A Comprehensive Review of the Role of Rho-Kinase Inhibitors in Corneal Diseases
Abstract
1. Introduction
2. Literature Search Strategy and Review Methodology
3. Mechanism of Action
4. Pharmacological Properties
5. Safety Profile
5.1. Conjunctival Hyperaemia
5.2. Blepharitis and Periocular Irritation
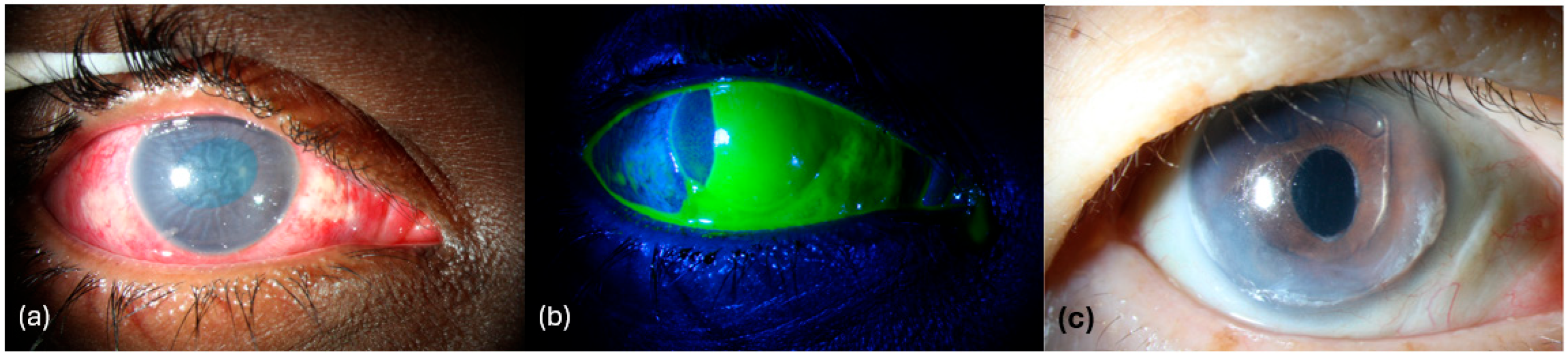
5.3. Corneal Verticillata (Vortex Keratopathy)
5.4. Conjunctival Haemorrhages
5.5. Honeycomb Keratopathy
5.6. Other Mild Ocular Effects
5.7. Serious Adverse Events
5.8. Contraindications and Precautions
6. Corneal Oedema
6.1. Fuchs’ Endothelial Corneal Dystrophy
6.1.1. Pathophysiology
6.1.2. Mechanistic Rationale for ROCKI
6.1.3. Preclinical Evidence
6.1.4. Clinical Studies
6.1.5. Summary and Limitations
6.2. Pseudophakic Bullous Keratopathy
6.2.1. Pathophysiology
6.2.2. Mechanistic Rationale for ROCKI
6.2.3. Preclinical Evidence
6.2.4. Clinical Studies
6.2.5. Summary and Limitations
6.3. Iridocorneal Endothelial Syndrome
6.3.1. Pathophysiology
6.3.2. Mechanistic Rationale for ROCKI
6.3.3. Preclinical Evidence
6.3.4. Clinical Studies
6.3.5. Summary and Limitations
7. Corneal Neovascularization
7.1. Pathophysiology
7.2. Mechanistic Rationale for ROCKI
7.3. Preclinical Evidence
7.4. Clinical Studies
7.5. Summary and Limitations
8. Post-Cataract Surgery Corneal Healing
8.1. Pathophysiology
8.2. Mechanistic Rationale for ROCKI
8.3. Preclinical Evidence
8.4. Clinical Studies
8.5. Summary and Limitations
9. Corneal Fibrosis and Wound Healing
9.1. Pathophysiology
9.2. Mechanistic Rationale for ROCKI
9.3. Preclinical Evidence
9.4. Clinical Studies
9.5. Summary
10. Post-Corneal Transplantation (Graft Survival and Immune Modulation)
10.1. Pathophysiology
10.2. Mechanistic Rationale for ROCKI
10.3. Preclinical Evidence
10.4. Clinical Studies
10.5. Summary and Limitations
11. Emerging Therapies (ROCKI + Cell Therapy, Sustained Delivery)
12. Limitations and Future Directions
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROCK | Rho-associated protein kinase |
| ROCKI | Rho-associated protein kinase inhibitor |
| CEC | Corneal endothelial cell |
| FECD | Fuchs endothelial corneal dystrophy |
| PBK | Pseudophakic bullous keratopathy |
| RBD | Rho-binding domain |
| MLC | Myosin light chain |
| MYPT1 | Myosin phosphatase target subunit |
| α-SMA | Alpha smooth muscle actin |
| DSO | Descemet stripping only |
| NF-κB | Nuclear factor kappa B |
| IL | Interleukin |
| TNF-α | Tumour necrosis factor alpha |
| TGF-β | Transforming growth factor beta |
| TLR4 | Toll like receptor 4 |
| Treg | Regulatory T-cells |
| Th17 | T helper 17 |
| VEGF | Vascular endothelial growth factor |
| IOP | Intraocular pressure |
| NET | Norepinephrine transporter |
| DSAEK | Descemet stripping automated endothelial keratoplasty |
| EK | Endothelial keratoplasty |
| DMEK | Descemet membrane endothelial keratoplasty |
| EMT | Epithelial–mesenchymal transition |
| CCT | Central corneal thickness |
| BCVA | Best corrected visual acuity |
| ECD | Endothelial cell density |
| CDVA | Corrected distance visual acuity |
| ICE | Iridocorneal endothelial syndrome |
| Na+/K+-ATPase | Sodium–potassium adenosine triphosphatase |
| ZO-1 | Zonula occludens-1 |
References
- Moshirfar, M.; Parker, L.; Birdsong, O.C.; Ronquillo, Y.C.; Hofstedt, D.; Shah, T.J.; Gomez, A.T.; Hoopes, P.C., Sr. Use of Rho Kinase Inhibitors in Ophthalmology: A Review of the Literature. Med. Hypothesis Discov. Innov. Ophthalmol. 2018, 7, 101–111. [Google Scholar]
- Yin, J.; Yu, F.-S.X. Rho Kinases Regulate Corneal Epithelial Wound Healing. Am. J. Physiol. Cell Physiol. 2008, 295, C378–C387. [Google Scholar] [CrossRef]
- Meekins, L.C.; Rosado-Adames, N.; Maddala, R.; Zhao, J.J.; Rao, P.V.; Afshari, N.A. Corneal Endothelial Cell Migration and Proliferation Enhanced by Rho Kinase (ROCK) Inhibitors in In Vitro and In Vivo Models. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6731–6738. [Google Scholar] [CrossRef] [PubMed]
- Petroll, W.M.; Lakshman, N. Fibroblastic Transformation of Corneal Keratocytes by Rac Inhibition Is Modulated by Extracellular Matrix Structure and Stiffness. J. Funct. Biomater. 2015, 6, 222–240. [Google Scholar] [CrossRef]
- Pagano, L.; Lee, J.W.; Posarelli, M.; Giannaccare, G.; Kaye, S.; Borgia, A. ROCK Inhibitors in Corneal Diseases and Glaucoma—A Comprehensive Review of These Emerging Drugs. J. Clin. Med. 2023, 12, 6736. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U.; Zenkel, M.; Strunz, M.; Gießl, A.; Schondorf, H.; da Silva, H.; Schmidt, G.A.; Greiner, M.A.; Okumura, N.; Koizumi, N.; et al. Potential Functional Restoration of Corneal Endothelial Cells in Fuchs Endothelial Corneal Dystrophy by ROCK Inhibitor (Ripasudil). Am. J. Ophthalmol. 2021, 224, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Moloney, G.; Garcerant Congote, D.; Hirnschall, N.; Arsiwalla, T.; Luiza Mylla Boso, A.; Toalster, N.; D’Souza, M.; Devasahayam, R.N. Descemet Stripping Only Supplemented with Topical Ripasudil for Fuchs Endothelial Dystrophy 12-Month Outcomes of the Sydney Eye Hospital Study. Cornea 2021, 40, 320–326. [Google Scholar] [CrossRef]
- Rao, P.V.; Pattabiraman, P.P.; Kopczynski, C. Role of the Rho GTPase/Rho Kinase Signaling Pathway in Pathogenesis and Treatment of Glaucoma: Bench to Bedside Research. Exp. Eye Res. 2017, 158, 23–32. [Google Scholar] [CrossRef]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-Kinase/ROCK: A Key Regulator of the Cytoskeleton and Cell Polarity. Cytoskelet 2010, 67, 545–554. [Google Scholar] [CrossRef]
- Julian, L.; Olson, M.F. Rho-Associated Coiled-Coil Containing Kinases (ROCK). Small GTPases 2014, 5, e29846. [Google Scholar] [CrossRef]
- Liao, J.K.; Seto, M.; Noma, K. Rho Kinase (ROCK) Inhibitors. J. Cardiovasc. Pharmacol. 2007, 50, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Umetsu, A.; Furuhashi, M.; Watanabe, M.; Tsugeno, Y.; Suzuki, S.; Hikage, F.; Ohguro, H. ROCK 1 and 2 Affect the Spatial Architecture of 3D Spheroids Derived from Human Corneal Stromal Fibroblasts in Different Manners. Sci. Rep. 2022, 12, 7419. [Google Scholar] [CrossRef] [PubMed]
- Croze, R.H.; Thi, W.J.; Clegg, D.O. ROCK Inhibition Promotes Attachment, Proliferation, and Wound Closure in Human Embryonic Stem Cell–Derived Retinal Pigmented Epithelium. Transl. Vis. Sci. Technol. 2016, 5, 7. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Gao, H.; Nabeka, H.; Shimokawa, T.; Wakisaka, H.; Matsuda, S.; Kobayashi, N. Rho Kinase Inhibitors Stimulate the Migration of Human Cultured Osteoblastic Cells by Regulating Actomyosin Activity. Cell Mol. Biol. Lett. 2011, 16, 279–295. [Google Scholar] [CrossRef]
- Yamamoto, M.; Quantock, A.J.; Young, R.D.; Okumura, N.; Ueno, M.; Sakamoto, Y.; Kinoshita, S.; Koizumi, N. A Selective Inhibitor of the Rho Kinase Pathway, Y-27632, and Its Influence on Wound Healing in the Corneal Stroma. Mol. Vis. 2012, 18, 1727–1739. [Google Scholar] [PubMed]
- Petroll, W.M.; Miron-Mendoza, M. Mechanical Interactions and Crosstalk between Corneal Keratocytes and the Extracellular Matrix. Exp. Eye Res. 2015, 133, 49–57. [Google Scholar] [CrossRef]
- Okumura, N.; Koizumi, N.; Kay, E.P.; Ueno, M.; Sakamoto, Y.; Nakamura, S.; Hamuro, J.; Kinoshita, S. The ROCK Inhibitor Eye Drop Accelerates Corneal Endothelium Wound Healing. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2493–2502. [Google Scholar] [CrossRef]
- Okumura, N.; Fujii, K.; Kagami, T.; Makiko, N.; Kitahara, M.; Kinoshita, S.; Koizumi, N. Activation of the Rho/Rho Kinase Signaling Pathway Is Involved in Cell Death of Corneal Endothelium. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6843–6851. [Google Scholar] [CrossRef]
- Okumura, N.; Nakano, S.; Kay, E.P.; Numata, R.; Ota, A.; Sowa, Y.; Sakai, T.; Ueno, M.; Kinoshita, S.; Koizumi, N. Involvement of Cyclin D and P27 in Cell Proliferation Mediated by ROCK Inhibitors Y-27632 and Y-39983 during Corneal Endothelium Wound Healing. Investig. Ophthalmol. Vis. Sci. 2014, 55, 318–329. [Google Scholar] [CrossRef]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Tsuchiya, H.; Hamuro, J.; Kinoshita, S. ROCK Inhibitor Converts Corneal Endothelial Cells into a Phenotype Capable of Regenerating in Vivo Endothelial Tissue. Am. J. Pathol. 2012, 181, 268–277. [Google Scholar] [CrossRef]
- Okumura, N.; Inoue, R.; Okazaki, Y.; Nakano, S.; Nakagawa, H.; Kinoshita, S.; Koizumi, N. Effect of the Rho Kinase Inhibitor Y-27632 on Corneal Endothelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6067–6074. [Google Scholar] [CrossRef]
- Parekh, M.; Miall, A.; Chou, A.; Buhl, L.; Deshpande, N.; Price, M.O.; Price, F.W.; Jurkunas, U.V. Enhanced Migration of Fuchs Corneal Endothelial Cells by Rho Kinase Inhibition: A Novel Ex Vivo Descemet’s Stripping Only Model. Cells 2024, 13, 1218. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Guan, L.; Tian, P.; Li, C.; Zhang, Y. Rho Kinase Type 1 (ROCK1) Promotes Lipopolysaccharide-Induced Inflammation in Corneal Epithelial Cells by Activating Toll-Like Receptor 4 (TLR4)-Mediated Signaling. Med. Sci. Monit. 2018, 24, 3514–3523. [Google Scholar] [CrossRef]
- Słoniecka, M.; Danielson, P. Substance P Induces Fibrotic Changes through Activation of the RhoA/ROCK Pathway in an in Vitro Human Corneal Fibrosis Model. J. Mol. Med. 2019, 97, 1477–1489. [Google Scholar] [CrossRef]
- Ho, W.-T.; Chang, J.-S.; Lei, C.-J.; Chen, T.-C.; Wang, J.-K.; Chang, S.-W.; Yang, M.-H.; Jou, T.-S.; Wang, I.-J. ROCK Inhibitor Enhances Resilience Against Metabolic Stress Through Increasing Bioenergetic Capacity in Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2025, 66, 51. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Feng, Y.; Li, X.; Lu, Z.; Gu, H.; Li, W.; Hill, L.J.; Ou, S. Evolution of Therapeutic Strategy Based on Oxidant-Antioxidant Balance for Fuchs Endothelial Corneal Dystrophy. Ocul. Surf. 2024, 34, 247–261. [Google Scholar] [CrossRef]
- Li, S.; Zhang, P.; Li, A.; Bao, J.; Pan, Z.; Jie, Y. Rho-Kinase Inhibitor Alleviates CD4+T Cell Mediated Corneal Graft Rejection by Modulating Its STAT3 and STAT5 Activation. Exp. Eye Res. 2024, 242, 109857. [Google Scholar] [CrossRef]
- Inomata, T.; Fujimoto, K.; Okumura, Y.; Zhu, J.; Fujio, K.; Shokirova, H.; Miura, M.; Okano, M.; Funaki, T.; Sung, J.; et al. Novel Immunotherapeutic Effects of Topically Administered Ripasudil (K-115) on Corneal Allograft Survival. Sci. Rep. 2020, 10, 19817. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Pi, R.; Li, P.; Chen, R.; Lin, L.; He, H.; Zhou, S. Fasudil Hydrochloride, a Potent ROCK Inhibitor, Inhibits Corneal Neovascularization after Alkali Burns in Mice. Mol. Vis. 2015, 21, 688–698. [Google Scholar] [PubMed]
- Renault, M.-A.; Roncalli, J.; Tongers, J.; Thorne, T.; Klyachko, E.; Misener, S.; Volpert, O.V.; Mehta, S.; Burg, A.; Luedemann, C.; et al. Sonic Hedgehog Induces Angiogenesis via Rho Kinase-Dependent Signaling in Endothelial Cells. J. Mol. Cell. Cardiol. 2010, 49, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Dang, Y. Rho-Kinase Inhibitors as Emerging Targets for Glaucoma Therapy. Ophthalmol. Ther. 2023, 12, 2943–2957. [Google Scholar] [CrossRef]
- Al-Humimat, G.; Marashdeh, I.; Daradkeh, D.; Kooner, K. Investigational Rho Kinase Inhibitors for the Treatment of Glaucoma. J. Exp. Pharmacol. 2021, 13, 197–212. [Google Scholar] [CrossRef]
- Inamoto, Y.; Kato, K.; Kawakita, T.; Onishi, Y.; Matsuoka, K.-I.; Shiratori, S.; Ikegame, K.; Hiramoto, N.; Toyosaki, M.; Katayama, Y.; et al. An Open-Label Study of Belumosudil, a Selective ROCK2 Inhibitor, as Second or Subsequent Line of Therapy for Steroid-Dependent/Steroid-Resistant Chronic GVHD. Am. J. Hematol. 2024, 99, 1917–1926. [Google Scholar] [CrossRef]
- Ishizaki, T.; Uehata, M.; Tamechika, I.; Keel, J.; Nonomura, K.; Maekawa, M.; Narumiya, S. Pharmacological Properties of Y-27632, a Specific Inhibitor of Rho-Associated Kinases. Mol. Pharmacol. 2000, 57, 976–983. [Google Scholar] [CrossRef]
- Bond, L.M.; Sellers, J.R.; McKerracher, L. Rho Kinase as a Target for Cerebral Vascular Disorders. Future Med. Chem. 2015, 7, 1039–1053. [Google Scholar] [CrossRef]
- Hahmann, C.; Schroeter, T. Rho-Kinase Inhibitors as Therapeutics: From Pan Inhibition to Isoform Selectivity. Cell. Mol. Life Sci. 2009, 67, 171–177. [Google Scholar] [CrossRef]
- ROCK Inhibitor Y-27632 Dihydrochloride|Rho-Kinase Inhibitor|Tocris. Available online: https://www.tocris.com/products/y-27632-dihydrochloride_1254 (accessed on 18 May 2025).
- Muto, T.; Machida, S. Intravitreal Fasudil for Treatment of Diabetic Macular Edema with an Unfavorable Response. Semin. Ophthalmol. 2022, 37, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Kasai, T.; Kawai, H. Ocular Penetration and Pharmacokinetics of Ripasudil Following Topical Administration to Rabbits. J. Ocul. Pharmacol. Ther. 2016, 32, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Ramirez, N.; Novack, G.D.; Kopczynski, C. Ocular Hypotensive Safety and Systemic Absorption of AR-13324 Ophthalmic Solution in Normal Volunteers. Am. J. Ophthalmol. 2015, 159, 980–985.e1. [Google Scholar] [CrossRef]
- Ismaiel, Z.F.; Abdel Hamed, M.A.; Fawzy, S.M. The Future of Glaucoma Treatment Ripasudil. Egypt. J. Hosp. Med. 2017, 68, 1184–1188. [Google Scholar] [CrossRef]
- Maruyama, Y.; Ikeda, Y.; Mori, K.; Yoshii, K.; Ueno, M.; Yoshikawa, H.; Sotozono, C.; Kinoshita, S. Morphological Change and Recovery of Corneal Endothelial Cells after Rho-Associated Protein Kinase Inhibitor Eye-Drop (Ripasudil 0.4%) Instillation. Br. J. Ophthalmol. 2021, 105, 169–173. [Google Scholar] [CrossRef]
- Lin, C.-W.; Sherman, B.; Moore, L.A.; Laethem, C.L.; Lu, D.-W.; Pattabiraman, P.P.; Rao, P.V.; deLong, M.A.; Kopczynski, C.C. Discovery and Preclinical Development of Netarsudil, a Novel Ocular Hypotensive Agent for the Treatment of Glaucoma. J. Ocul. Pharmacol. Ther. 2018, 34, 40–51. [Google Scholar] [CrossRef]
- Patel, P.; Patel, B.C. Netarsudil Ophthalmic Solution. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Hirata, K.; Torii, R.; Hamuro, J.; Kinoshita, S. Enhancement of Corneal Endothelium Wound Healing by Rho-Associated Kinase (ROCK) Inhibitor Eye Drops. Br. J. Ophthalmol. 2011, 95, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Colby, K.A.; Kruse, F.E. A Close Look at the Clinical Efficacy of Rho-Associated Protein Kinase Inhibitor Eye Drops for Fuchs Endothelial Corneal Dystrophy. Cornea 2021, 40, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inatani, M.; Honjo, M.; Tokushige, H.; Azuma, J.; Araie, M. Intraocular Pressure-Lowering Effects and Safety of Topical Administration of a Selective ROCK Inhibitor, SNJ-1656, in Healthy Volunteers. Arch. Ophthalmol. 2008, 126, 309–315. [Google Scholar] [CrossRef]
- PubChem Y-39983. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11507964 (accessed on 18 May 2025).
- Hsu, C.-R.; Chen, Y.-H.; Liu, C.-P.; Chen, C.-H.; Huang, K.-K.; Huang, J.-W.; Lin, M.-N.; Lin, C.-L.; Chen, W.-R.; Hsu, Y.-L.; et al. A Highly Selective Rho-Kinase Inhibitor (ITRI-E-212) Potentially Treats Glaucoma Upon Topical Administration With Low Incidence of Ocular Hyperemia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 624–633. [Google Scholar] [CrossRef]
- Mulder, I.A.; Abbinanti, M.; Woller, S.A.; Ruschel, J.; Coutinho, J.M.; de Vries, H.E.; van Bavel, E.; Rosen, K.; McKerracher, L.; Ayata, C. The Novel ROCK2 Selective Inhibitor NRL-1049 Preserves the Blood-Brain Barrier after Acute Injury. J. Cereb. Blood Flow Metab. 2024, 44, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Ohta, M.; Kaneko, Y.; Kawai, H. Species Differences in Metabolism of Ripasudil (K-115) Are Attributed to Aldehyde Oxidase. Xenobiotica 2016, 46, 579–590. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A Comprehensive Insight On Ocular Pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Mietzner, R.; Kade, C.; Froemel, F.; Pauly, D.; Stamer, W.D.; Ohlmann, A.; Wegener, J.; Fuchshofer, R.; Breunig, M. Fasudil Loaded PLGA Microspheres as Potential Intravitreal Depot Formulation for Glaucoma Therapy. Pharmaceutics 2020, 12, 706. [Google Scholar] [CrossRef]
- Stein, S.; Auel, T.; Kempin, W.; Bogdahn, M.; Weitschies, W.; Seidlitz, A. Influence of the Test Method on in Vitro Drug Release from Intravitreal Model Implants Containing Dexamethasone or Fluorescein Sodium in Poly (d,l-Lactide-Co-Glycolide) or Polycaprolactone. Eur. J. Pharm. Biopharm. 2018, 127, 270–278. [Google Scholar] [CrossRef]
- Khallaf, A.M.; El-Moslemany, R.M.; Ahmed, M.F.; Morsi, M.H.; Khalafallah, N.M. Exploring a Novel Fasudil-Phospholipid Complex Formulated as Liposomal Thermosensitive In Situ Gel for Glaucoma. Int. J. Nanomed. 2022, 17, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Araie, M.; Sugiyama, K.; Aso, K.; Kanemoto, K.; Iwata, R.; Hollander, D.A.; Senchyna, M.; Kopczynski, C.C. Phase 3 Clinical Trial Comparing the Safety and Efficacy of Netarsudil to Ripasudil in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension: Japan Rho Kinase Elevated Intraocular Pressure Treatment Trial (J-ROCKET). Adv. Ther. 2023, 40, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Serle, J.B.; Katz, L.J.; McLaurin, E.; Heah, T.; Ramirez-Davis, N.; Usner, D.W.; Novack, G.D.; Kopczynski, C.C.; ROCKET-1 and ROCKET-2 Study Groups. Two Phase 3 Clinical Trials Comparing the Safety and Efficacy of Netarsudil to Timolol in Patients with Elevated Intraocular Pressure: Rho Kinase Elevated IOP Treatment Trial 1 and 2 (ROCKET-1 and ROCKET-2). Am. J. Ophthalmol. 2018, 186, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, E.; Ishida, W.; Sumi, T.; Kishimoto, T.; Tada, K.; Fukuda, K.; Yoneda, T.; Kuroiwa, H.; Terao, E.; Fujisawa, Y.; et al. Evaluation of Offset of Conjunctival Hyperemia Induced by a Rho-Kinase Inhibitor; 0.4% Ripasudil Ophthalmic Solution Clinical Trial. Sci. Rep. 2019, 9, 3755. [Google Scholar] [CrossRef]
- Asrani, S.; Bacharach, J.; Holland, E.; McKee, H.; Sheng, H.; Lewis, R.A.; Kopczynski, C.C.; Heah, T. Fixed-Dose Combination of Netarsudil and Latanoprost in Ocular Hypertension and Open-Angle Glaucoma: Pooled Efficacy/Safety Analysis of Phase 3 MERCURY-1 and -2. Adv. Ther. 2020, 37, 1620–1631. [Google Scholar] [CrossRef]
- Saito, H.; Kagami, S.; Mishima, K.; Mataki, N.; Fukushima, A.; Araie, M. Long-Term Side Effects Including Blepharitis Leading to Discontinuation of Ripasudil. J. Glaucoma 2019, 28, 289. [Google Scholar] [CrossRef]
- Tanihara, H.; Kakuda, T.; Sano, T.; Kanno, T.; Kurihara, Y. Long-Term Intraocular Pressure-Lowering Effects and Adverse Events of Ripasudil in Patients with Glaucoma or Ocular Hypertension over 24 Months. Adv. Ther. 2022, 39, 1659–1677. [Google Scholar] [CrossRef]
- Walters, T.R.; Ahmed, I.I.K.; Lewis, R.A.; Usner, D.W.; Lopez, J.; Kopczynski, C.C.; Heah, T. Once-Daily Netarsudil/Latanoprost Fixed-Dose Combination for Elevated Intraocular Pressure in the Randomized Phase 3 MERCURY-2 Study. Ophthalmol. Glaucoma 2019, 2, 280–289. [Google Scholar] [CrossRef]
- Singh, I.P.; Fechtner, R.D.; Myers, J.S.; Kim, T.; Usner, D.W.; McKee, H.; Sheng, H.; Lewis, R.A.; Heah, T.; Kopczynski, C.C. Pooled Efficacy and Safety Profile of Netarsudil Ophthalmic Solution 0.02% in Patients with Open-Angle Glaucoma or Ocular Hypertension. J. Glaucoma 2020, 29, 878. [Google Scholar] [CrossRef]
- Kahook, M.Y.; Serle, J.B.; Mah, F.S.; Kim, T.; Raizman, M.B.; Heah, T.; Ramirez-Davis, N.; Kopczynski, C.C.; Usner, D.W.; Novack, G.D.; et al. Long-Term Safety and Ocular Hypotensive Efficacy Evaluation of Netarsudil Ophthalmic Solution: Rho Kinase Elevated IOP Treatment Trial (ROCKET-2). Am. J. Ophthalmol. 2019, 200, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Tazoe, F.; Watanabe, Y.; Inoue, N.; Kanazawa, M.; Kasano, A.; Inoue, T. Ripasudil Does Not Induce Phospholipid Accumulation in Human Corneal Epithelial Cells. Exp. Eye Res. 2025, 255, 110351. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Kakuda, T.; Sano, T.; Kanno, T.; Gunji, R. Safety and Efficacy of Ripasudil in Japanese Patients with Glaucoma or Ocular Hypertension: 12-Month Interim Analysis of ROCK-J, a Post-Marketing Surveillance Study. BMC Ophthalmol. 2020, 20, 275. [Google Scholar] [CrossRef]
- Inoue, K.; Ishida, K.; Tomita, G. Effectiveness and Safety of Switching from Prostaglandin Analog Monotherapy to Prostaglandin/Timolol Fixed Combination Therapy or Adding Ripasudil. Jpn. J. Ophthalmol. 2018, 62, 508–516. [Google Scholar] [CrossRef]
- Rashad, R.; Lee, H.J. Netarsudil-Associated Reticular Epithelial Edema Directly Influenced by Endothelial Dysfunction in a Post-Endothelial Keratoplasty Patient. Cornea Open 2023, 2, e0024. [Google Scholar] [CrossRef]
- Bhargava, M.; Sen, S.; Bhambhani, V.; Paul, R.S.; Dutta, C. Reticular Epithelial Corneal Edema as a Novel Side-Effect of Rho Kinase Inhibitors: An Indian Scenario. Indian J. Ophthalmol. 2022, 70, 1163–1170. [Google Scholar] [CrossRef]
- Lyons, L.J.; Wu, K.Y.; Baratz, K.H.; Sit, A.J. Honeycomb Epithelial Edema Associated With Rho Kinase Inhibition: A Case Series and Review of the Literature. Cornea 2022, 41, 243–248. [Google Scholar] [CrossRef] [PubMed]
- LoBue, S.A.; Moustafa, G.A.; Vu, A.; Amin, M.; Nguyen, T.; Goyal, H. Transient Reticular Cystic Corneal Epithelial Edema With Topical Netarsudil: A Case Series and Review. Cornea 2021, 40, 1048–1054. [Google Scholar] [CrossRef]
- Tran, J.A.; Jurkunas, U.V.; Yin, J.; Davies, E.C.; Sola-Del Valle, D.A.; Chen, T.C.; Lin, M.M. Netarsudil-Associated Reticular Corneal Epithelial Edema. Am. J. Ophthalmol. Case Rep. 2022, 25, 101287. [Google Scholar] [CrossRef]
- Mandlik, K.; Christy, S.J.; Ravisankar, R. Netarsudil-Induced Honeycomb Hypertrophy. Asia. Pac. J. Ophthalmol. 2023, 12, 504–505. [Google Scholar] [CrossRef]
- Jeang, L.J.; Shah, A.S.; Hammer, J.D.; Tuli, S.S. Reticular Epithelial Edema after Penetrating Keratoplasty in a Patient Taking Netarsudil. Digit. J. Ophthalmol. 2022, 28, 34–37. [Google Scholar] [CrossRef]
- Wisely, C.E.; Liu, K.C.; Gupta, D.; Carlson, A.N.; Asrani, S.G.; Kim, T. Reticular Bullous Epithelial Edema in Corneas Treated with Netarsudil: A Case Series. Am. J. Ophthalmol. 2020, 217, 20–26. [Google Scholar] [CrossRef]
- Jain, N.; Singh, A.; Mishra, D.K.; Murthy, S.I. Honeycomb Epithelial Oedema Due to Ripasudil: Clinical, Optical Coherence Tomography and Histopathological Correlation. BMJ Case Rep. 2022, 15, e251074. [Google Scholar] [CrossRef]
- Dasso, L.; Al-Khaled, T.; Sonty, S.; Aref, A.A. Profile of Netarsudil Ophthalmic Solution and Its Potential in the Treatment of Open-Angle Glaucoma: Evidence to Date. Clin. Ophthalmol. 2018, 12, 1939–1944. [Google Scholar] [CrossRef]
- Mehran, N.A.; Sinha, S.; Razeghinejad, R. New Glaucoma Medications: Latanoprostene Bunod, Netarsudil, and Fixed Combination Netarsudil-Latanoprost. Eye 2020, 34, 72–88. [Google Scholar] [CrossRef]
- Li, X.; Balas, M.; Mathew, D.J. A Review of Ocular and Systemic Side Effects in Glaucoma Pharmacotherapy. J. Clin. Transl. Ophthalmol. 2025, 3, 2. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, X.; Tang, F.; Huang, T. A Real-World Pharmacovigilance Study of Netarsudil Based on the FDA Adverse Event Reporting System (FAERS). BMC Pharmacol. Toxicol. 2025, 26, 88. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Fukushima, A.; Suganami, H.; Araie, M. K-115 Clinical Study Group One-Year Clinical Evaluation of 0.4% Ripasudil (K-115) in Patients with Open-Angle Glaucoma and Ocular Hypertension. Acta. Ophthalmol. 2016, 94, e26–e34. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-C.; Chen, Y.-H.; Lu, D.-W. The Application of Rho Kinase Inhibitors in the Management of Glaucoma. Int. J. Mol. Sci. 2024, 25, 5576. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, D.; Coleman, A.L.; Shibayama, V.P.; Tseng, V.L. Netarsudil-Induced Corneal Flattening in a Child with Secondary Open-Angle Glaucoma. Case Rep. Ophthalmol. 2022, 13, 330–335. [Google Scholar] [CrossRef]
- Guzman Aparicio, M.A.; Liebman, D.L.; Chodosh, J.; Freitag, S.K.; Kazlas, M.; Mai, D.D.; Marando, C.M.; Mukai, S.; Wu, A.M.; Chen, T.C. Two Pediatric Cases of Reticular Corneal Epithelial Edema Associated with Netarsudil. Am. J. Ophthalmol. Case Rep. 2022, 27, 101638. [Google Scholar] [CrossRef]
- Tone, S.O.; Kocaba, V.; Böhm, M.; Wylegala, A.; White, T.L.; Jurkunas, U.V. Fuchs Endothelial Corneal Dystrophy: The Vicious Cycle of Fuchs Pathogenesis. Prog. Retin. Eye Res. 2021, 80, 100863. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Cho, K.; Srikumaran, D. Fuchs Dystrophy and Cataract: Diagnosis, Evaluation and Treatment. Ophthalmol. Ther. 2023, 12, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C. Proliferative Capacity of Corneal Endothelial Cells. Exp. Eye Res. 2012, 95, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Peh, G.S.L.; Chng, Z.; Ang, H.-P.; Cheng, T.Y.D.; Adnan, K.; Seah, X.-Y.; George, B.L.; Toh, K.-P.; Tan, D.T.; Yam, G.H.F.; et al. Propagation of Human Corneal Endothelial Cells: A Novel Dual Media Approach. Cell Transpl. 2015, 24, 287–304. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Adnan, K.; George, B.L.; Ang, H.-P.; Seah, X.-Y.; Tan, D.T.; Mehta, J.S. The Effects of Rho-Associated Kinase Inhibitor Y-27632 on Primary Human Corneal Endothelial Cells Propagated Using a Dual Media Approach. Sci. Rep. 2015, 5, 9167. [Google Scholar] [CrossRef]
- Nakagawa, H.; Alemi, H.; Wang, S.; Kahale, F.; Blanco, T.; Liu, C.; Yin, J.; Dohlman, T.H.; Dana, R. Descemet Stripping Only (DSO) Technique for Corneal Endothelial Damage in Mice. Cornea 2023, 42, 470–475. [Google Scholar] [CrossRef]
- Davies, E.; Jurkunas, U.; Pineda, R. Pilot Study of Corneal Clearance With the Use of a Rho-Kinase Inhibitor After Descemetorhexis Without Endothelial Keratoplasty for Fuchs Endothelial Corneal Dystrophy. Cornea 2021, 40, 899–902. [Google Scholar] [CrossRef]
- Macsai, M.S.; Shiloach, M. Use of Topical Rho Kinase Inhibitors in the Treatment of Fuchs Dystrophy After Descemet Stripping Only. Cornea 2019, 38, 529–534. [Google Scholar] [CrossRef]
- Soh, Y.Q.; Peh, G.; George, B.L.; Seah, X.Y.; Primalani, N.K.; Adnan, K.; Mehta, J.S. Predicative Factors for Corneal Endothelial Cell Migration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Xi, L.W.Q.; Luu, W.; Enkhbat, M.; Neo, D.; Mehta, J.S.; Peh, G.S.L.; Yim, E.K.F. Preclinical Models for Studying Fuchs Endothelial Corneal Dystrophy. Cells 2025, 14, 505. [Google Scholar] [CrossRef]
- Lindstrom, R.L.; Lewis, A.E.; Holland, E.J.; Sheppard, J.D.; Hovanesian, J.A.; Senchyna, M.; Hollander, D.A. Phase 2, Randomized, Open-Label Parallel-Group Study of Two Dosing Regimens of Netarsudil for the Treatment of Corneal Edema Due to Fuchs Corneal Dystrophy. J. Ocul. Pharmacol. Ther. 2022, 38, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Price, M.O.; Price, F.W. Randomized, Double-Masked, Pilot Study of Netarsudil 0.02% Ophthalmic Solution for Treatment of Corneal Edema in Fuchs Dystrophy. Am. J. Ophthalmol. 2021, 227, 100–105. [Google Scholar] [CrossRef]
- Tomioka, Y.; Kitazawa, K.; Fukuoka, H.; Ueno, M.; Koizumi, N.; Sotozono, C.; Kinoshita, S. Twelve-Year Outcome of Rho-Associated Protein Kinase Inhibitor Eye Drop Treatment for Fuchs Endothelial Corneal Dystrophy: A Case Study. Am. J. Ophthalmol. Case Rep. 2023, 30, 101839. [Google Scholar] [CrossRef]
- Lin, C.C.; Chamberlain, W.; Benetz, B.A.; Gensheimer, W.; Li, J.Y.; Jeng, B.H.; Clover, J.; Varnado, N.; Abdelrahman, S.; Srinivasan, A.; et al. Descemet Endothelial Thickness Comparison Trial II (DETECT II): Multicentre, Outcome Assessor-Masked, Placebo-Controlled Trial Comparing Descemet Membrane Endothelial Keratoplasty (DMEK) to Descemet Stripping Only (DSO) with Adjunctive Ripasudil for Fuchs Dystrophy. BMJ Open Ophthalmol. 2024, 9, e001725. [Google Scholar] [CrossRef]
- Ripasudil Treatment after Simultaneous DSO and Cataract Surgery in Subjects with FECD. Available online: https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/ripasudil-treatment-after-simultaneous-dso-and-cataract-surgery-in-subjects-with-fecd/ (accessed on 21 May 2025).
- Liu, J.-X.; Chiang, T.-L.; Hung, K.-F.; Sun, Y.-C. Therapeutic Future of Fuchs Endothelial Corneal Dystrophy: An Ongoing Way to Explore. Taiwan J. Ophthalmol. 2024, 14, 15–26. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K. Pseudophakic Bullous Keratopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Singh, N.K.; Sahu, S.K. Rho-Kinase Inhibitors: Role in Corneal Endothelial Disorders. Semin. Ophthalmol. 2023, 38, 9–14. [Google Scholar] [CrossRef]
- Tseng, M.; Feder, R. Topical Ripasudil for the Treatment of Segmental Corneal Edema: A Case Series. Cornea 2023, 42, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Erdinest, N.; Shemesh, N.; Weill, Y.; Morad, S.; Nitzan, I.; Smadja, D.; Landau, D.; Lavy, I. Managing Pseudophakic Bullous Keratopathy with a Topical Rho Kinase Inhibitor: A Case Series. J. Med. Case Rep. 2025, 19, 214. [Google Scholar] [CrossRef]
- Davies, E. Case Series: Novel Utilization of Rho-Kinase Inhibitor for the Treatment of Corneal Edema. Cornea 2020, 40, 116–120. [Google Scholar] [CrossRef]
- The Effect of ROCK Inhibitors on Corneas of Patients with Glaucoma and Pseudophakic Bulbous Keratopathy (PBK). Available online: https://ctv.veeva.com/study/the-effect-of-rock-inhibitors-on-corneas-of-patients-with-glaucoma-and-pseudophakic-bulbous-keratopa (accessed on 21 May 2025).
- Das, S.; Tur, K.; Tripathy, K. Iridocorneal Endothelial Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Schlötzer-Schrehardt, U.; Gießl, A.; Zenkel, M.; Bartsch, A.; Okumura, N.; Koizumi, N.; Kinoshita, S.; Tourtas, T.; Kruse, F.E. Drug- and Cell-Type-Specific Effects of ROCK Inhibitors as a Potential Cause of Reticular Corneal Epithelial Edema. Cells 2025, 14, 258. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M. Phase 2 Randomized Clinical Study of a Rho Kinase Inhibitor, K-115, in Primary Open-Angle Glaucoma and Ocular Hypertension. Am. J. Ophthalmol. 2013, 156, 731–736.e2. [Google Scholar] [CrossRef] [PubMed]
- Sit, A.J.; Gupta, D.; Kazemi, A.; McKee, H.; Challa, P.; Liu, K.C.; Lopez, J.; Kopczynski, C.; Heah, T. Netarsudil Improves Trabecular Outflow Facility in Patients with Primary Open Angle Glaucoma or Ocular Hypertension: A Phase 2 Study. Am. J. Ophthalmol. 2021, 226, 262–269. [Google Scholar] [CrossRef]
- Chang, J.-H.; Garg, N.K.; Lunde, E.; Han, K.-Y.; Jain, S.; Azar, D.T. Corneal Neovascularization: An Anti-VEGF Therapy Review. Surv. Ophthalmol. 2012, 57, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Gabison, E.E.; Kato, T.; Azar, D.T. Corneal Neovascularization. Curr. Opin. Ophthalmol. 2001, 12, 242–249. [Google Scholar] [CrossRef]
- Dietrich, T.; Bock, F.; Yuen, D.; Hos, D.; Bachmann, B.O.; Zahn, G.; Wiegand, S.; Chen, L.; Cursiefen, C. Cutting Edge: Lymphatic Vessels, Not Blood Vessels, Primarily Mediate Immune Rejections After Transplantation. J. Immunol. 2010, 184, 535–539. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Lee, S.-M.; Sung, J.; Niutta, M.; Coassin, M.; Mashaghi, A.; Inomata, T. Variable Responses to Corneal Grafts: Insights from Immunology and Systems Biology. J. Clin. Med. 2020, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chan, K.E.; Lim, B.X.H.; Lim, D.K.-A.; Wong, W.M.; Chai, C.; Manotosh, R.; Lim, C.H.L. Management of Corneal Neovascularization: Current and Emerging Therapeutic Approaches. Indian J. Ophthalmol. 2024, 72, S354–S371. [Google Scholar] [CrossRef]
- Kimura, K.; Ito, M.; Amano, M.; Chihara, K.; Fukata, Y.; Nakafuku, M.; Yamamori, B.; Feng, J.; Nakano, T.; Okawa, K.; et al. Regulation of Myosin Phosphatase by Rho and Rho-Associated Kinase (Rho-Kinase). Science 1996, 273, 245–248. [Google Scholar] [CrossRef]
- Bryan, B.A.; Dennstedt, E.; Mitchell, D.C.; Walshe, T.E.; Noma, K.; Loureiro, R.; Saint-Geniez, M.; Campaigniac, J.-P.; Liao, J.K.; D’Amore, P.A. RhoA/ROCK Signaling Is Essential for Multiple Aspects of VEGF-Mediated Angiogenesis. FASEB J. 2010, 24, 3186–3195. [Google Scholar] [CrossRef]
- van Nieuw Amerongen, G.P.; Koolwijk, P.; Versteilen, A.; van Hinsbergh, V.W.M. Involvement of RhoA/Rho Kinase Signaling in VEGF-Induced Endothelial Cell Migration and Angiogenesis in Vitro. Arter. Thromb. Vasc. Biol. 2003, 23, 211–217. [Google Scholar] [CrossRef]
- Zhao, Z.; Manser, E. PAK and Other Rho-Associated Kinases--Effectors with Surprisingly Diverse Mechanisms of Regulation. Biochem. J. 2005, 386, 201–214. [Google Scholar] [CrossRef]
- Loirand, G.; Guérin, P.; Pacaud, P. Rho Kinases in Cardiovascular Physiology and Pathophysiology. Circ. Res. 2006, 98, 322–334. [Google Scholar] [CrossRef]
- Zhang, F.; Zarkada, G.; Yi, S.; Eichmann, A. Lymphatic Endothelial Cell Junctions: Molecular Regulation in Physiology and Diseases. Front. Physiol. 2020, 11, 509. [Google Scholar] [CrossRef]
- Sijnave, D.; Van Bergen, T.; Castermans, K.; Kindt, N.; Vandewalle, E.; Stassen, J.-M.; Moons, L.; Stalmans, I. Inhibition of Rho-Associated Kinase Prevents Pathological Wound Healing and Neovascularization After Corneal Trauma. Cornea 2015, 34, 1120–1129. [Google Scholar] [CrossRef]
- Boland, M.V.; Ervin, A.M.; Friedman, D.; Jampel, H.; Hawkins, B.; Volenweider, D.; Chelladurai, Y.; Ward, D.; Suarez-Cuervo, C.; Robinson, K.A. Treatment for Glaucoma: Comparative Effectiveness. In AHRQ Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012. [Google Scholar]
- Corneal Endothelial Cell Loss—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/corneal-endothelial-cell-loss?utm_source=chatgpt.com (accessed on 22 May 2025).
- Briceno-Lopez, C.; Burguera-Giménez, N.; García-Domene, M.C.; Díez-Ajenjo, M.A.; Peris-Martínez, C.; Luque, M.J. Corneal Edema after Cataract Surgery. J. Clin. Med. 2023, 12, 6751. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, T.; Zhou, S.; Li, F.; Guo, W.; Li, M.; Liu, T. Changes in Aqueous Humor Cytokines and Metabolomics in Contralateral Eye after Unilateral Cataract Surgery. BMC Ophthalmol. 2025, 25, 137. [Google Scholar] [CrossRef]
- Dong, N.; Xu, B.; Wang, B.; Chu, L.; Tang, X. Aqueous Cytokines as Predictors of Macular Edema in Patients with Diabetes Following Uncomplicated Phacoemulsification Cataract Surgery. Biomed. Res. Int. 2015, 2015, 126984. [Google Scholar] [CrossRef]
- Chen, K.; Xu, W.-Y.; Sun, S.-S.; Zhou, H.-W. Corneal Endothelial Cells and Acoustic Cavitation in Phacoemulsification. World J. Clin. Cases 2023, 11, 1712–1718. [Google Scholar] [CrossRef]
- Igarashi, N.; Honjo, M.; Kaburaki, T.; Aihara, M. Effects of ROCK Inhibitors on Apoptosis of Corneal Endothelial Cells in CMV-Positive Posner–Schlossman Syndrome Patients. Investig. Ophthalmol. Vis. Sci. 2020, 61, 5. [Google Scholar] [CrossRef]
- Uchida, T.; Honjo, M.; Yamagishi, R.; Aihara, M. The Anti-Inflammatory Effect of Ripasudil (K-115), a Rho Kinase (ROCK) Inhibitor, on Endotoxin-Induced Uveitis in Rats. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5584–5593. [Google Scholar] [CrossRef]
- Okumura, N.; Ueno, M.; Koizumi, N.; Sakamoto, Y.; Hirata, K.; Hamuro, J.; Kinoshita, S. Enhancement on Primate Corneal Endothelial Cell Survival In Vitro by a ROCK Inhibitor. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3680–3687. [Google Scholar] [CrossRef]
- Fujimoto, H.; Setoguchi, Y.; Kiryu, J. The ROCK Inhibitor Ripasudil Shows an Endothelial Protective Effect in Patients with Low Corneal Endothelial Cell Density After Cataract Surgery. Transl. Vis. Sci. Technol. 2021, 10, 18. [Google Scholar] [CrossRef]
- Alkharashi, M.; Abusayf, M.M.; Otaif, W.; Alkharashi, A. The Protective Effect of Rho-Associated Kinase Inhibitor Eye Drops (Ripasudil) on Corneal Endothelial Cells After Cataract Surgery: A Prospective Comparative Study. Ophthalmol. Ther. 2024, 13, 1773–1781. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Marino, G.K.; Santhiago, M.R.; Wilson, S.E. The Corneal Basement Membranes and Stromal Fibrosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4044–4053. [Google Scholar] [CrossRef]
- Xing, D.; Bonanno, J.A. Effect of cAMP on TGFβ1-Induced Corneal Keratocyte–Myofibroblast Transformation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 626–633. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal Myofibroblasts and Fibrosis. Exp. Eye Res. 2020, 201, 108272. [Google Scholar] [CrossRef]
- Guo, X.; Sriram, S.; Tran, J.A.; Hutcheon, A.E.K.; Zieske, J.D. Inhibition of Human Corneal Myofibroblast Formation. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3511–3520. [Google Scholar] [CrossRef]
- Qi, X.-J.; Ning, W.; Xu, F.; Dang, H.-X.; Fang, F.; Li, J. Fasudil, an Inhibitor of Rho-Associated Coiled-Coil Kinase, Attenuates Hyperoxia-Induced Pulmonary Fibrosis in Neonatal Rats. Int. J. Clin. Exp. Pathol. 2015, 8, 12140–12150. [Google Scholar]
- Li, Q.; Cheng, Y.; Zhang, Z.; Bi, Z.; Ma, X.; Wei, Y.; Wei, X. Inhibition of ROCK Ameliorates Pulmonary Fibrosis by Suppressing M2 Macrophage Polarisation through Phosphorylation of STAT3. Clin. Transl. Med. 2022, 12, e1036. [Google Scholar] [CrossRef]
- Chen, J.; Guerriero, E.; Sado, Y.; SundarRaj, N. Rho-Mediated Regulation of TGF-Beta1- and FGF-2-Induced Activation of Corneal Stromal Keratocytes. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3662–3670. [Google Scholar] [CrossRef]
- Ichikawa, K.; Tanaka, S.-I.; Miyajima, M.; Okada, Y.; Saika, S. Inhibition of Rho Kinase Suppresses Capsular Contraction Following Lens Injury in Mice. Taiwan J. Ophthalmol. 2020, 10, 100–105. [Google Scholar] [CrossRef]
- Dai, J.; Rayana, N.P.; Peng, M.; Sugali, C.K.; Harvey, D.H.; Dhamodaran, K.; Yu, E.; Dalloul, J.M.; Liu, S.; Mao, W. Inhibition of Pterygium Cell Fibrosis by the Rho Kinase Inhibitor. Sci. Rep. 2024, 14, 30930. [Google Scholar] [CrossRef]
- Tan, J.K.; Steel, D.H.; Ahmad, S.; Viswanathan, A.; Mathew, R.G.; Khaw, P.T.; Henein, C. Exploring the Potential of Rho Kinase Inhibitors in Ophthalmology: From Mechanisms to Clinical Practice. Surv. Ophthalmol. 2025, 70, 900–917. [Google Scholar] [CrossRef]
- Shadmani, A.; Wu, A.Y. Navigating the Path to Corneal Healing Success and Challenges: A Comprehensive Overview. Eye 2025, 39, 1047–1055. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in Corneal Wound Healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Gurnani, B.; Czyz, C.N.; Mahabadi, N.; Havens, S.J. Corneal Graft Rejection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Amouzegar, A.; Chauhan, S.K. Effector and Regulatory T Cell Trafficking in Corneal Allograft Rejection. Mediat. Inflamm. 2017, 2017, 8670280. [Google Scholar] [CrossRef]
- Dana, M.R.; Qian, Y.; Hamrah, P. Twenty-Five-Year Panorama of Corneal Immunology: Emerging Concepts in the Immunopathogenesis of Microbial Keratitis, Peripheral Ulcerative Keratitis, and Corneal Transplant Rejection. Cornea 2000, 19, 625–643. [Google Scholar] [CrossRef]
- Niederkorn, J.Y.; Larkin, D.F.P. Immune Privilege of Corneal Allografts. Ocul. Immunol. Inflamm. 2010, 18, 162–171. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. Immune Mechanisms of Corneal Allograft Rejection. Curr. Eye Res. 2007, 32, 1005–1016. [Google Scholar] [CrossRef]
- Bardi, G.; Niggli, V.; Loetscher, P. Rho Kinase Is Required for CCR7-Mediated Polarization and Chemotaxis of T Lymphocytes. FEBS Lett. 2003, 542, 79–83. [Google Scholar] [CrossRef]
- To Study How Ripasudil Can Help for Graft Survival after Corneal Transplant. Available online: https://trial.medpath.com/clinical-trial/386b8baf95b34d88/ctri/2024/06/069429-prospective-study-rho-kinase-inhibitors-keratoplasty (accessed on 23 May 2025).
- Chamberlain, W.; Lin, C.C.; Li, J.Y.; Gensheimer, W.; Clover, J.; Jeng, B.H.; Varnado, N.; Abdelrahman, S.; Arnold, B.F.; Lietman, T.M.; et al. Descemet Endothelial Thickness Comparison Trial 1 (DETECT 1): Outcome Masked, Placebo-Controlled Trial Comparing Two Types of Corneal Transplant Surgeries and Effect of Rho Kinase Inhibitors on Endothelial Cell Loss Protocol. BMJ Open Ophthalmol. 2024, 9, e001454. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Ong, H.S.; Adnan, K.; Ang, H.-P.; Lwin, C.N.; Seah, X.-Y.; Lin, S.-J.; Mehta, J.S. Functional Evaluation of Two Corneal Endothelial Cell-Based Therapies: Tissue-Engineered Construct and Cell Injection. Sci. Rep. 2019, 9, 6087. [Google Scholar] [CrossRef] [PubMed]
- Peh, G.S.L.; Bandeira, F.; Neo, D.; Adnan, K.; Hartono, Y.; Ong, H.S.; Naso, S.; Venkatraman, A.; Gomes, J.A.P.; Kocaba, V.; et al. Effects of Rho-Associated Kinase (Rock) Inhibitors (Alternative to Y-27632) on Primary Human Corneal Endothelial Cells. Cells 2023, 12, 1307. [Google Scholar] [CrossRef]
- So, S.; Park, Y.; Kang, S.S.; Han, J.; Sunwoo, J.H.; Lee, W.; Kim, J.; Ye, E.A.; Kim, J.Y.; Tchah, H.; et al. Therapeutic Potency of Induced Pluripotent Stem-Cell-Derived Corneal Endothelial-like Cells for Corneal Endothelial Dysfunction. Int. J. Mol. Sci. 2022, 24, 701. [Google Scholar] [CrossRef]
- Zhang, W.; Shao, C.; Yu, F.; Chen, J.; Fu, Y.; Fan, X. Y-27632 Promotes the Repair Effect of Umbilical Cord Blood-Derived Endothelial Progenitor Cells on Corneal Endothelial Wound Healing. Cornea 2021, 40, 203–214. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Kureshi, A.; Daniels, J.T. Tissue Engineered Corneal Endothelium Transplantation in an Ex Vivo Human Cornea Organ Culture Model. Sci. Rep. 2025, 15, 12571. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Akhbanbetova, A.; Quantock, A.J.; Heard, C.M. Topical Delivery of a Rho-Kinase Inhibitor to the Cornea via Mucoadhesive Film. Eur. J. Pharm. Sci. 2016, 91, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Koda, S.; Okumura, N.; Kitano, J.; Koizumi, N.; Tabata, Y. Development of Poly Lactic/Glycolic Acid (PLGA) Microspheres for Controlled Release of Rho-Associated Kinase Inhibitor. J. Ophthalmol. 2017, 2017, 1598218. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Harnessing the Tunable Cavity of Nanoceria for Enhancing Y-27632-Mediated Alleviation of Ocular Hypertension. Theranostics 2021, 11, 5447–5463. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Akbar, D.; Giunta, M.; Kalevar, A.; Tran, S.D. Hydrogels in Ophthalmology: Novel Strategies for Overcoming Therapeutic Challenges. Materials 2024, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- SpyGlass Pharma Reports Positive 18-Month Data on Glaucoma Sustained Delivery System. Available online: https://glance.eyesoneyecare.com/stories/2025-04-28/spyglass-pharma-reports-positive-18-month-data-on-glaucoma-sustained-delivery-system/ (accessed on 24 May 2025).
- Peyman, G.A. Ophthalmic Drug Delivery Method 2020. US Patent US10842669B2, 29 April 2019. [Google Scholar]
- Borrás, T.; Buie, L.K.; Spiga, M.-G.; Carabana, J. Prevention of Nocturnal Elevation of Intraocular Pressure by Gene Transfer of Dominant-Negative RhoA in Rats. JAMA Ophthalmol. 2015, 133, 182–190. [Google Scholar] [CrossRef] [PubMed]
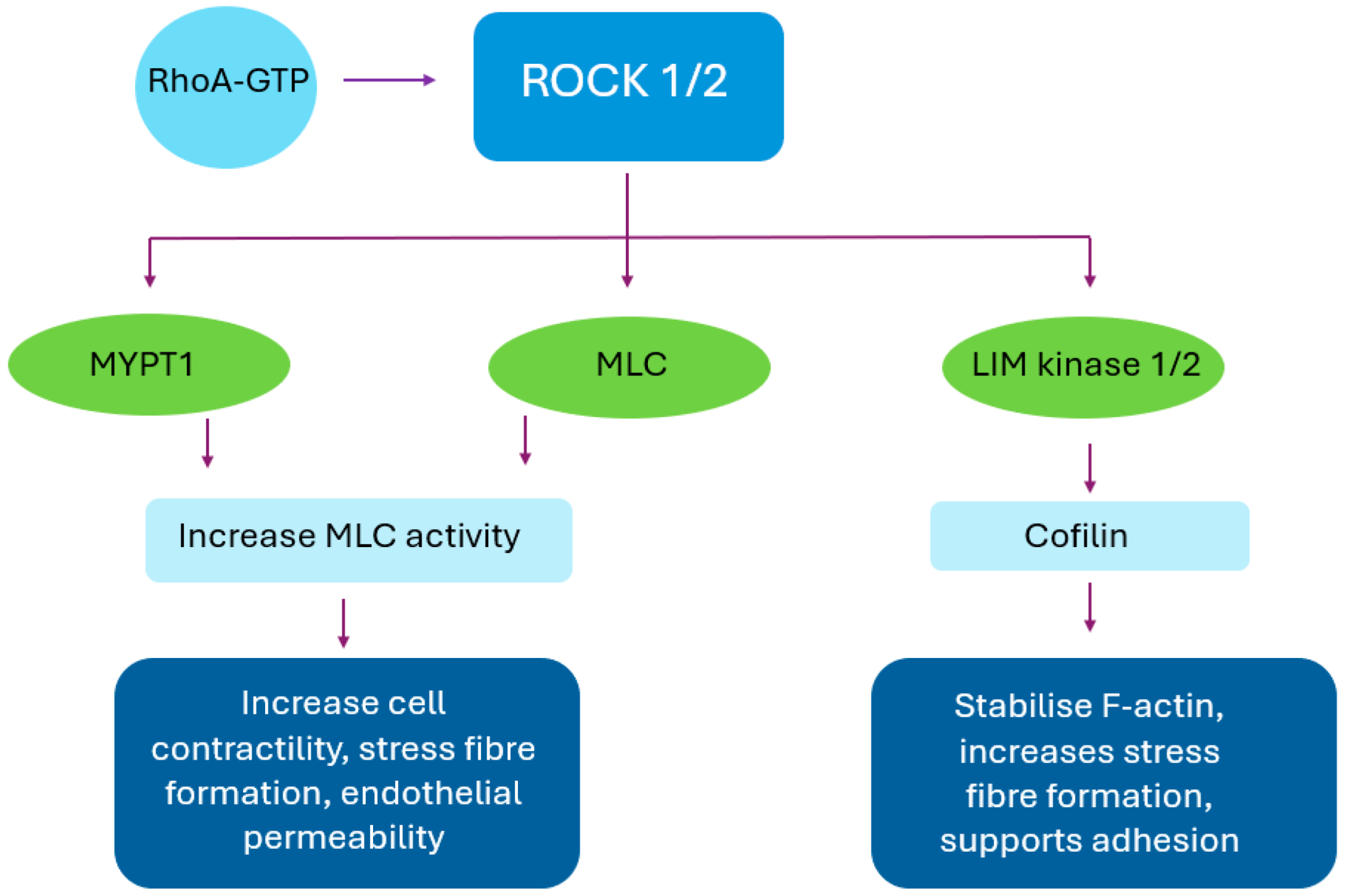
| Corneal Layer | Basal Role of ROCK | Pathological Over-Activation | Key Effects of ROCK Inhibition |
|---|---|---|---|
| Epithelium | Coordinates cortical F-actin and adherens/tight junctions during sheet migration and barrier renewal | NF-κB-driven cytokine surge (IL-1β, IL-6, TNF-α) and leukocyte influx after infection or chemical injury | Y-27632 relaxes actomyosin, boosts migration/adhesion and closes scratch defects faster than control |
| Stroma (keratocytes /fibroblasts) | Couples TGF-β signalling to α-SMA expression, myofibroblast contractility and extracellular matrix deposition | Fibrotic haze and high-tension scars after surgery or burns | Topical or in-matrix Y-27632 blocks keratocyte-to-myofibroblast switch, normalises collagen to an embryonic-like lattice |
| Endothelium | Maintains hexagonal architecture and pump barrier by stabilising F-actin, ZO-1 and Na+/K+-ATPase | Oxidative/inflammatory stress → contraction, junction loss, caspase-3 apoptosis → oedema | Y-27632 / ripasudil relax cytoskeleton, up-shift cyclin-D, enhance spreading & Rac1-driven migration; accelerate clearance after DSO or CEC injection; reactivate FECD endothelia |
| Immune & inflammatory axis | ROCK 1 amplifies TLR4-NF-κB signalling | Persistent stromal inflammation, delayed healing | Inhibitors dampen cytokines, favour Treg over Th17, reduce graft rejection |
| Angiogenesis | Up-stream of VEGF and Shh–Rac pathways in limbal ECs | Pathologic corneal neovascularisation | Fasudil or netarsudil eye-drops reduce corneal neovascularization area in alkali-burn mice |
| ROCKI | Selectivity | Formulation/Administration | Key Pharmacologic Features | Preferred/Investigated Indications |
|---|---|---|---|---|
| Netarsudil | Non-selective (ROCK1 & ROCK2) + NET inhibitor | 0.02% topical solution, once daily | Dual ROCK and NET inhibition; long corneal half-life | Glaucoma, ocular hypertension, corneal endothelial disorders, post-surgical recovery |
| Ripasudil | Non-selective (ROCK1 & ROCK2) | 0.4% topical solution, twice daily | Rapid corneal uptake; shorter corneal effect (~6 h); rapid systemic clearance | Glaucoma, corneal endothelial regeneration, wound healing |
| Fasudil | Non-selective (ROCK1 & ROCK2) | Intravenous (approved in Japan for cerebral vasospasm); intravitreal in trials | ATP-competitive inhibitor; reversible binding; off-target at high dose | Cerebral vasospasm, retinal edema (in trials) |
| Y-27632 | Non-selective (ROCK1 & ROCK2) | Experimental; topical in pre-clinical/animal studies | ATP-competitive inhibitor; nanomolar potency; off-target at high dose | Pre-clinical glaucoma and corneal research |
| SNJ-1656 (Y-39983) | Non-selective (ROCK1 & ROCK2) | Experimental; topical in pre-clinical/animal studies, Phase II for glaucoma | More potent derivative of Y-27632 | Glaucoma (Phase II), corneal disease (experimental) |
| Study (Year, Ref.) | Design | Subjects/Model (n) | Intervention & Dosing | Key Findings | Follow-up |
|---|---|---|---|---|---|
| Schlotzer-Schrehardt et al. (2021) [7] | Experimental (ex vivo/in vitro) | FECD lamellae (n = 450), wound model (n = 30), intact (n = 20), FECD cell lines (n = 3) | Single 30 µM ripasudil dose | ↑ Cell-cycle, adhesion/migration, barrier & pump genes/proteins; ↓ EMT markers in FECD & normal samples | 24–72 h |
| Parekh et al. (2024) [23] | Experimental (ex vivo DSO model & in vitro) | Immortalized CECs from cadaveric normal and FECD donors | Ripasudil (0.3 µM, 1 µM, and 10 µM) | Enhanced migration & wound closure | 24–72 h |
| Okumura et al. (2013) [18] | Preclinical + clinical case series | Monkeys (n = 7); human corneal oedema (central n = 4, diffuse n = 4) | Y-27632 drops 6×/day | Central oedema: CCT ↓ at 6 months; Diffuse oedema: no CCT change; ED density: ↑ to ~3000 cells/mm2 with restored ZO-1 & Na+/K+-ATPase | 4 weeks for primates, 6 months for humans |
| Lindstrom et al. (2022) [96] | Phase 2 RCT, open-label, parallel-group | FECD patients (n = 40; CCT ≥ 600 µm, BCVA 20/40–20/400) | Netarsudil 1×/day vs. 2×/day for 8 weeks | At week 4: CCT ↓ 28.4 µm (x1/day dosing), ↓ 20.1 µm (x2/day dosing); 12.5% oedema resolution; 25% gained ≥ 10 letters; benefits persisted to week 8; no significant differences in dosing groups; well tolerated | 8 weeks |
| Moloney et al. (2021) [8] | Pre-post clinical with historical control | FECD eyes with DSO (n = 23) | Ripasudil 0.4% 6×/day until clearance | 96% (22/23) cleared at mean 4.1 weeks; VA +0.20 LogMAR, BCVA +0.156; 1 failure | 12 months |
| Davies et al. (2021) [92] | Intra- & inter-patient pilot | FECD eyes with DSO + cataract surgery (n = 20 eyes, 10 patients) | Netarsudil immediately vs. delayed post-DSO | Immediate use: clearance 4.6 ± 1.7 weeks vs. 8 ± 1.9 weeks (p < 0.01); “rescue” clearance 1–2 weeks after; higher ECC with immediate use (p = 0.05) | Until clearance |
| Macsai & Shiloach (2019) [93] | Prospective (non-randomized controlled) | FECD patients DSO ± cataract (ripasudil n = 9 vs. control n = 9) | Ripasudil 0.4% 4×/day for 2 months vs. none | Clearance 4.6 vs. 6.5 weeks (p < 0.01); ripasudil group maintained peripheral ECD (no change), controls lost 10% ECD by 12 months (p < 0.05) | 12 months |
| Price et al. (2021) [97] | Prospective, randomized, double-masked pilot trial | FECD patients (n = 29) | Netarsudil 0.02% vs. placebo for 3 months | CCT reduction: –20 µm at 1 month; –26 µm at 3 months; scotopic CDVA + 1.6 lines; no change in disability scores; one withdrew (glare) | 3 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leong, E.Y.X.; Ding, J.; Wu, D.; Lim, B.X.H.; Ang, A.; Wong, E.; Morlet, N.; Mehta, J.S.; Lim, C.H.L. A Comprehensive Review of the Role of Rho-Kinase Inhibitors in Corneal Diseases. Life 2025, 15, 1283. https://doi.org/10.3390/life15081283
Leong EYX, Ding J, Wu D, Lim BXH, Ang A, Wong E, Morlet N, Mehta JS, Lim CHL. A Comprehensive Review of the Role of Rho-Kinase Inhibitors in Corneal Diseases. Life. 2025; 15(8):1283. https://doi.org/10.3390/life15081283
Chicago/Turabian StyleLeong, Elizabeth Y. X., Jianbin Ding, Duoduo Wu, Blanche X. H. Lim, Andrea Ang, Evan Wong, Nigel Morlet, Jodhbir S. Mehta, and Chris H. L. Lim. 2025. "A Comprehensive Review of the Role of Rho-Kinase Inhibitors in Corneal Diseases" Life 15, no. 8: 1283. https://doi.org/10.3390/life15081283
APA StyleLeong, E. Y. X., Ding, J., Wu, D., Lim, B. X. H., Ang, A., Wong, E., Morlet, N., Mehta, J. S., & Lim, C. H. L. (2025). A Comprehensive Review of the Role of Rho-Kinase Inhibitors in Corneal Diseases. Life, 15(8), 1283. https://doi.org/10.3390/life15081283






