Single-Night Sleep Extension Enhances Morning Physical and Cognitive Performance Across Time of Day in Physically Active University Students: A Randomized Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
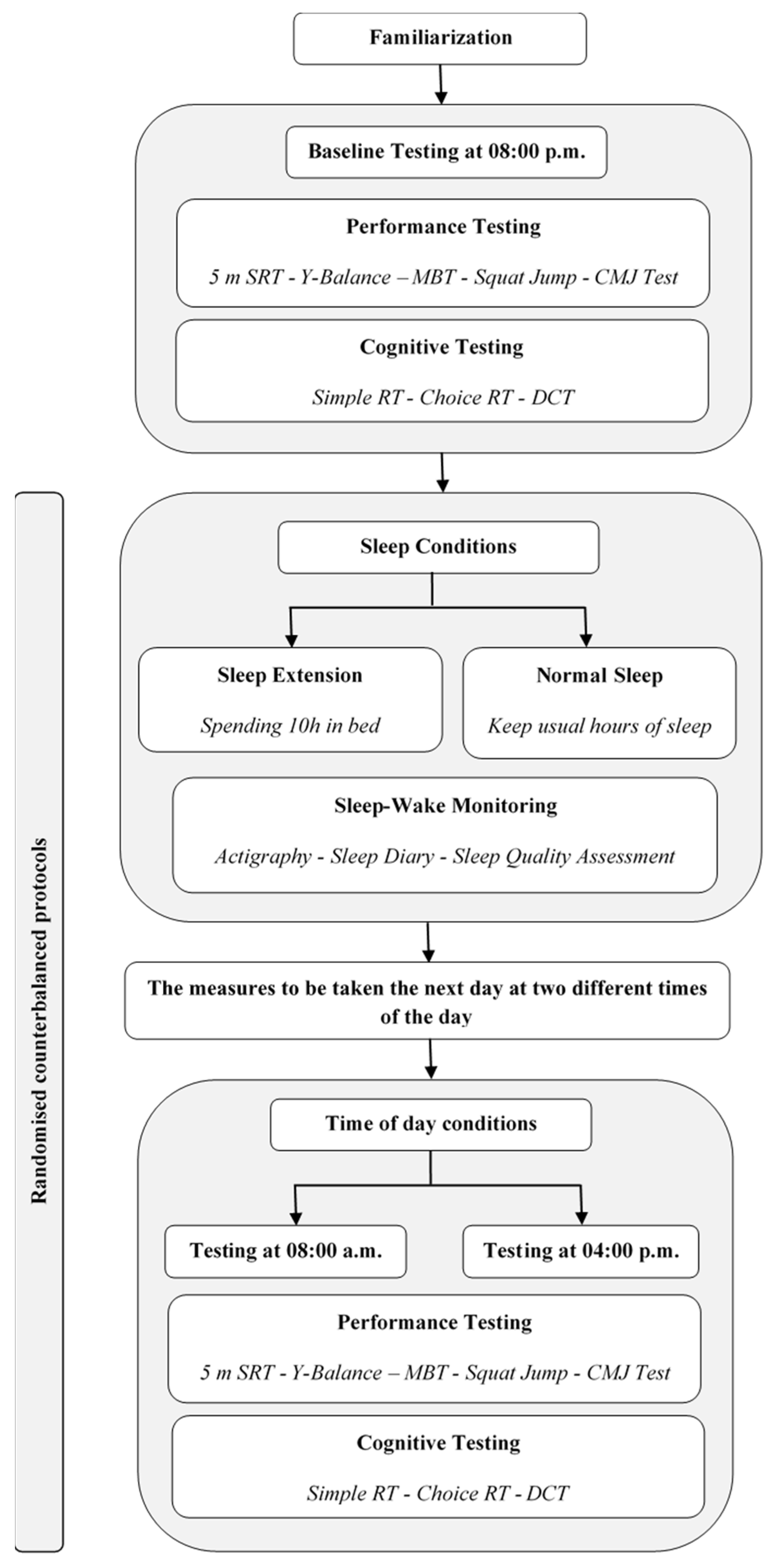
2.2. Study Design
2.2.1. Experimental Design
2.2.2. Experimental Protocol
2.3. Assessment Procedures
2.3.1. Sleep–Wake Monitoring
2.3.2. Performance Testing
2.3.3. Cognitive Testing
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Sleep Parameters
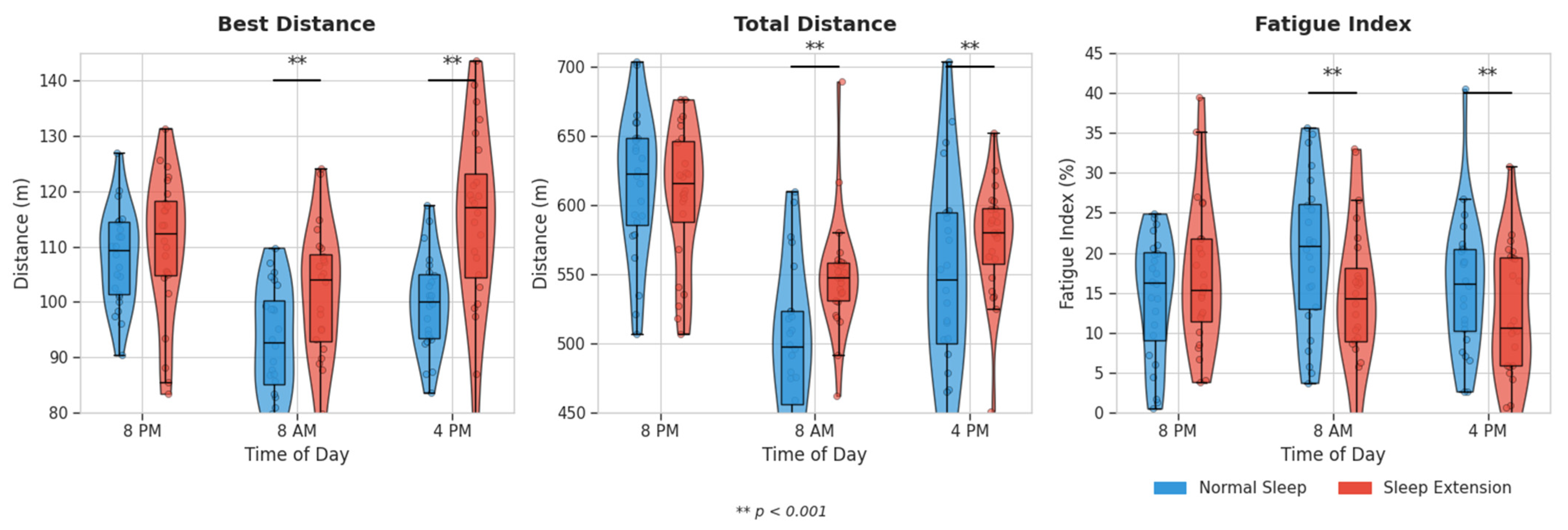
3.3. Physical Performance Outcomes
3.3.1. 5 m Shuttle-Run Test
3.3.2. Jump Performance
3.3.3. Medicine Ball Throw and Y-Balance Test
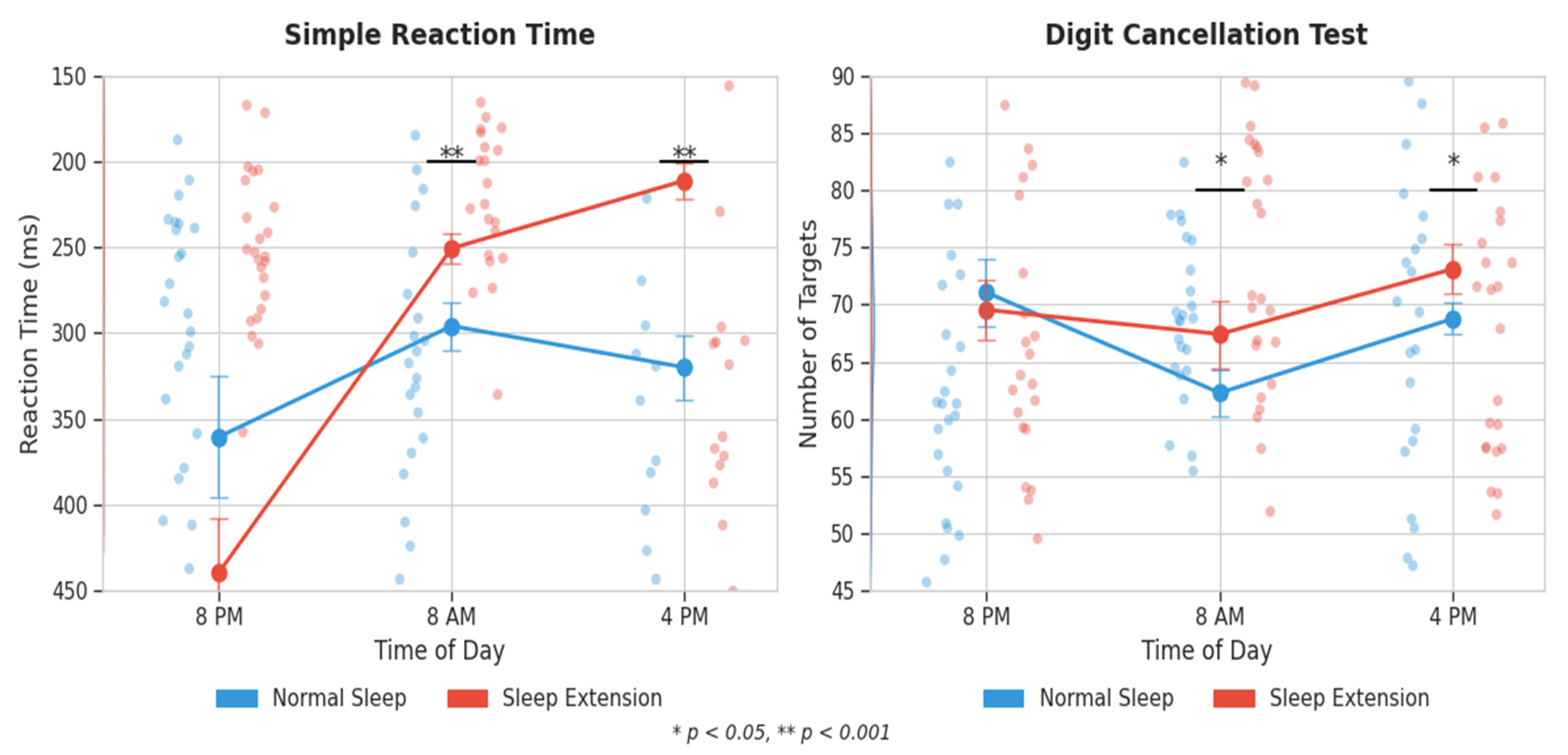
3.4. Cognitive Outcomes
3.4.1. Reaction Time Tests
3.4.2. Digit Cancellation Test
3.5. Time-of-Day Effects and Sleep–Performance Relationships
4. Discussion
4.1. Effects on Physical Performance
4.2. Effects on Cognitive Performance
4.3. Time-of-Day Effects and Chronobiological Considerations
4.4. Neurophysiological Mechanisms
4.5. Relationship Between Sleep Parameters and Performance
4.6. Limitations
4.7. Practical Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BD | best distance |
| BMI | body mass index |
| CI | confidence interval |
| CMJ | countermovement jump |
| CRT | choice reaction time |
| CV | coefficient of variation |
| DCT | digit cancellation test |
| EXT | sleep extension |
| FI | fatigue index |
| ICC | intraclass correlation coefficient |
| LLC | Limited Liability Company |
| min | minutes |
| ms | milliseconds |
| NS | normal sleep |
| PSQI | Pittsburgh Sleep Quality Index |
| REM | rapid eye movement |
| RPE | rating of perceived exertion |
| RT | reaction time |
| SJ | squat jump |
| SRT | shuttle-run test |
| TD | total distance |
| η2p | partial eta squared |
References
- Fullagar, H.H.; Skorski, S.; Duffield, R.; Hammes, D.; Coutts, A.J.; Meyer, T. Sleep and athletic performance: The effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2015, 45, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.M. Sleep and Athletic Performance. Curr. Sports Med. Rep. 2017, 16, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.S.H.; Teo, W.P.; Warmington, S.A. Effects of training and competition on the sleep of elite athletes: A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.A.; Costa, J.A.; Marques, E.A.; Brito, J.; Lastella, M.; Figueiredo, P. The Impact of Sleep Interventions on Athletic Performance: A Systematic Review. Sports Med. Open 2023, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Bonnar, D.; Bartel, K.; Kakoschke, N.; Lang, C. Sleep Interventions Designed to Improve Athletic Performance and Recovery: A Systematic Review of Current Approaches. Sports Med. 2018, 48, 683–703. [Google Scholar] [CrossRef] [PubMed]
- Simpson, N.S.; Gibbs, E.L.; Matheson, G.O. Optimizing sleep to maximize performance: Implications and recommendations for elite athletes. Scand. J. Med. Sci. Sports 2017, 27, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.D.; Mah, K.E.; Kezirian, E.J.; Dement, W.C. The effects of sleep extension on the athletic performance of collegiate basketball players. Sleep 2011, 34, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Simon, R.D., Jr. Sleep extension improves serving accuracy: A study with college varsity tennis players. Physiol. Behav. 2015, 151, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Charest, J.; Grandner, M.A. Sleep and Athletic Performance: Impacts on Physical Performance, Mental Performance, Injury Risk and Recovery, and Mental Health. Sleep Med. Clin. 2020, 15, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Duraccio, K.M.; Kamhout, S.; Baron, K.G.; Reutrakul, S.; Depner, C.M. Sleep extension and cardiometabolic health: What it is, possible mechanisms and real-world applications. J. Physiol. 2024, 602, 6571–6586. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Halson, S.L.; Weakley, J.; Hawley, J.A. Sleep, circadian biology and skeletal muscle interactions: Implications for metabolic health. Sleep Med. Rev. 2022, 66, 101700. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Embang, J.E.G.; Tan, Y.H.V.; Ng, Y.X.; Loyola, G.J.P.; Wong, L.-W.; Guo, Y.; Dong, Y. Role of sleep and neurochemical biomarkers in synaptic plasticity related to neurological and psychiatric disorders: A scoping review. J. Neurochem. 2025, 169, e16270. [Google Scholar] [CrossRef] [PubMed]
- Drust, B.; Waterhouse, J.; Atkinson, G.; Edwards, B.; Reilly, T. Circadian rhythms in sports performance—An update. Chronobiol. Int. 2005, 22, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, S.; Shibata, S. Time-of-Day-Dependent Physiological Responses to Meal and Exercise. Front. Nutr. 2020, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, S.J.; Esser, K.A. The clockwork of champions: Influence of circadian biology on exercise performance. Free Radic. Biol. Med. 2024, 224, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Bonato, M.; Galasso, L.; La Torre, A.; Merati, G.; Montaruli, A.; Roveda, E.; Carandente, F. Sleep quality and high intensity interval training at two different times of day: A crossover study on the influence of the chronotype in male collegiate soccer players. Chronobiol. Int. 2017, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Lichstein, K.L.; Morin, C.M. Recommendations for a standard research assessment of insomnia. Sleep 2006, 29, 1155–1173. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, H.; Souissi, N. The effect of training at a specific time of day: A review. J. Strength. Cond. Res. 2012, 26, 1984–2005. [Google Scholar] [CrossRef] [PubMed]
- Rabat, A.; Arnal, P.J.; Monnard, H.; Erblang, M.; Van Beers, P.; Bougard, C.; Drogou, C.; Guillard, M.; Sauvet, F.; Leger, D.; et al. Limited Benefit of Sleep Extension on Cognitive Deficits During Total Sleep Deprivation: Illustration with Two Executive Processes. Front. Neurosci. 2019, 13, 591. [Google Scholar] [CrossRef] [PubMed]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Ait-Aoudia, M.; Levy, P.P.; Bui, E.; Insana, S.; de Fouchier, C.; Germain, A.; Jehel, L. Validation of the French version of the Pittsburgh Sleep Quality Index Addendum for posttraumatic stress disorder. Eur. J. Psychotraumatol. 2013, 4, 19298. [Google Scholar] [CrossRef] [PubMed]
- Karakoç, B.; Eken, Ö.; Kurtoğlu, A.; Arslan, O.; Eken, I.; Elkholi, S.M. Time-of-Day Effects on Post-Activation Potentiation Protocols: Effects of Different Tension Loads on Agility and Vertical Jump Performance in Judokas. Medicina 2025, 61, 426. [Google Scholar] [CrossRef] [PubMed]
- Spieszny, M.; Trybulski, R.; Biel, P.; Zając, A.; Krzysztofik, M. Post-Isometric Back Squat Performance Enhancement of Squat and Countermovement Jump. Int. J. Environ. Res. Public Health 2022, 19, 12720. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, S.W.; Teyhen, D.S.; Lorenson, C.L.; Warren, R.L.; Koreerat, C.M.; Straseske, C.A.; Childs, J.D. Y-balance test: A reliability study involving multiple raters. Mil. Med. 2013, 178, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Stockbrugger, B.A.; Haennel, R.G. Validity and reliability of a medicine ball explosive power test. J. Strength. Cond. Res. 2001, 15, 431–438. [Google Scholar] [PubMed]
- Boddington, M.K.; Lambert, M.I.; St Clair Gibson, A.; Noakes, T.D. Reliability of a 5-m multiple shuttle test. J. Sports Sci. 2001, 19, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Polechoński, J.; Langer, A. Assessment of the Relevance and Reliability of Reaction Time Tests Performed in Immersive Virtual Reality by Mixed Martial Arts Fighters. Sensors 2022, 22, 4762. [Google Scholar] [CrossRef] [PubMed]
- Hatta, T.; Yoshizaki, K.; Ito, Y.; Mase, M.; Kabasawa, H. Reliability and validity of the digit cancellation test, a brief screen of attention. Psychologia 2012, 55, 246–256. [Google Scholar] [CrossRef]
- Mong, J.A.; Cusmano, D.M. Sex differences in sleep: Impact of biological sex and sex steroids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150110. [Google Scholar] [CrossRef] [PubMed]
- Patrick, Y.; Lee, A.; Raha, O.; Pillai, K.; Gupta, S.; Sethi, S.; Mukeshimana, F.; Gerard, L.; Moghal, M.U.; Saleh, S.N.; et al. Effects of sleep deprivation on cognitive and physical performance in university students. Sleep. Biol. Rhythm. 2017, 15, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Dutil, C.; De Pieri, J.; Sadler, C.M.; Maslovat, D.; Chaput, J.-P.; Carlsen, A.N. Chronic short sleep duration lengthens reaction time, but the deficit is not associated with motor preparation. J. Sleep Res. 2025, 34, e14231. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.E.; Benasi, G.; Hale, C.; Yeung, L.-K.; Cochran, J.; Brickman, A.M.; St-Onge, M.-P. The effects of insufficient sleep and adequate sleep on cognitive function in healthy adults. Sleep Health 2024, 10, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Tai, X.Y. Sleep Duration and Executive Function in Adults. Curr. Neurol. Neurosci. Rep. 2023, 23, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Léger, D.; Debellemaniere, E.; Rabat, A.; Bayon, V.; Benchenane, K.; Chennaoui, M. Slow-wave sleep: From the cell to the clinic. Sleep Med. Rev. 2018, 41, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Lastella, M.; Roach, G.D.; Halson, S.L.; Sargent, C. Sleep/wake behaviours of elite athletes from individual and team sports. Eur. J. Sport Sci. 2015, 15, 94–100. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Normal Sleep | Sleep Extension | Mean Difference [95% CI] | p-Value | Cohen’s d |
|---|---|---|---|---|---|
| Time in bed (min) | 514.2 ± 58.9 | 563.6 ± 62.4 | 49.4 [39.3, 59.5] | <0.001 | 0.82 |
| Total sleep time (min) | 476.5 ± 64.2 | 531.3 ± 56.8 | 54.8 [43.3, 66.3] | <0.001 | 0.91 |
| Sleep efficiency (%) | 93.7 ± 4.3 | 95.1 ± 3.8 | 1.4 [0.1, 2.7] | 0.045 | 0.34 |
| Sleep latency (min) | 22.6 ± 16.8 | 19.5 ± 13.2 | −3.1 [−6.9, 0.7] | 0.105 | 0.21 |
| Variable | Time | Normal Sleep | Sleep Extension | Condition Effect | Time Effect | Interaction Effect | |
|---|---|---|---|---|---|---|---|
| 5 m SRT | Best Distance (m) | 8:00 PM | 108.5 ± 10.3 | 109.7 ± 10.5 | p < 0.001 η2p = 0.561 | p < 0.001 η2p = 0.591 | p = 0.012 η2p = 0.174 |
| 8:00 AM | 93.3 ± 8.5 | 102.8 ± 11.9 ** | |||||
| 4:00 PM | 98.2 ± 9.8 | 110.6 ± 11.4 ** | |||||
| Total Distance (m) | 8:00 PM | 591.2 ± 57.1 | 598.3 ± 58.2 | p < 0.001 η2p = 0.598 | p < 0.001 η2p = 0.567 | p = 0.026 η2p = 0.147 | |
| 8:00 AM | 510.3 ± 49.6 | 547.3 ± 43.2 ** | |||||
| 4:00 PM | 539.0 ± 55.3 | 586.0 ± 45.3 ** | |||||
| Fatigue Index (%) | 8:00 PM | 15.8 ± 7.6 | 15.1 ± 7.7 | p < 0.001 η2p = 0.431 | p < 0.001 η2p = 0.457 | p = 0.006 η2p = 0.199 | |
| 8:00 AM | 21.2 ± 9.5 | 13.1 ± 8.3 ** | |||||
| 4:00 PM | 16.5 ± 8.4 | 10.2 ± 7.0 ** | |||||
| RPE | 8:00 PM | 7.5 ± 0.6 | 7.2 ± 0.5 | p = 0.005 η2p = 0.290 | p < 0.001 η2p = 0.417 | p = 0.166 η2p = 0.075 | |
| 8:00 AM | 8.4 ± 0.6 | 7.8 ± 0.4 ** | |||||
| 4:00 PM | 8.0 ± 0.5 | 7.5 ± 0.3 * | |||||
| Y-Balance Test | Right (%) | 8:00 PM | 115.5 ± 13.8 | 115.9 ± 14.0 | p = 0.010 η2p = 0.255 | p = 0.031 η2p = 0.140 | p = 0.158 η2p = 0.077 |
| 8:00 AM | 109.3 ± 12.2 | 112.8 ± 12.9 | |||||
| 4:00 PM | 110.1 ± 13.4 | 115.0 ± 12.6 * | |||||
| Left (%) | 8:00 PM | 103.5 ± 19.7 | 103.8 ± 19.5 | p = 0.031 η2p = 0.186 | p = 0.071 η2p = 0.109 | p = 0.203 η2p = 0.067 | |
| 8:00 AM | 97.7 ± 16.3 | 99.5 ± 16.8 | |||||
| 4:00 PM | 98.1 ± 17.0 | 101.7 ± 16.3 * | |||||
| Medicine Ball Throw (m) | 8:00 PM | 9.8 ± 2.0 | 9.9 ± 2.1 | p = 0.008 η2p = 0.269 | p = 0.019 η2p = 0.159 | p = 0.121 η2p = 0.088 | |
| 8:00 AM | 8.9 ± 2.2 | 9.4 ± 2.2 * | |||||
| 4:00 PM | 9.8 ± 2.3 | 10.5 ± 2.4 * | |||||
| Squat Jump Height (cm) | 8:00 PM | 28.5 ± 8.1 | 29.0 ± 8.4 | p = 0.003 η2p = 0.329 | p = 0.002 η2p = 0.231 | p = 0.029 η2p = 0.143 | |
| 8:00 AM | 26.3 ± 7.2 | 28.2 ± 8.0 * | |||||
| 4:00 PM | 27.7 ± 7.3 | 29.6 ± 7.5 * | |||||
| CMJ Height (cm) | 8:00 PM | 30.4 ± 8.7 | 30.6 ± 8.6 | p = 0.006 η2p = 0.287 | p = 0.010 η2p = 0.183 | p = 0.043 η2p = 0.128 | |
| 8:00 AM | 28.0 ± 8.1 | 29.4 ± 8.6 * | |||||
| 4:00 PM | 29.3 ± 8.7 | 31.1 ± 9.0 * | |||||
| Simple RT (ms) | 8:00 PM | 362.8 ± 159.8 | 359.7 ± 151.9 | p < 0.001 η2p = 0.504 | p < 0.001 η2p = 0.415 | p < 0.001 η2p = 0.261 | |
| 8:00 AM | 296.4 ± 75.2 | 252.8 ± 55.3 ** | |||||
| 4:00 PM | 334.1 ± 91.2 | 209.5 ± 47.8 ** | |||||
| Choice RT Accuracy (%) | 8:00 PM | 95.1 ± 2.9 | 95.8 ± 2.6 | p = 0.004 η2p = 0.310 | p = 0.008 η2p = 0.189 | p = 0.124 η2p = 0.087 | |
| 8:00 AM | 96.4 ± 2.4 | 97.9 ± 1.6 * | |||||
| 4:00 PM | 95.2 ± 3.2 | 98.0 ± 1.4 ** | |||||
| DCT (targets) | 8:00 PM | 70.9 ± 15.3 | 70.3 ± 14.7 | p = 0.002 η2p = 0.360 | p = 0.009 η2p = 0.186 | p = 0.003 η2p = 0.229 | |
| 8:00 AM | 63.0 ± 10.0 | 67.6 ± 12.6 * | |||||
| 4:00 PM | 68.0 ± 10.3 | 73.3 ± 11.0 * | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzouraa, E.; Dhahbi, W.; Ferchichi, A.; Geantă, V.A.; Kunszabo, M.I.; Chtourou, H.; Souissi, N. Single-Night Sleep Extension Enhances Morning Physical and Cognitive Performance Across Time of Day in Physically Active University Students: A Randomized Crossover Study. Life 2025, 15, 1178. https://doi.org/10.3390/life15081178
Bouzouraa E, Dhahbi W, Ferchichi A, Geantă VA, Kunszabo MI, Chtourou H, Souissi N. Single-Night Sleep Extension Enhances Morning Physical and Cognitive Performance Across Time of Day in Physically Active University Students: A Randomized Crossover Study. Life. 2025; 15(8):1178. https://doi.org/10.3390/life15081178
Chicago/Turabian StyleBouzouraa, Eya, Wissem Dhahbi, Aymen Ferchichi, Vlad Adrian Geantă, Mihai Ioan Kunszabo, Hamdi Chtourou, and Nizar Souissi. 2025. "Single-Night Sleep Extension Enhances Morning Physical and Cognitive Performance Across Time of Day in Physically Active University Students: A Randomized Crossover Study" Life 15, no. 8: 1178. https://doi.org/10.3390/life15081178
APA StyleBouzouraa, E., Dhahbi, W., Ferchichi, A., Geantă, V. A., Kunszabo, M. I., Chtourou, H., & Souissi, N. (2025). Single-Night Sleep Extension Enhances Morning Physical and Cognitive Performance Across Time of Day in Physically Active University Students: A Randomized Crossover Study. Life, 15(8), 1178. https://doi.org/10.3390/life15081178






