Expression of 15-PGDH Regulates Body Weight and Body Size by Targeting JH in Honeybees (Apis mellifera)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sampling
2.3. Inhibition of 15-PGDH in Honey Bee Workers Larvae
2.4. RNA Isolation and Real Time Quantitative PCR
2.5. Detecting of JH Titer
2.6. Morphological Measurement of Workers
2.7. Statistical Analyses
3. Results
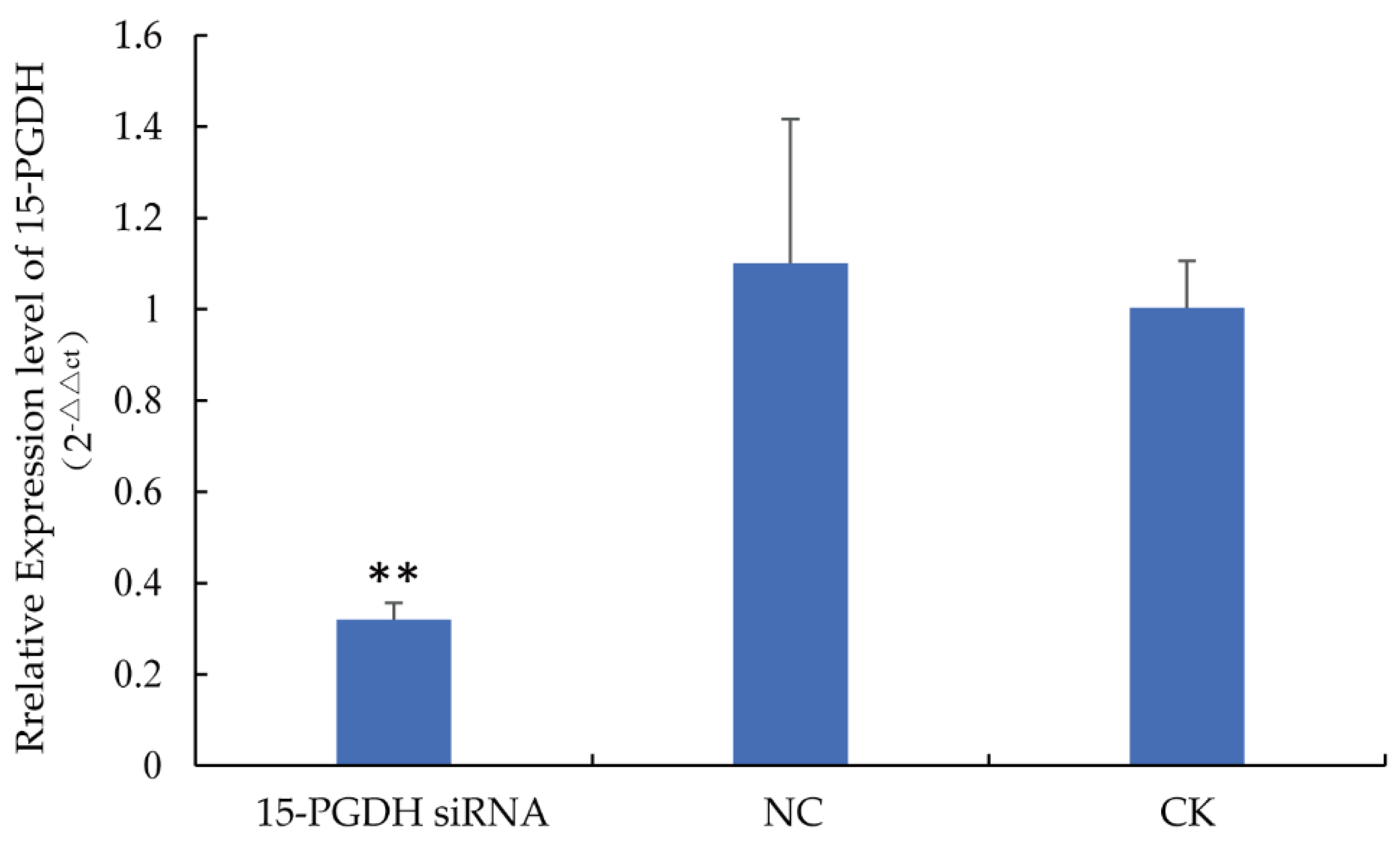
3.1. The Expression Level of 15-PGDH
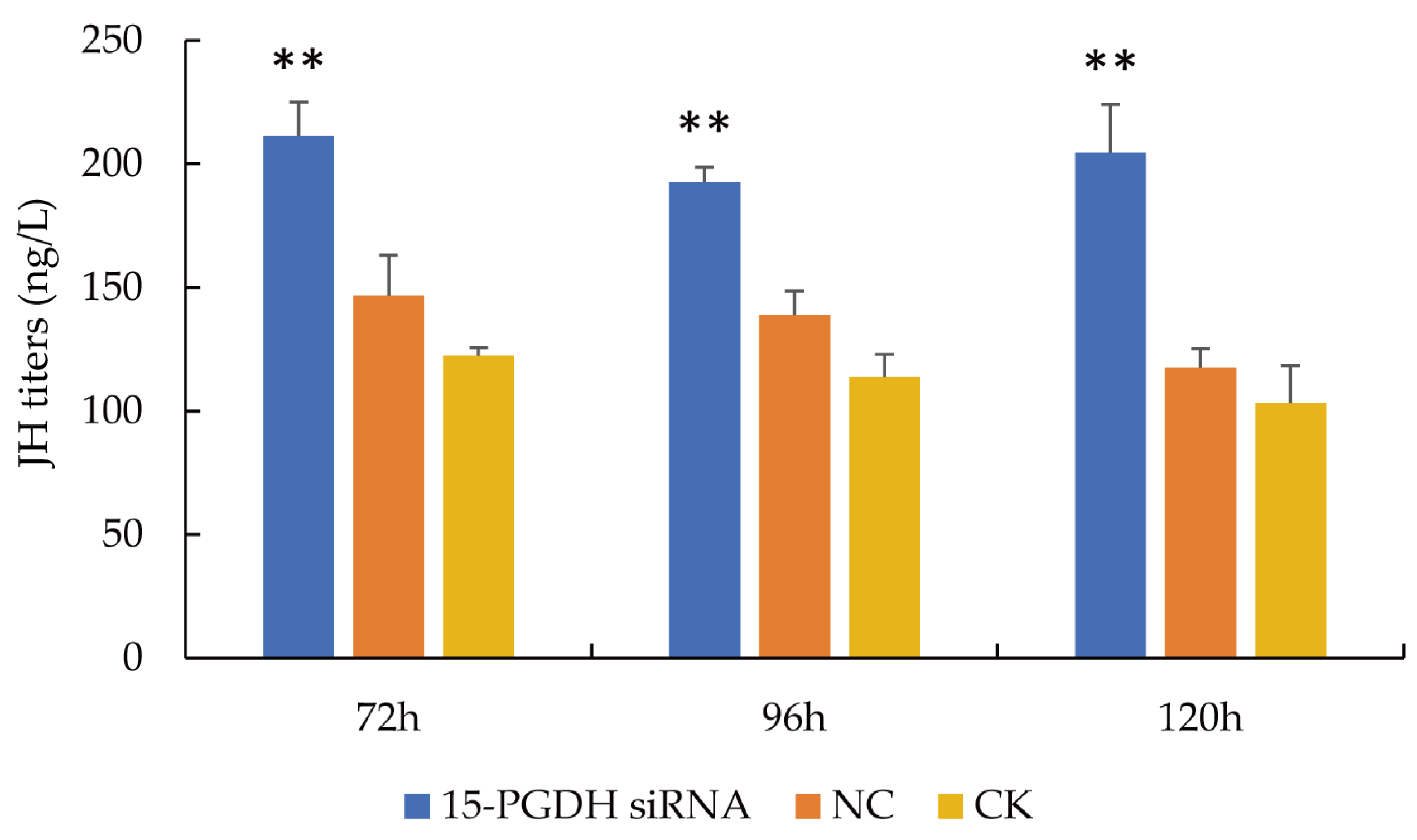
3.2. JH Titers
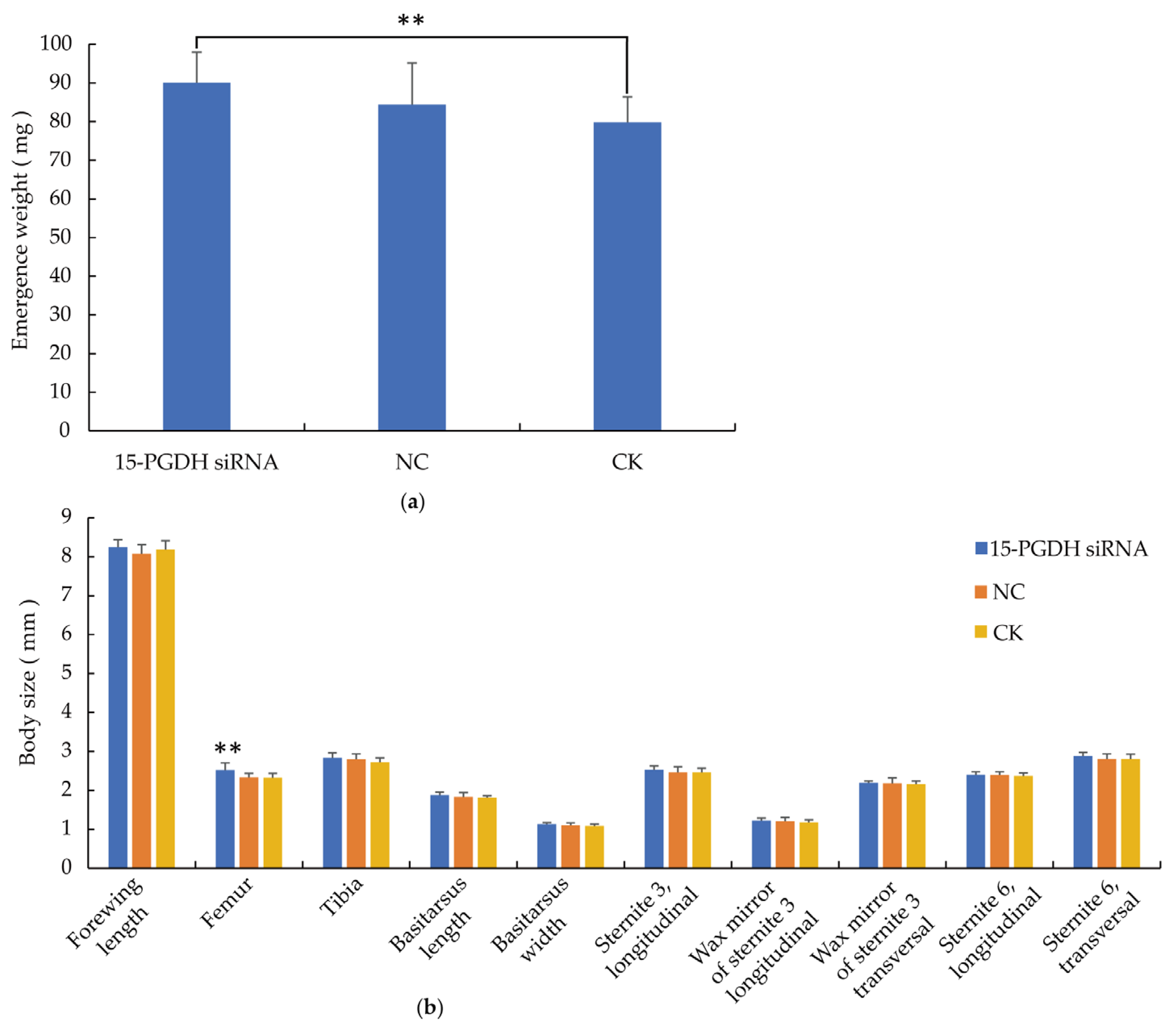
3.3. Morphological Characteristics of Workers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reilly, J.; Bartomeus, I.; Simpson, D.; Allen-Perkins, A.; Garibaldi, L.; Winfree, R. Wild insects and honey bees are equally important to crop yields in a global analysis. Glob. Ecol. Biogeogr. 2024, 33, e13843. [Google Scholar] [CrossRef]
- Martin, K.; Anderson, B.; Minnaar, C.; Jager, M.D. Honey bees are important pollinators of South African blueberries despite their inability to sonicate. S. Afr. J. Bot. 2021, 137, 46–51. [Google Scholar] [CrossRef]
- Classen, A.; Peters, M.K.; Ferger, S.W.; Helbig-Bonitz, M.; Schmack, J.M.; Maassen, G.; Schleuning, M.; Kalko, E.K.; Böhning-Gaese, K.; Steffan-Dewenter, I. Complementary ecosystem services provided by pest predators and pollinators increase quantity and quality of coffee yields. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133148. [Google Scholar] [CrossRef]
- Fekadie, B.; Getachew, A.; Ayalew, W.; Jenberie, A. Evaluating the effect of honeybee pollination on production of watermelon (Citrullus lantatus), in Northern Ethiopia. Int. J. Trop. Insect Sci. 2023, 43, 1431–1449. [Google Scholar] [CrossRef]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef]
- Bishop, J.A.; Armbruster, W.S. Thermoregulatory abilities of Alaskan bees: Effects of size, phylogeny and ecology. Funct. Ecol 1999, 13, 711–724. [Google Scholar] [CrossRef]
- Chole, H.; Woodard, S.H.; Bloch, G. Body size variation in bees: Regulation, mechanisms, and relationship to social organization. Curr. Opin. Insect Sci. 2019, 35, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.M.; Dietz, A. Pyridoxine requirement of the honey bee (Apis mellifera) for brood rearing. Apidologie 1976, 7, 67–84. [Google Scholar] [CrossRef]
- Stabentheiner, A.; Kovac, H.; Brodschneider, R. Honeybee colony thermoregulation–regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE 2010, 5, e8967. [Google Scholar] [CrossRef]
- Wirtz, P.; Beetsma, J. Induction of caste differentiation in the honeybee (Apis mellifera) by juvenile hormone. Entomol. Exp. Et Appl. 1972, 15, 517–520. [Google Scholar] [CrossRef]
- Kucharski, R.; Maleszka, J.; Foret, S.; Maleszka, R. Nutritional control of reproductive status in honeybees via DNA methylation. Science 2008, 319, 1827–1830. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Huang, Z.Y.; Zeng, Z.J.; Wang, Z.L.; Wu, X.B.; Yan, W.Y. Diet and cell size both affect queen-worker differentiation through DNA methylation in honey bees (Apis mellifera, Apidae). PLoS ONE 2011, 6, e18808. [Google Scholar] [CrossRef]
- Barchuk, A.R.; Cristino, A.S.; Kucharski, R.; Costa, L.F.; Simões, Z.L.; Maleszka, R. Molecular determinants of caste differentiation in the highly eusocial honeybee Apis mellifera. BMC Dev. Biol. 2007, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Fondrk, M.K.; Kaftanoglu, O.; Emore, C.; Hunt, G.; Frederick, K.; Amdam, G.V. The making of a queen: TOR pathway is a key player in diphenic caste development. PLoS ONE 2007, 2, e509. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, G.R.; Davey, K.G. Cellular and molecular actions of juvenile hormone. II. Roles of juvenile hormone in adult insects. Adv. Insect Physiol. 1996, 26, 1–155. [Google Scholar]
- Fei, H.; Martin, T.R.; Jaskowiak, K.M.; Hatle, J.D.; Whitman, D.W.; Borst, D.W. Starvation affects vitellogenin production but not vitellogenin mRNA levels in the lubber grasshopper, Romalea microptera. J. Insect Physiol. 2005, 51, 435–443. [Google Scholar] [CrossRef]
- Xiao, H.-J.; Fu, X.-W.; Liu, Y.-Q.; Wu, K.-M. Synchronous vitellogenin expression and sexual maturation during migration are negatively correlated with juvenile hormone levels in Mythimna separata. Sci. Rep. 2016, 6, 33309. [Google Scholar] [CrossRef]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Negroni, M.A.; LeBoeuf, A.C. Social administration of juvenile hormone to larvae increases body size and nutritional needs for pupation. R. Soc. Open Sci. 2023, 10, 231471. [Google Scholar] [CrossRef]
- Capella, I.C.S.; Hartfelder, K. Juvenile hormone effect on DNA synthesis and apoptosis in caste-specific differentiation of the larval honey bee (Apis mellifera L.) ovary. J. Insect Physiol. 1998, 44, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Hartfelder, K.; Steinbrück, G. Germ cell cluster formation and cell death are alternatives in caste-specific differentiation of the larval honey bee ovary. Invertebr. Reprod. Dev. 1997, 31, 237–250. [Google Scholar] [CrossRef]
- Pandey, A.; Bloch, G. Juvenile hormone and ecdysteroids as major regulators of brain and behavior in bees. Curr. Opin. Insect Sci. 2015, 12, 26–37. [Google Scholar] [CrossRef]
- Dong, S.; Li, K.; Zang, H.; Song, Y.; Kang, J.; Chen, Y.; Du, L.; Wang, N.; Chen, D.; Luo, Q. ame-miR-5119-Eth axis modulates larval-pupal transition of western honeybee worker. Front. Physiol. 2024, 15, 1475306. [Google Scholar] [CrossRef]
- Wang, Y.; Azevedo, S.V.; Hartfelder, K.; Amdam, G.V. Insulin-like peptides (AmILP1 and AmILP2) differentially affect female caste development in the honey bee (Apis mellifera L.). J. Exp. Biol. 2013, 216, 4347–4357. [Google Scholar] [CrossRef]
- Sandoughi, M.; Saravani, M.; Rokni, M.; Nora, M.; Mehrabani, M.; Dehghan, A. Association between COX-2 and 15-PGDH polymorphisms and SLE susceptibility. Int. J. Rheum. Dis. 2020, 23, 627–632. [Google Scholar] [CrossRef]
- Hassannia, B.; Wiernicki, B.; Ingold, I.; Qu, F.; Van Herck, S.; Tyurina, Y.Y.; Bayır, H.; Abhari, B.A.; Angeli, J.P.F.; Choi, S.M. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Investig. 2018, 128, 3341–3355. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Yan, M.; Rerko, R.M.; Platzer, P.; Dawson, D.; Willis, J.; Tong, M.; Lawrence, E.; Lutterbaugh, J.; Lu, S.; Willson, J.K.V. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-β-induced suppressor of human gastrointestinal cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 17468–17473. [Google Scholar] [CrossRef]
- Castro-Sánchez, L.; Agra, N.; Izquierdo, C.L.; Motiño, O.; Casado, M.; Boscá, L.; Martín-Sanz, P. Regulation of 15-hydroxyprostaglandin dehydrogenase expression in hepatocellular carcinoma. Int. J. Biochem. Cell Biol. 2013, 45, 2501–2511. [Google Scholar] [CrossRef] [PubMed]
- Schmehl, D.R.; Tomé, H.V.; Mortensen, A.N.; Martins, G.F.; Ellis, J.D. Protocol for the in vitro rearing of honey bee (Apis mellifera L.) workers. J. Apic. Res. 2016, 55, 113–129. [Google Scholar] [CrossRef]
- Chen, X.; Fu, J. The microRNA miR-14 Regulates Egg-Laying by Targeting EcR in Honeybees (Apis mellifera). Insects 2021, 12, 351. [Google Scholar] [CrossRef]
- Friedrich, R. Morphometric Analysis and Classiffcation. In Biogeography and Taxonomy of Honeybees; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1988; pp. 66–78. [Google Scholar]
- Meixner, M.D.; Pinto, M.A.; Bouga, M.; Kryger, P.; Ivanova, E.; Fuchs, S. Standard methods for characterising subspecies and ecotypes of Apis mellifera. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Willmer, P.G.; Cunnold, H.; Ballantyne, G. Insights from measuring pollen deposition: Quantifying the pre-eminence of bees as flower visitors and effective pollinators. Arthropod-Plant Interact. 2017, 11, 411–425. [Google Scholar] [CrossRef]
- Lemanski, N.J.; Williams, N.M.; Rachael, W. Greater bee diversity is needed to maintain crop pollination over time. Nat. Ecol. Evol. 2022, 6, 1516–1523. [Google Scholar] [CrossRef]
- Raza, M.F.; Li, W. Biogenic amines in honey bee cognition: Neurochemical pathways and stress impacts. Curr. Opin. Insect Sci. 2025, 70, 101376. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Ghosh, S.; Sun, S.; Cheon, K.J.; Mohamadzade Namin, S.; Jung, C. Chlorella-supplemented diet improves the health of honey bee (Apis mellifera). Front. Ecol. Evol. 2022, 10, 922741. [Google Scholar] [CrossRef]
- Retschnig, G.; Rich, J.; Crailsheim, K.; Pfister, J.; Perreten, V.; Neumann, P. You are what you eat: Relative importance of diet, gut microbiota and nestmates for honey bee, Apis mellifera, worker health. Apidologie 2021, 52, 632–646. [Google Scholar] [CrossRef]
- Retschnig, G.; Williams, G.R.; Mehmann, M.M.; Yanez, O.; de Miranda, J.R.; Neumann, P. Sex-specific differences in pathogen susceptibility in honey bees (Apis mellifera). PLoS ONE 2014, 9, e85261. [Google Scholar] [CrossRef]
- Kapustjanskij, A.; Streinzer, M.; Paulus, H.; Spaethe, J. Bigger is better: Implications of body size for flight ability under different light conditions and the evolution of alloethism in bumblebees. Funct. Ecol. 2007, 21, 1130–1136. [Google Scholar] [CrossRef]
- Liao, W.; Wang, Y.; Qiao, X.; Zhang, X.; Deng, H.; Zhang, C.; Li, J.; Yuan, X.; Zhang, H. A Poly (dA: dT) Tract in the IGF1 Gene Is a Genetic Marker for Growth Traits in Pigs. Animals 2022, 12, 3316. [Google Scholar] [CrossRef]
- Zeng, B.; Huang, Y.; Xu, J.; Shiotsuki, T.; Bai, H.; Palli, S.R.; Huang, Y.; Tan, A. The FOXO transcription factor controls insect growth and development by regulating juvenile hormone degradation in the silkworm, Bombyx mori. J. Biol. Chem. 2017, 292, 11659–11669. [Google Scholar] [CrossRef]
- Santos, C.G.; Hartfelder, K. Insights into the dynamics of hind leg development in honey bee (Apis mellifera L.) queen and worker larvae—A morphology/differential gene expression analysis. Genet. Mol. Biol. 1900, 38, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Asencot, M.; Lensky, Y. Juvenile hormone induction of ‘queenliness’ on female honey bee (Apis mellifera L.) larvae reared on worker jelly and on stored royal jelly. Comp. Biochem. Physiol. Part B Comp. Biochem. 1984, 78, 109–117. [Google Scholar] [CrossRef]
- Dietz, A.; Hermann, H.; Blum, M. The role of exogenous JH I, JH III and Anti-JH (precocene II) on queen induction of 4.5-day-old worker honey bee larvae. J. Insect Physiol. 1979, 25, 503–512. [Google Scholar] [CrossRef]
- Rembold, H.; Czoppelt, C.; Rao, P. Effect of juvenile hormone treatment on caste differentiation in the honeybee, Apis mellifera. J. Insect Physiol. 1974, 20, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Schmidt Capella, I.C.; Hartfelder, K. Juvenile-hormone-dependent interaction of actin and spectrin is crucial for polymorphic differentiation of the larval honey bee ovary. Cell Tissue Res. 2002, 307, 265–272. [Google Scholar] [CrossRef]
- Ho, W.J.; Smith, J.N.P.; Park, Y.S.; Hadiono, M.; Christo, K.; Jogasuria, A.; Zhang, Y.; Broncano, A.V.; Kasturi, L.; Dawson, D.M. 15-PGDH Regulates Hematopoietic and Gastrointestinal Fitness During Aging. PLoS ONE 2022, 17, e0268787. [Google Scholar] [CrossRef]
- Koo, J.; Palli, S.R. Recent advances in understanding of the mechanisms of RNA interference in insects. Insect Mol. Biol. 2025, 34, 491–504. [Google Scholar] [CrossRef]
- Rachinsky, A.; Tobe, S.S.; Feldlaufer, M.F. Terminal steps in JH biosynthesis in the honey bee (Apis mellifera L.): Developmental changes in sensitivity to JH precursor and allatotropin. Insect Biochem. Mol. Biol. 2000, 30, 729–737. [Google Scholar] [CrossRef]
- Hartfelder, K.; Engels, W. Social insect polymorphism: Hormonal regulation of plasticity in development and reproduction in the honeybee. Curr. Top. Dev. Biol. 1998, 40, 45–77. [Google Scholar] [PubMed]
| Primer Name | Primer Sequence | Ref. |
|---|---|---|
| 15-PGDH-F 15-PGDH-R | 5′-CGGGTTTACCCCATGGTTTC-3′ 5′-CCGTCCCCATAAATCGCTGA-3′ | Designed by this study |
| β-actin-F β-actin-R | 5′-CTGCTGCATCATCCTCAAGC-3′ 5′-GAAAAGAGCCTCGGGACAAC-3′ | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, X.; Zhang, X.; Lu, H.; Xin, L.; Liu, R.; Chen, X. Expression of 15-PGDH Regulates Body Weight and Body Size by Targeting JH in Honeybees (Apis mellifera). Life 2025, 15, 1230. https://doi.org/10.3390/life15081230
Qu X, Zhang X, Lu H, Xin L, Liu R, Chen X. Expression of 15-PGDH Regulates Body Weight and Body Size by Targeting JH in Honeybees (Apis mellifera). Life. 2025; 15(8):1230. https://doi.org/10.3390/life15081230
Chicago/Turabian StyleQu, Xinying, Xinru Zhang, Hanbing Lu, Lingjun Xin, Ran Liu, and Xiao Chen. 2025. "Expression of 15-PGDH Regulates Body Weight and Body Size by Targeting JH in Honeybees (Apis mellifera)" Life 15, no. 8: 1230. https://doi.org/10.3390/life15081230
APA StyleQu, X., Zhang, X., Lu, H., Xin, L., Liu, R., & Chen, X. (2025). Expression of 15-PGDH Regulates Body Weight and Body Size by Targeting JH in Honeybees (Apis mellifera). Life, 15(8), 1230. https://doi.org/10.3390/life15081230






