.751 | 0.05 |
| 34 | Caryophyllene oxide | 1582 | 32.158 | 2.28 |
| 35 | Humulene oxide II | 1608 | 33.186 | 0.23 |
| 36 | 14-hydroxy-(Z)-Caryophyllene | 1670 | 35.575 | 0.07 |
| No. | Compound | RI | R.T. (min) | Relative Percentage (%) |
|---|---|---|---|---|
| 1 | α-Thujene | 926 | 5.688 | 0.02 |
| 2 | α-Pinene | 931 | 5.864 | 0.80 |
| 3 | Camphene | 946 | 6.283 | 0.02 |
| 4 | Thuja-2,4(10)-diene | 951 | 6.431 | 0.02 |
| 5 | Sabinene | 970 | 6.965 | 0.65 |
| 6 | β-Pinene | 974 | 7.078 | 1.11 |
| 7 | Myrcene | 989 | 7.476 | 0.16 |
| 8 | α-Phellandrene | 997 | 7.600 | 0.02 |
| 9 | α-Terpinene | 1016 | 8.380 | 0.02 |
| 10 | p-Cymene | 1022 | 8.627 | 0.23 |
| 11 | Limonene | 1026 | 8.764 | 1.91 |
| 12 | 1,8-Cineole | 1028 | 8.856 | 6.13 |
| 13 | (Z)-beta-Ocimene | 1034 | 9.080 | 0.12 |
| 14 | γ-Terpinene | 1056 | 9.879 | 0.06 |
| 15 | cis-Sabinene hydrate | 1064 | 10.171 | 0.12 |
| 16 | Terpinolene | 1088 | 11.055 | 0.02 |
| 17 | Linalool | 1099 | 11.464 | 0.12 |
| 18 | 2-Methylbutyl 2-methylbutanoate | 1103 | 11.628 | 0.05 |
| 19 | cis-Thujone | 1105 | 11.708 | 0.14 |
| 20 | trans-Thujone | 1115 | 12.186 | 0.03 |
| 21 | trans-Sabinol | 1139 | 13.109 | 0.04 |
| 22 | Camphor | 1142 | 13.330 | 0.06 |
| 23 | neo-Isopulegol | 1144 | 13.397 | 0.09 |
| 24 | Menthone | 1155 | 13.890 | 53.66 |
| 25 | Menthofuran | 1162 | 14.194 | 6.53 |
| 26 | iso-Menthone | 1163 | 14.245 | 6.64 |
| 27 | Menthol | 1172 | 14.623 | 13.52 |
| 28 | Terpinen-4-ol | 1176 | 14.777 | 0.58 |
| 29 | iso-Menthol | 1181 | 15.018 | 0.14 |
| 30 | α-Terpineol | 1189 | 15.343 | 0.05 |
| 31 | Myrtenal | 1195 | 15.595 | 0.02 |
| 32 | Pulegone | 1237 | 17.467 | 1.68 |
| 33 | Piperitone | 1252 | 18.118 | 0.54 |
| 34 | neo-Menthyl acetate | 1273 | 19.086 | 0.08 |
| 35 | Bornyl acetate | 1284 | 19.559 | 0.03 |
| 36 | Dihydroedulan | 1287 | 19.673 | 0.04 |
| 37 | Menthyl acetate | 1292 | 19.927 | 1.27 |
| 38 | iso-Menthyl acetate | 1307 | 20.568 | 0.05 |
| 39 | Menthofurolactone 1 | 1346 | 22.258 | 0.06 |
| 40 | Menthofurolactone 2 | 1348 | 22.367 | 0.07 |
| 41 | β-Bourbonene | 1385 | 23.938 | 0.09 |
| 42 | β-Elemene | 1392 | 24.265 | 0.04 |
| 43 | (E)-Caryophyllene | 1419 | 25.422 | 1.58 |
| 44 | α-Humulene | 1454 | 26.86 | 0.26 |
| 45 | (E)-β-Farnesene | 1458 | 27.020 | 0.03 |
| 46 | Germacrene D | 1482 | 28.020 | 0.61 |
| 47 | Bicyclogermacrene | 1497 | 28.668 | 0.12 |
| 48 | δ-Cadinene | 1524 | 29.766 | 0.05 |
| 49 | Spathulenol | 1577 | 31.933 | 0.02 |
| 50 | Caryophyllene oxide | 1582 | 32.142 | 0.12 |
| 51 | Viridiflorol | 1591 | 32.500 | 0.12 |
| 52 | 6-methoxy-Elemicin | 1598 | 32.773 | 0.02 |
| 53 | Humulene epoxide II | 1608 | 33.200 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milosevic Markovic, M.; Anicic, B.; Lazarevic, M.; Jaksic Karisik, M.; Mitic, D.; Milovanovic, B.; Ivanovic, S.; Pecinar, I.; Petrovic, M.; Petrovic, M.; et al. Cytotoxic Effects of Thymus serpyllum L. and Mentha × piperita L. Essential Oils on Basal Cell Carcinoma—An In Vitro Study. Life 2025, 15, 1296. https://doi.org/10.3390/life15081296
Milosevic Markovic M, Anicic B, Lazarevic M, Jaksic Karisik M, Mitic D, Milovanovic B, Ivanovic S, Pecinar I, Petrovic M, Petrovic M, et al. Cytotoxic Effects of Thymus serpyllum L. and Mentha × piperita L. Essential Oils on Basal Cell Carcinoma—An In Vitro Study. Life. 2025; 15(8):1296. https://doi.org/10.3390/life15081296
Chicago/Turabian StyleMilosevic Markovic, Maja, Boban Anicic, Milos Lazarevic, Milica Jaksic Karisik, Dijana Mitic, Branislav Milovanovic, Stefan Ivanovic, Ilinka Pecinar, Milan Petrovic, Masa Petrovic, and et al. 2025. "Cytotoxic Effects of Thymus serpyllum L. and Mentha × piperita L. Essential Oils on Basal Cell Carcinoma—An In Vitro Study" Life 15, no. 8: 1296. https://doi.org/10.3390/life15081296
APA StyleMilosevic Markovic, M., Anicic, B., Lazarevic, M., Jaksic Karisik, M., Mitic, D., Milovanovic, B., Ivanovic, S., Pecinar, I., Petrovic, M., Petrovic, M., Markovic, N., Bojic, M., Petrovic, N., Petrovic, S., & Milasin, J. (2025). Cytotoxic Effects of Thymus serpyllum L. and Mentha × piperita L. Essential Oils on Basal Cell Carcinoma—An In Vitro Study. Life, 15(8), 1296. https://doi.org/10.3390/life15081296










