The Role of Rehabilitation Program in Managing the Triad of Sarcopenia, Obesity, and Chronic Pain
Abstract
1. Introduction
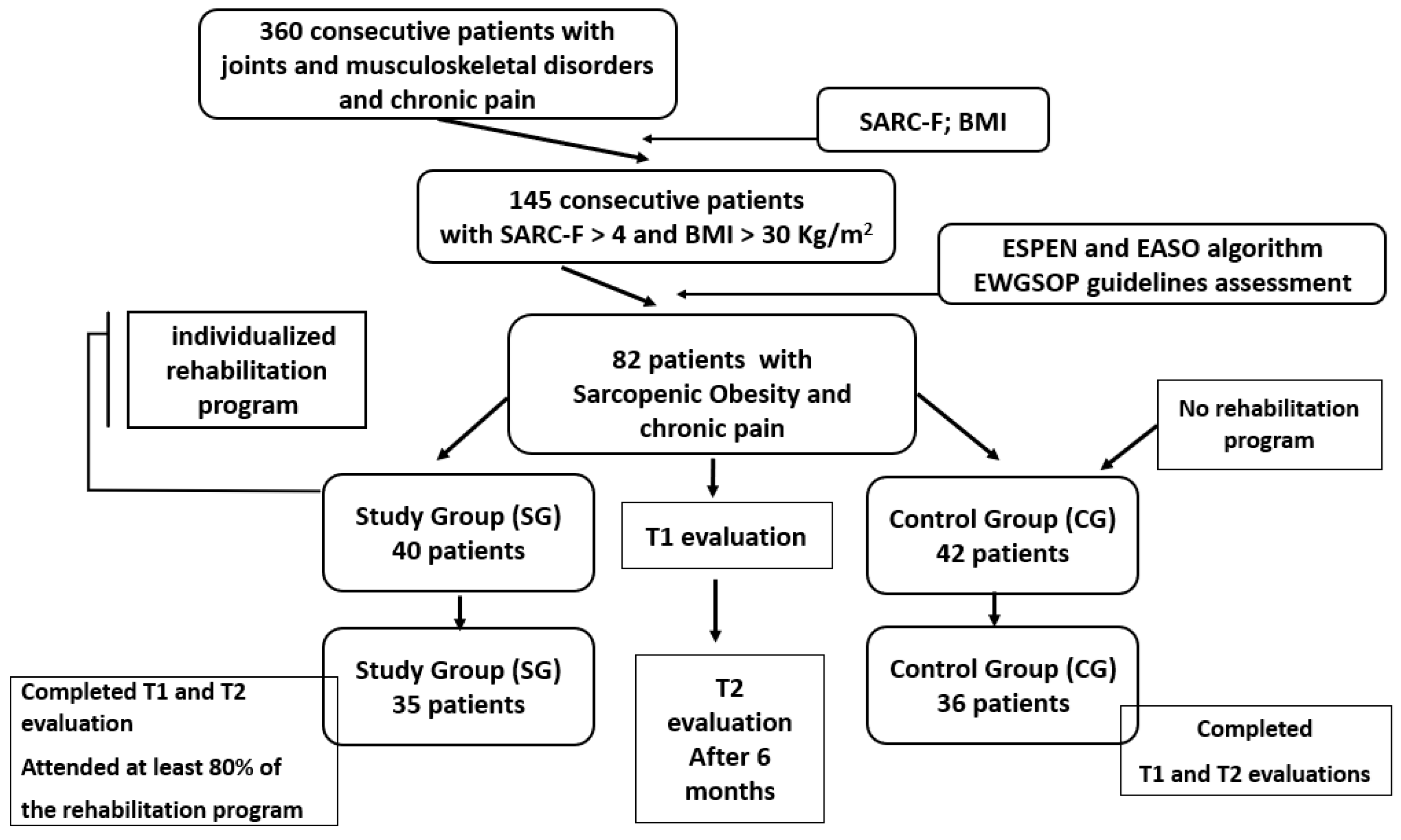
2. Materials and Methods
2.1. Design Overview
- -
- Study group (SG): 40 participants engaged in a 6-month tailored rehabilitation program;
- -
- Control group (CG): 42 participants maintained their usual daily routine.
2.2. Participants
2.2.1. Eligibility Criteria
2.2.2. Recruitment and Initial Screening
2.2.3. Sarcopenia Diagnosis
- Step 1—Muscle Strength and Physical Performance:
- -
- Handgrip strength (HGS) was measured with a Saehan SH5008 dynamometer. Patients were instructed to stand upright with the dynamometer beside them, and the elbow was flexed to a 90° angle. Maximal isometric effort for 5 s was performed three times for the dominant side. The best of all attempts was used for study; the cut-off value for men: <27 kg; for women: <16 kg [30];
- -
- Gait velocity was assessed by instructing the participants to traverse an 8 m path. The timing commenced when the participant crossed the 1 m mark and concluded at the 7 m mark, covering a span of 6 m. This procedure was repeated twice, and the superior performance was noted. A threshold speed of 0.8 m per second was established as the criterion [31].
- Step 2—Body Composition Analysis:
- -
- Patients with reduced HGS or gait speed underwent Bioelectrical Impedance Analysis (BIA) using the Omron BF511 device. Parameters included skeletal muscle mass (SMM), BF%, and SMMI. To minimize variability and ensure accurate results, the bioimpedance analysis was conducted under standardized conditions: at the same time of day, following similar food intake patterns, at least a few hours after any physical activity. The measurements were performed by the same evaluator, and results were interpreted using standardized values adjusted for age and sex [32];
- -
- Pathological values were defined as SMM < 24% of body weight or SMMI < 7 kg/m2 for men and <5.7 kg/m2 for women [33];
- -
- Obesity was defined as body fat > 60th percentile: >27% for men and >38% for women [34].
2.2.4. Clinical and Functional Assessments
- -
- Participants underwent laboratory analysis, including C-reactive protein, fibrinogen, lipid profile, adiponectin, leptin, and TNF-α using ELISA kits (Biovendor R&D, Brno, Czech Republic);
- -
- Physical performance was assessed with SPPB, evaluating balance, gait speed, and chair rise ability. The SPPB is used to objectively assess lower limb function in older adults through three tests: static body balance, lower limb muscle strength (chair stand test), and gait (4 m gait walk) [36]. For balance, the patient had to maintain three different positions for 10 s: (a) Feet together, (b) semi-tandem position (the ankle of one foot behind the joint of the other foot), and (c) tandem position (the toes of one foot directly behind the heel of the other foot and touching it). For the chair stand test and 4 m gait walk, the same procedure was followed as explained above. For each of the tests, scores range from 0 to 4 points, with a maximum score on the instrument of 12 points. A higher score indicated a better physical performance. A score less than or equal to 8 is indicative of severe sarcopenia [37];
- -
- Pain severity was evaluated with NRS, a validated 0–10 scale (0 = no pain; 10 = worst imaginable pain) [38];
- -
- To assess central sensitization, PPT was measured bilaterally at the lumbar region using an Algometer II (SBMedic Electronics, Sweden). The probe (1 cm2) was applied perpendicularly with pressure increasing at ~1 N/s until pain was reported. The mean of three trials at 30 s intervals was recorded [39,40]. The lumbar location was selected due to literature evidence linking chronic low back pain with muscle degradation and systemic sensitization mechanisms in musculoskeletal disorders [41].
2.2.5. Sarcopenia-Specific Quality of Life
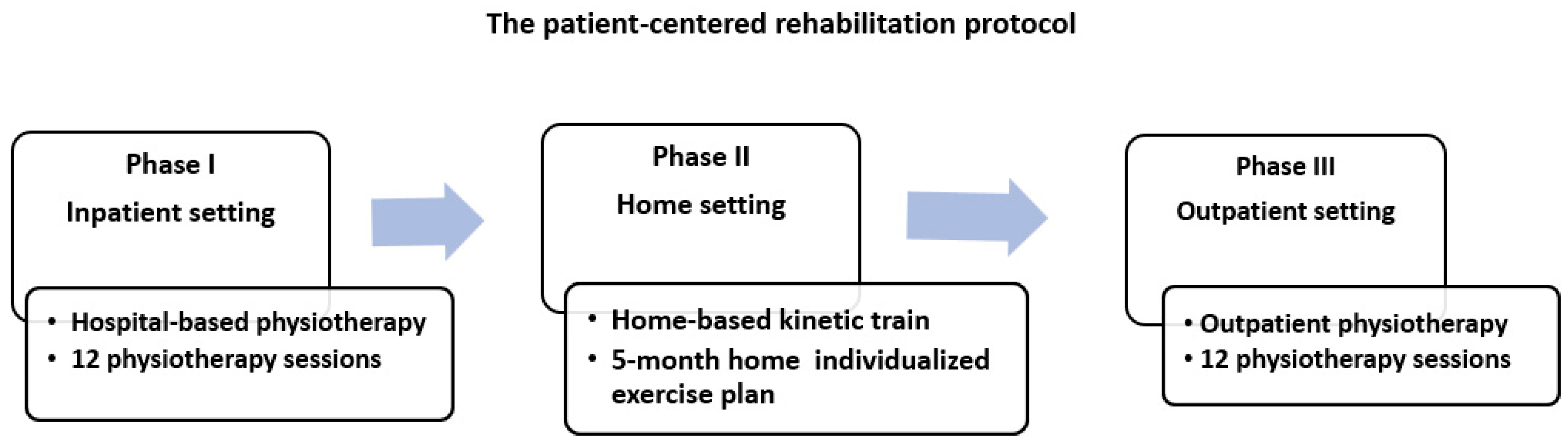
2.3. Study Treatment
- -
- Patient education and support: Counseling regarding the impact of obesity and sarcopenia on functional health, and the pivotal role of physical activity in improving body composition and reducing chronic pain;
- -
- Nutritional support: Emphasis on protein intake (1.2–1.5 g/kg body weight/day), distributed evenly across meals to enhance muscle protein synthesis. Patients were advised on tailored dietary strategies to simultaneously address sarcopenia and obesity to prevent further decompensations [44];
- -
- Pharmacological treatment: Supplementation with vitamin D and calcium was recommended to support musculoskeletal integrity. The administration of additional analgesic treatment was not permitted during the study period.
- Phase I (hospital-based physiotherapy): 12 physiotherapy sessions conducted in an inpatient setting;
- Phase II (home-based kinetic training): 5-month individualized exercise plan performed at home, with monthly in-person monitoring by outpatient services;
- Phase III (outpatient physiotherapy): A second round of 12 physiotherapy sessions provided through the outpatient clinic.
- -
- Pulsed Electromagnetic Field Therapy (PEMF) and focused magnetic field (FMF) were incorporated due to their anti-inflammatory, analgesic, and regenerative effects at the cellular level. PEMF promotes microcirculation, supports soft tissue repair, and modulates inflammatory cascades, which is particularly relevant in chronic musculoskeletal conditions. FMF application allows focused energy delivery to targeted areas such as thighs or lumbar spine, enhancing localized healing responses [53,54,55];
- -
- Electrical muscle stimulation (EMS) was employed to enhance skeletal muscle function through the stimulation of type II muscle fibers, which are typically lost during sarcopenia. Low-frequency stimulation focused on improving neuromuscular activation and strength, while higher-frequency protocols promoted muscle mass preservation. The method is especially useful for patients with limited physical capacity, offering muscle engagement without joint overload [56,57,58];
- -
- Low-Level Laser Therapy (LLLT) was selected for its myoregenerative and anti-inflammatory properties. The use of infrared wavelengths (808 nm) has demonstrated beneficial effects on mitochondrial function and ATP synthesis, supporting muscle tissue regeneration and reducing oxidative stress—both relevant in the treatment of sarcopenia-related muscle degeneration [59,60,61,62];
- -
- Deep oscillation therapy via manual applicator was chosen for its dual-phase therapeutic impact: an initial high-frequency phase (100 Hz) for analgesia and muscle relaxation, followed by a low-frequency range (5–25 Hz) to stimulate local metabolism and enhance lymphatic flow, contributing to pain relief and functional recovery in soft tissues [62,63,64];
- -
- Kinesiotherapy, delivered in the form of resistance training, balance and gait exercises, and aerobic training, was integrated to comprehensively address muscle endurance, postural control, cardiovascular health, and mobility [65,66,67,68]. The protocols emphasized low-impact, progressive load strategies to prevent pain exacerbation and ensure safety, particularly considering comorbid low back pain in the target population.
| Components | Description | |
|---|---|---|
| The first stage (2 weeks) 12 sessions of physio- and kinesiotherapy Inpatient | Pulsed Electromagnetic Field Therapy (PEMF) with focused magnetic field (FMF) (BTL-5000 Czech Republic by BTL Industries (Prague, Czech Republic)) | Lumbar solenoid (60 cm) and disc applicator (13 × 13 × 3 cm) on both thighs (BTL-239-1). Key parameters: Rectangular pulses with 10 Hz frequency, 50 µT intensity. For FMF, applied through disc applicator, the total intensity used was 128 mT. 30 min per session, twice daily (morning session, after waking up, at 9 a.m. and evening session before going to bed at 7 p.m.). |
| Electrical muscle stimulation (EMS) of the lumbar region, quadriceps muscles, and calf muscles (Endomed 482, device series 42.400, Enraf-Nonius, Netherlands) | Biphasic rectangular pulses, pulse frequency 100 Hz (Hertz), in steps of 1 Hz, pulse-width of 150 µs, 2 milliseconds phase duration, 30 Hz current frequency were used. Established load ratio of 3 s of current followed by 3 s of rest (ratio 1:1). Intensity: 10 mA (adjusted to patient comfort, typically to the point of visible muscle contraction without causing pain). 30 min daily session of EMS for 6 days each week using two pairs of 10 cm × 15 cm carbon rubber electrodes (150 cm2), one for each side, stimulated both muscle groups and lumbar region, 10 min each region. | |
| Low-Level Laser Therapy (LLLT) 20 min, daily (ASTAR PhysioGo 500I/501I Poland, PhysioGo series) | Wavelength of 808 nm, a power of 100 mW, an energy dosage of 7 J/cm2, and energy per point of 0.003 J. Applied to shoulder girdle muscles, quadriceps, and major gluteus, left and right. | |
| Deep oscillation therapy with manual applicator Personal device DOP1.1.—INDIVID—Physiomed (device series—2442007) Germany, Physiomed Elektromedizin AG | First, 10 min—high frequency 100 Hz—in the lumbosacral region. Then, 10 min—low frequency 5–25 Hz. 5 cm oscillator head applied in both thigh muscles. Total: 30 min daily. | |
Kinesiotherapy:
40 min | Resistance exercises (strength training)—performed a.m. Muscle groups: Focus on low-impact exercises that target both the upper and lower body but are gentle on the back. Include exercises that strengthen the core, as this can help alleviate low back pain. Load: Start with very light weights or body-weight exercises, particularly for the lower back and core. The initial load should be about 20–30% of baseline strength to avoid straining the back. Repetitions and sets: Perform higher repetitions (10–15) at a lower intensity to focus on muscle endurance, crucial for sarcopenia. Keep to 2–3 sets to avoid fatigue. Rest intervals: Extend rest intervals to 120–180 s between sets to ensure full recovery, especially important for patients with low back pain. Equipment: Utilize resistance bands (TheraBand Latex Resistance Bands) and body-weight exercises to minimize stress on the lumbar spine. Balance and gait training—performed in a.m. Supportive equipment: Incorporate balance exercises that can be performed while seated or holding onto a stable object to reduce the risk of falls and lower back strain. Duration and intensity: Start with short sessions of simple balance exercises (mono- and bipedal walking), gradually increasing the complexity as the patient’s balance improves. Endurance training (aerobic training)—performed in p.m. Many repetitions, low resistance, using large muscle groups. Exercise selection: Choose non-weight-bearing activities such as walking or cycling on a recumbent bike to reduce impact on the back and joints. Intensity and duration: Begin with very low intensity (20–30% of maximum heart rate) for short durations (5 min). Gradually increase as tolerated without exacerbating pain. Monitoring: Keep a close watch on the patient’s back pain during exercises, adjusting the program as needed to avoid discomfort. Warm-up and cool-down: Emphasize gentle stretching, particularly of the lower back and core muscles, and include breathing exercises to help relax the muscles and reduce pain. | |
| The second stage (5 months)—see Table 2 Home training kinetic program with monthly monitoring through the outpatient service | ||
| The third stage (2 weeks) 12 physio- and kinesiotherapy sessions Outpatient | Pulsed Electromagnetic Field Therapy (PEMF) with focused magnetic field (FMF) (BTL-5000 Czech Republic by BTL Industries) | Lumbar solenoid (60 cm) and disc applicator (13 × 13 × 3 cm) on both thighs (BTL-239-1). Key parameters: Rectangular pulses with 10 Hz frequency, 50 µT intensity. For FMF, applied through disc applicator, the total intensity used was 128 mT. 30 min per session, twice daily (morning session, after waking up, at 9 a.m. and evening session before going to bed at 7 p.m.) |
| Low-Level Laser Therapy (LLLT) 10 min, daily ASTAR PhysioGo 500I/501I Poland, PhysioGo series | Wavelength of 660 nm, a power of 100 mW, an energy dosage of 5 J/cm2, and energy per point of 0.003 J. | |
| Deep oscillation—therapy with manual applicator Personal device DOP1.1.—INDIVID—Physiomed (device series—2442007) Germany, Physiomed Elektromedizin AG | First, 10 min—high frequency at 100 Hz. Then, 10 min—low frequency at 5–25 Hz. 5 cm oscillator head applied in both thigh muscles. Total: 30 min daily. | |
Kinesiotherapy
| Implement balance training such as standing on one foot, walking heel-to-toe, or using balance boards to reduce fall risk and improve body coordination. (10 min) Practice functional movements that mimic daily activities, such as stepping up and down a stair, sitting down and standing up from a chair, and carrying weights to simulate grocery bags (10 min). Pedaling on the elliptical bike. Begin with short durations (10–15 min) and gradually increase to 20 min. | |
- -
- Progression: Gradual increase in exercise intensity and duration, adjusted to each patient’s tolerance;
- -
- Safety: Emphasis on using support or supervision during training to prevent falls or injuries;
- -
- Hydration and nutrition: Reminders to maintain adequate hydration and consume a balanced, protein-rich diet to support recovery and muscle mass preservation.
| Day | Type of Training | Activities | Duration |
|---|---|---|---|
| Day 1 | Aerobic training | Warm-up (10 min): Gentle stretching and slow walking or stationary cycling Main activity (20 min): Walking on flat surface or treadmill Cool-down (10 min): Slow walking and stretching | 40 min |
| Day 2 | Resistance training | Warm-up (10 min): Light stretching and mobility exercises Focus on gentle movements; avoid any that cause discomfort Main activity (20 min):
| 40 min |
| Day 3 | Balance training | Warm-up (10 min): Gentle stretching of the upper and lower body. To prepare the muscles and joints for exercise, reducing the risk of injury. Ensure that stretches are performed gently and within a comfortable range of motion, especially for the lower back. Include dynamic stretches that mimic the movements of the main activity to better prepare the body. Main activity (20 min): Seated leg extensions to strengthen the quadriceps, which are crucial for knee stability and balance. Sit in a sturdy chair, extend one leg out straight, hold for a few seconds, and then lower it back down. Repeat with the other leg for 6 repetitions, 30–60 s rest periods, perform slowly, ensuring each leg is extended fully before lowering Chair squats to strengthen the lower body, including the hips, thighs, and buttocks, which support balance. Stand in front of a chair with feet hip-width apart. Slowly bend the knees and lower the body as if to sit, touch the chair lightly, then stand back up. 6 repetitions, 30–60 s rest periods, stand and lower to just touch the chair, then stand back up slowly Wall push-ups to enhance upper body strength, which helps in maintaining overall stability. Stand facing a wall, place hands on the wall at shoulder width and level, then bend the elbows to bring the chest towards the wall and push back to the starting position. 6 repetitions, 30–60 s rest periods, adjust the distance from the wall to maintain comfort; push up slowly. Cool-down (10 min): Gentle stretching focusing on the legs and lower back. To relax the muscles and gradually return the heart rate to normal, preventing muscle stiffness. Include stretches that specifically target the lower back, such as knee-to-chest stretches or pelvic tilts, to alleviate any tension built up during the exercise. Ensure that all movements are slow and controlled. | 40 min |
| Day 4 | Rest day | Activity: Light walking or leisure activities (gardening, shopping, light housework). | 40–60 min |
| Day 5 | Combined aerobic and light resistance training | Warm-up (10 min): See Day 3. Main Activity (40 min): Cycling on a stationary bike, light resistance circuit. Cool-down (10 min): See Day 3. | 60 min |
| Day 6 | Flexibility training | Warm-up (10 min): Light cardiovascular exercise like walking or stationary cycling at a very low intensity. Main activity (20 min): Dynamic stretches: Leg swings and arm circles to improve range of motion. Static stretches: Hold stretches for each major muscle group for 20–30 s, such as hamstring and quadriceps stretches and arm stretches. Cool-down (10 min): Deep breathing and relaxation techniques to enhance muscle relaxation. | 40 min |
| Day 7 | Rest day | Activity: Light, non-strenuous activities such as walking around the home or gardening, gentle walking in a park with low-intensity movements | 30–60 min |
2.4. Statistical Analysis
2.5. Ethics Approval
3. Results
3.1. Baseline Patient Characteristics
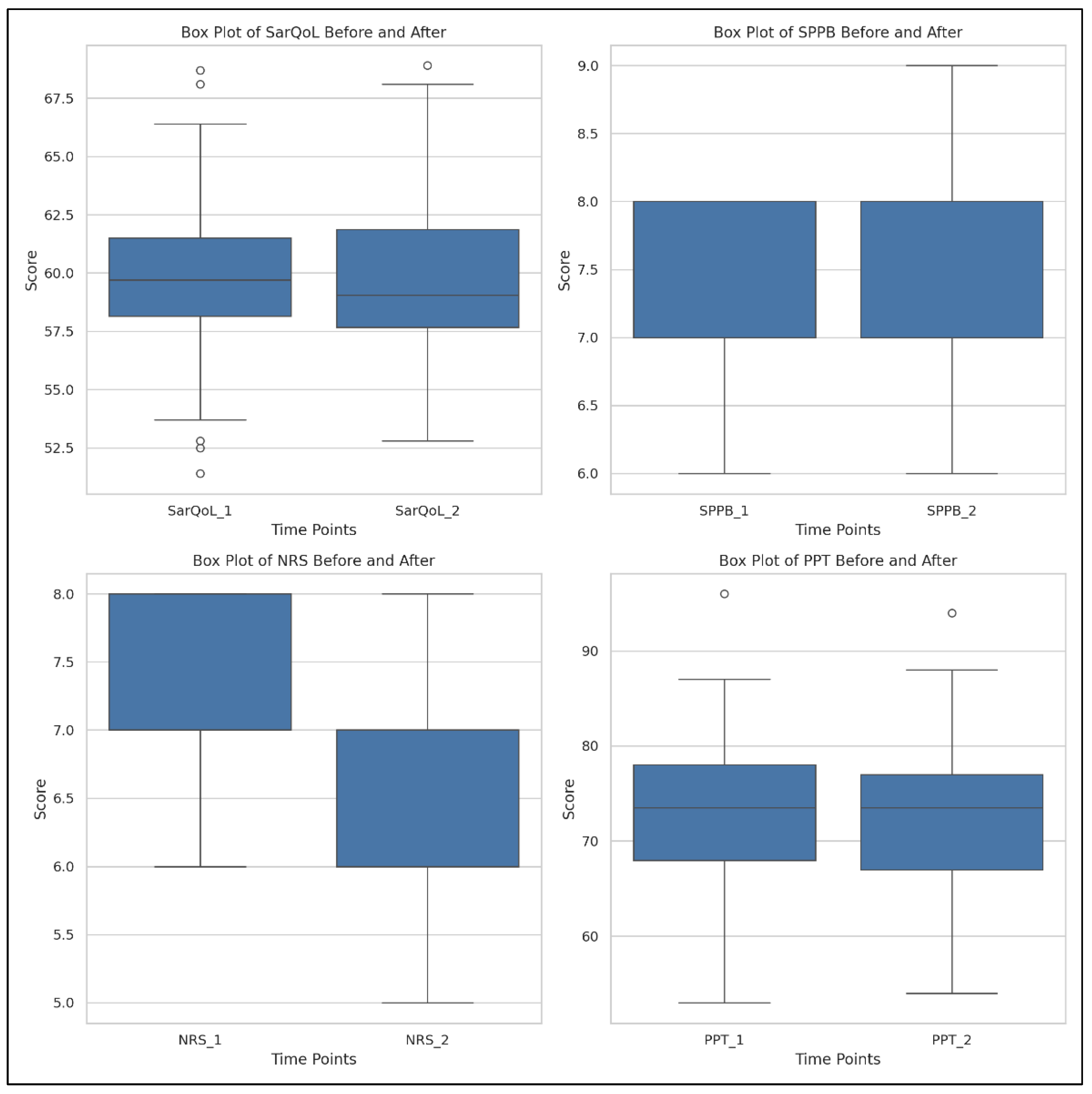
3.2. Study Group: Time Evolution
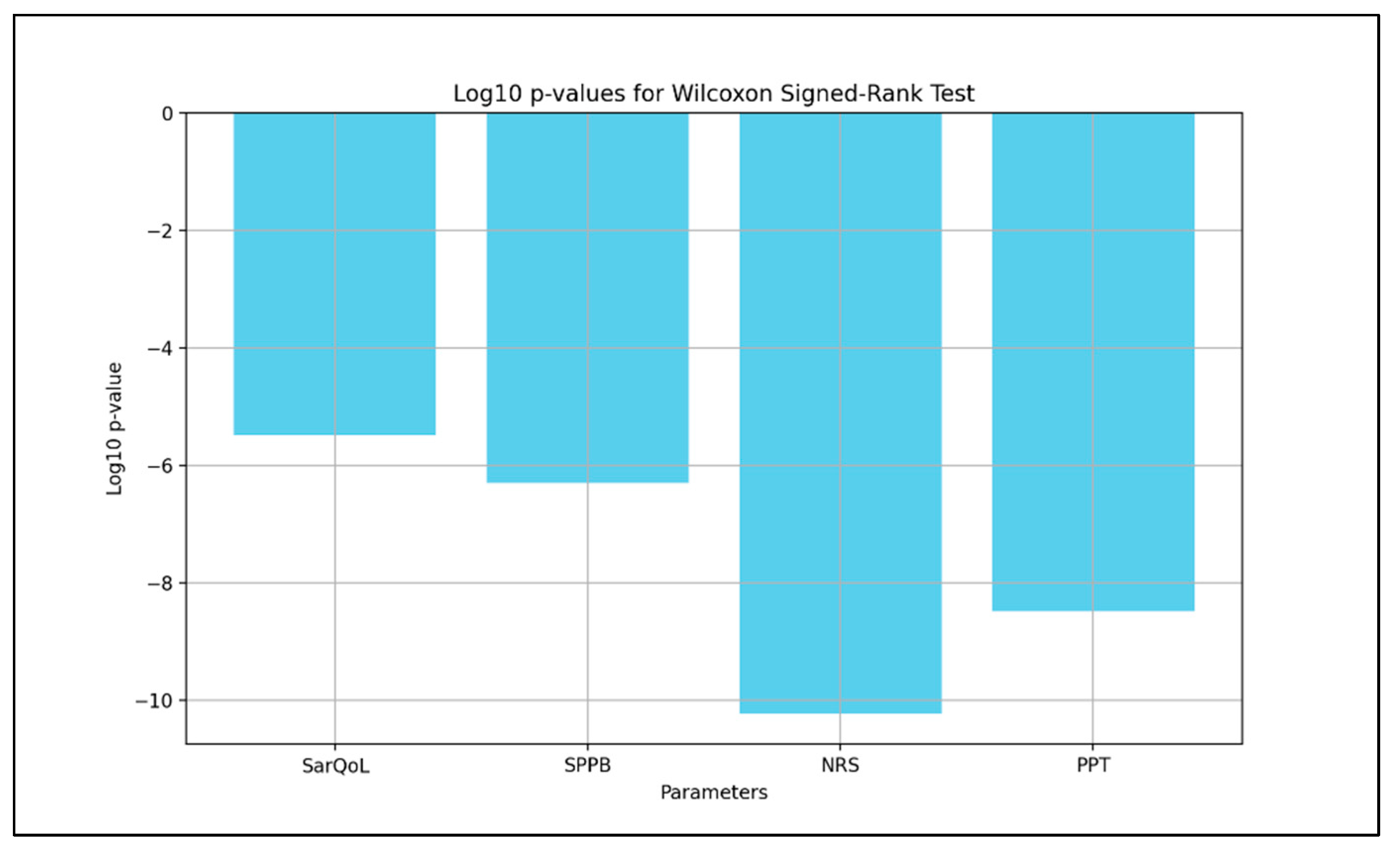
- SarQoL: Test statistic = 55.0, p = 3.25 × 10−6;
- SPPB: Test statistic = 0.0, p = 4.99 × 10−7;
- NRS: Test statistic = 0.0, p = 5.82 × 10−11;
- PPT: Test statistic = 11.0, p = 3.20 × 10−9.
- SarQoL: Highest improvement in urban men (+9.99), lowest in rural men (+4.94);
- SPPB: Relatively stable gains across all subgroups;
- NRS: Moderate reduction in all subgroups;
- PPT: Highest gains in rural men (+15.60).
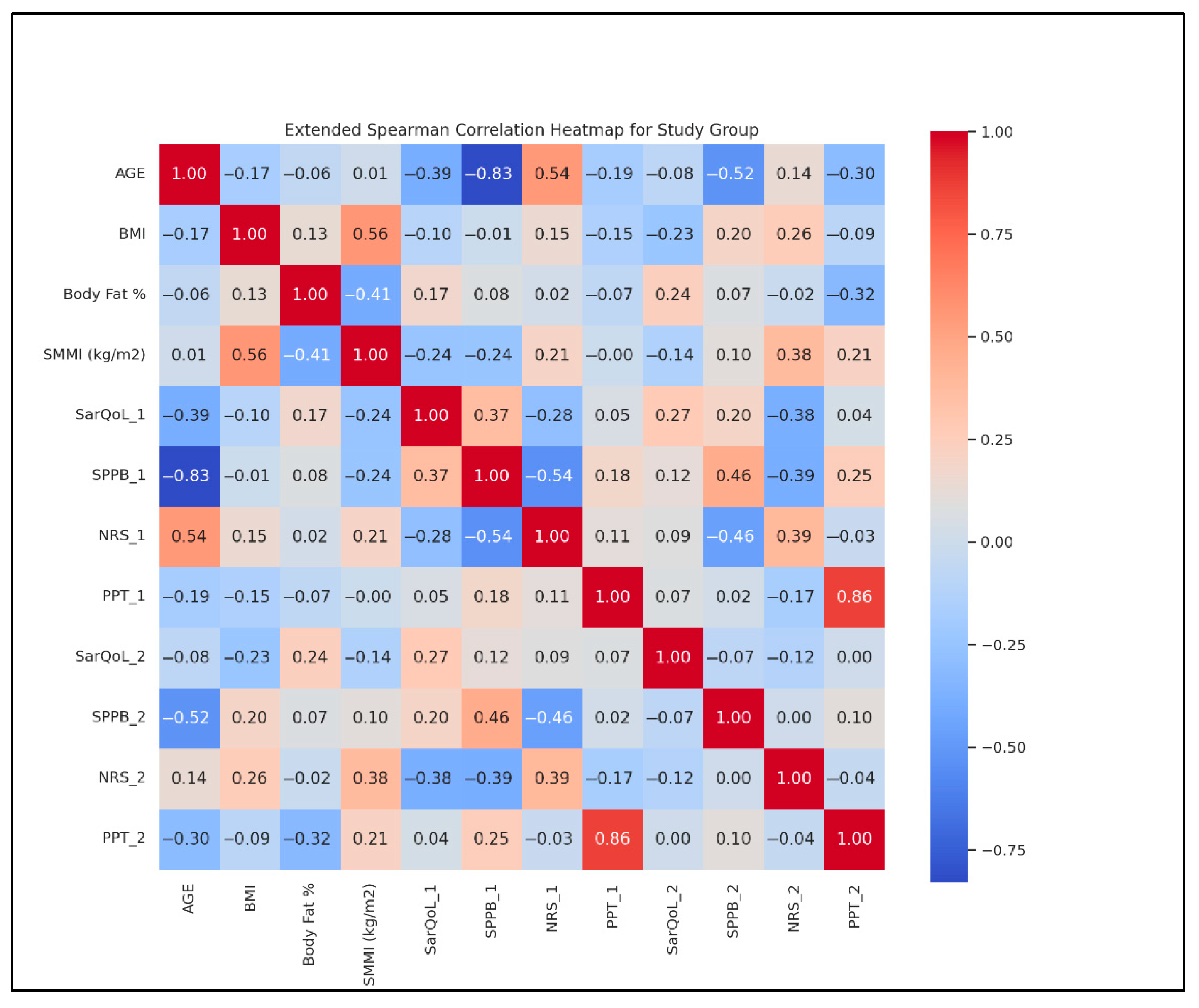
- Age and BMI: r = 0.45;
- BMI and BF %: r = 0.75;
- SMMI and SPPB: r = −0.30;
- SarQoL and NRS: r = −0.55;
- PPT and NRS: r = −0.65.
- Heatmap analysis also showed the following:
- Strengthened SarQoL-SPPB correlation from T1 (r = 0.45) to T2 (r = 0.65);
- Weakened BMI-NRS correlation from T1 (r = −0.30) to T2 (r = −0.50).
3.3. Control Group: Time Evolution
- SarQoL scores showed minor, non-significant changes (p > 0.05);
- SPPB values remained stable between the two time points (p > 0.05);
- NRS for pain and PPT also demonstrated no statistically significant variations (p > 0.05).
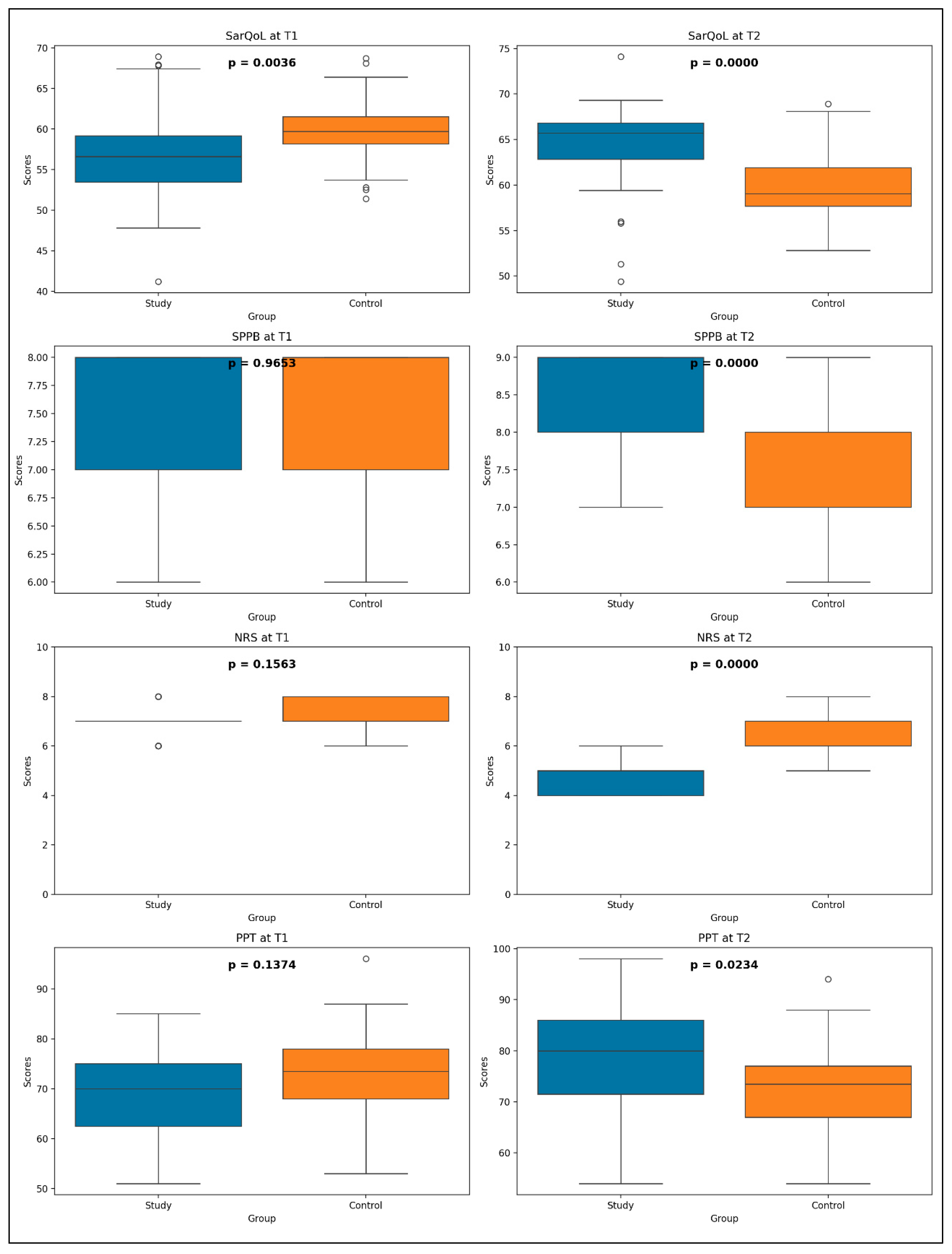
3.4. Study Group Versus Control Group
- SPPB (U = 634.0, p = 0.9653);
- NRS (U = 517.0, p = 0.1563);
- PPT (U = 500.5, p = 0.1374).
- SarQoL: U = 984.5, p < 0.0001;
- SPPB: U = 1072.0, p < 0.0001;
- NRS: U = 31.0, p < 0.0001;
- PPT: U = 827.5, p = 0.0234.
4. Discussion
- A replicable model of multimodal rehabilitation suitable for clinical use in geriatric or outpatient rehabilitation settings;
- Educational utility in training professionals on how to integrate physiotherapy techniques with kinetic exercise regimens;
- While the present study does not isolate the effects of electrotherapy from exercise, the combination mirrors standard clinical care pathways. Further trials with active control groups receiving only one modality may clarify the individual contributions of each component;
- A framework for developing interdisciplinary chronic care pathways combining medical, nutritional, and physical interventions;
- This study has several limitations that should be acknowledged when interpreting the results. First, the sample size, although adequate for detecting changes within groups, limits the statistical power for subgroup analyses or for exploring specific profiles of treatment responders. Additionally, the absence of a factorial design prevents the isolation of the individual contributions of each therapeutic component (kinesiotherapy, electrotherapy, neurotrophic supplementation), making it difficult to determine which modality contributed most to the observed outcomes. Also, due to the hands-on nature of physical therapy interventions, a placebo control or full blinding was not feasible. Nonetheless, both groups received equal attention at baseline, including counseling and educational materials, to reduce attention bias.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phelps, N.H.; Ezzati, M.; NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Underweight and Obesity from 1990 to 2022: A Pooled Analysis of 3663 Population-Representative Studies with 222 Million Children, Adolescents, and Adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Z.; Xu, Y.; He, M.; Gerber, B.S.; Wang, Z.; Liu, F.; Peng, C. Global Scientific Trends in Healthy Aging in the Early 21st Century: A Data-Driven Scientometric and Visualized Analysis. Heliyon 2024, 10, e23405. [Google Scholar] [CrossRef]
- Lim, K.I.; Yang, S.J.; Kim, T.N.; Yoo, H.J.; Kang, H.J.; Song, W.; Baik, S.H.; Choi, D.S.; Choi, K.M. The Association between the Ratio of Visceral Fat to Thigh Muscle Area and Metabolic Syndrome: The Korean Sarcopenic Obesity Study (KSOS). Clin. Endocrinol. 2010, 73, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Ingles, S.; Ashley, J.M.; Maxwell, M.H.; Lyons, R.F.; Elashoff, R.M. Clinical Detection of Sarcopenic Obesity by Bioelectrical Impedance Analysis. Am. J. Clin. Nutr. 1996, 64, 472S–477S. [Google Scholar] [CrossRef] [PubMed]
- Vladutu, B.M.; Matei, D.; Amzolini, A.M.; Kamal, C.; Traistaru, M.R. A Prospective Controlled Study on the Longitudinal Effects of Rehabilitation in Older Women with Primary Sarcopenia. Life 2025, 15, 609. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N. Body Composition in Healthy Aging. Ann. N. Y. Acad. Sci. 2000, 904, 437–448. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef]
- Bahat, G.; Kilic, C.; Ozkok, S.; Ozturk, S.; Karan, M.A. Associations of Sarcopenic Obesity versus Sarcopenia Alone with Functionality. Clin. Nutr. 2021, 40, 2851–2859. [Google Scholar] [CrossRef]
- Benz, E.; Pinel, A.; Guillet, C.; Capel, F.; Pereira, B.; De Antonio, M.; Pouget, M.; Cruz-Jentoft, A.J.; Eglseer, D.; Topinkova, E.; et al. Sarcopenia and Sarcopenic Obesity and Mortality Among Older People. JAMA Netw. Open 2024, 7, e243604. [Google Scholar] [CrossRef]
- Prado, C.M.; Wells, J.C.; Smith, S.R.; Stephan, B.C.M.; Siervo, M. Sarcopenic Obesity: A Critical Appraisal of the Current Evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef]
- Pinel, A.; Guillet, C.; Capel, F.; Pouget, M.; De Antonio, M.; Pereira, B.; Topinkova, E.; Eglseer, D.; Barazzoni, R.; Cruz-Jentoft, A.J.; et al. Identification of Factors Associated with Sarcopenic Obesity Development: Literature Review and Expert Panel Voting. Clin. Nutr. 2024, 43, 1414–1424. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic Obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef]
- Batsis, J.A.; Zbehlik, A.J.; Barre, L.K.; Bynum, J.P.; Pidgeon, D.; Bartels, S.J. Impact of Obesity on Disability, Function, and Physical Activity: Data from the Osteoarthritis Initiative. Scand. J. Rheumatol. 2015, 44, 495–502. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic Obesity: Definition, Cause and Consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Nguyen, T.T.; Zhang, Y.; Ryu, D.; Gariani, K. Sarcopenic Obesity: Epidemiology, Pathophysiology, Cardiovascular Disease, Mortality, and Management. Front. Endocrinol. 2023, 14, 1185221. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Stevens, J.; Cai, J. Abdominal Fat Distribution and Functional Limitations and Disability in a Biracial Cohort: The Atherosclerosis Risk in Communities Study. Int. J. Obes. 2005, 29, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, M324–M333. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Barbagallo, M. The Cardiometabolic Syndrome and Sarcopenic Obesity in Older Persons. J. Cardiometab. Syndr. 2007, 2, 183–189. [Google Scholar] [CrossRef]
- Popescu, C.; Matei, D.; Amzolini, A.M.; Trăistaru, M.R. Inflammation and Physical Performance in Overweight and Obese Schoolchildren. Life 2024, 14, 1583. [Google Scholar] [CrossRef]
- Aubertin-Leheudre, M.; Lord, C.; Goulet, E.D.B.; Khalil, A.; Dionne, I.J. Effect of Sarcopenia on Cardiovascular Disease Risk Factors in Obese Postmenopausal Women. Obesity 2006, 14, 2277–2283. [Google Scholar] [CrossRef]
- Yin, Y.-H.; Liu, J.Y.W.; Fan, T.M.; Leung, K.M.; Ng, M.W.; Tsang, T.Y.; Wong, S.Y.S.; Lee, R.Y.W.; Woo, J. Effectiveness of Nutritional Advice for Community-Dwelling Obese Older Adults with Frailty: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Ciudin, A.; Simó-Servat, A.; Palmas, F.; Barahona, M.J. Sarcopenic Obesity: A New Challenge in the Clinical Practice. Endocrinol. Diabetes Nutr. 2020, 67, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic Obesity in Older Adults: Aetiology, Epidemiology and Treatment Strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Farmer, R.E.; Mathur, R.; Schmidt, A.F.; Bhaskaran, K.; Fatemifar, G.; Eastwood, S.V.; Warren, H.R.; Shah, A.D.; Smeeth, L.; Chaturvedi, N.; et al. Associations between Measures of Sarcopenic Obesity and Risk of Cardiovascular Disease and Mortality: A Cohort Study and Mendelian Randomization Analysis Using the UK Biobank. J. Am. Heart Assoc. 2019, 8, e011638. [Google Scholar] [CrossRef]
- Yoo, J.J.; Cho, N.H.; Lim, S.H.; Kim, H.A. Relationships between Body Mass Index, Fat Mass, Muscle Mass, and Musculoskeletal Pain in Community Residents. Arthritis Rheumatol. 2014, 66, 3511–3520. [Google Scholar] [CrossRef]
- Eggermont, L.H.; Leveille, S.G.; Shi, L.; Kiely, D.K.; Kiel, D.P.; Jones, R.N.; Samelson, E.J.; Bean, J.F. Pain Characteristics Associated with the Onset of Disability in Older Adults: The Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly Boston Study. J. Am. Geriatr. Soc. 2014, 62, 1007–1016. [Google Scholar] [CrossRef]
- Cappellari, G.G.; Zanetti, M.; Donini, L.M.; Barazzoni, R. Detecting Sarcopenia in Obesity: Emerging New Approaches. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 402–409. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). About Adult BMI. Available online: https://www.cdc.gov/bmi/about/index.html (accessed on 15 June 2025).
- Guttikonda, D.; Smith, A.L. Sarcopenia Assessment Techniques. Clin. Liver Dis. 2021, 18, 189–192. [Google Scholar] [CrossRef]
- Ji, T.; Li, Y.; Ma, L. Sarcopenic Obesity: An Emerging Public Health Problem. Aging Dis. 2022, 13, 379–388. [Google Scholar] [CrossRef]
- Holmes, C.J.; Racette, S.B. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Wayne, S.J.; Waters, D.L.; Janssen, I.; Gallagher, D.; Morley, J.E. Sarcopenic Obesity Predicts Instrumental Activities of Daily Living Disability in the Elderly. Obes. Res. 2004, 12, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Simon, F.; Achiardi, O.; Vilos, C.; Cabrera, D.; Cabello-Verrugio, C. The Critical Role of Oxidative Stress in Sarcopenic Obesity. Oxid. Med. Cell. Longev. 2021, 2021, 4493817. [Google Scholar] [CrossRef] [PubMed]
- Jurdana, M.; Cemazar, M. Sarcopenic Obesity in Cancer. Radiol. Oncol. 2024, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; Di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and All-Cause Mortality: Systematic Review and Meta-Analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Breivik Hals, E.K.; Kvarstein, G.; Stubhaug, A. Assessment of Pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef]
- Imai, R.; Imaoka, M.; Nakao, H.; Hida, M.; Tazaki, F.; Inoue, T.; Orui, J.; Nakamura, M. Association between Chronic Pain with Presarcopenia and Central Sensitization in Japanese Community-Dwelling Older Adults: A Cross-Sectional Study. Medicine 2022, 101, e29998. [Google Scholar] [CrossRef]
- Trueba-Perdomo, J.H.; Gasparini, F.; Flores Cuautle, J.J.A. Pressure Pain Threshold Values Obtained Through Algometers. Rev. Mex. Ing. Bioméd. 2021, 42, 132–148. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Zhang, Y.; Luo, N.; Chen, Q.; Ge, M.; Shen, B. Association between Persistent Musculoskeletal Pain and Incident Sarcopenia in China: The Mediating Effect of Depressive Symptoms. Front. Public Health 2024, 12, 1416796. [Google Scholar] [CrossRef]
- Demonceau, C.; Voz, B.; Bruyère, O.; Reginster, J.-Y.; Beaudart, C. Content Validity of SarQoL, a Quality of Life Questionnaire Specific to Sarcopenia. Aging Clin. Exp. Res. 2024, 36, 101. [Google Scholar] [CrossRef]
- The SarQoL Questionnaire. Available online: http://www.sarqol.org (accessed on 15 June 2025).
- Cioboata, R.; Balteanu, M.A.; Zlatian, O.M.; Vlasceanu, S.G.; Driga, M.V.P.; Mitroi, D.M.; Catana, O.M.; Buciu, C.I.; Camen, G.; Mirea, A.A. Impact of vitamin C deficiency on imaging patterns and ventilatory function in pulmonary tuberculosis. Front. Med. 2025, 12, 1554723. [Google Scholar] [CrossRef]
- Nishida, M.M.; Tsuboyama, T.; Moritani, T.; Arai, H. Review of the Evidence on the Use of Electrical Muscle Stimulation to Treat Sarcopenia. Eur. Geriatr. Med. 2016, 7, 267–271. [Google Scholar] [CrossRef]
- Zarzeczny, R.; Nawrat-Szołtysik, A.; Polak, A. Effects of 12 Weeks of Neuromuscular Electrical Stimulation of the Quadriceps Muscles on the Function and Physio-Biochemical Traits in Functionally Fit Female Nursing-Home Residents Aged 75+ Years: A Pilot Study. Eur. J. Appl. Physiol. 2024, 124, 945–962. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, F.; Rodrigues, N.C.; Assis, L.L.; Peviani, S.S.; Durigan, J.L.; Moreira, F.M.; Hamblin, M.R.; Parizotto, N.A. Low intensity laser therapy accelerates muscle regeneration in aged rats. Photonics Lasers Med. 2012, 1, 287–297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trăistaru, R.; Alexandru, D.O.; Kamal, D.; Kamal, K.C.; Rogoveanu, O.C.; Postolache, P. Boswellia derivates and rehabilitation program in knee osteoarthritis patients. Rev. Chim. 2018, 69, 4105–4108. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Abdallah, G.A.; Hassan, S.S.; Nagy, E.N. Effect of exercise training with laser phototherapy on homeostasis balance resistant to hypercoagulability in seniors with obesity: A randomized trial. Sci. Rep. 2023, 13, 3592. [Google Scholar] [CrossRef] [PubMed]
- Trăistaru, R.; Alexandru, D.O.; Kamal, D.; Kamal, K.C.; Rogoveanu, O. The role of herbal extracts in knee osteoarthritis females rehabilitation. Farmacia 2018, 66, 507–513. [Google Scholar] [CrossRef]
- Matsuse, H.; Segal, N.A.; Rabe, K.G.; Shiba, N. The Effect of Neuromuscular Electrical Stimulation During Walking on Muscle Strength and Knee Pain in Obese Women With Knee Pain: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2020, 99, 56–64. [Google Scholar] [CrossRef]
- de Pedro Negri, A.M.; Ruiz Prieto, M.J.; Díaz-Mohedo, E.; Martín-Valero, R. Efficacy of Magnetic Therapy in Pain Reduction in Patients with Chronic Pelvic Pain: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5824. [Google Scholar] [CrossRef]
- Andrade, R.; Duarte, H.; Pereira, R.; Lopes, I.; Pereira, H.; Rocha, R.; Espregueira-Mendes, J. Pulsed electromagnetic field therapy effectiveness in low back pain: A systematic review of randomized controlled trials. Porto Biomed. J. 2016, 1, 156–163. [Google Scholar] [CrossRef]
- Elshiwi, A.M.; Hamada, H.A.; Mosaad, D.; Elsaid, N.; Abdelhalim, N.M.; Samhan, A.F.; Abdelbasset, W.K. Efficacy of pulsed low-frequency magnetic field therapy on patients with chronic low back pain: A randomized double-blind placebo-controlled trial. Asian Spine J. 2020, 14, 33–42. [Google Scholar] [CrossRef]
- Kull, P.; Keilani, M.; Remer, F.; Crevenna, R. Efficacy of pulsed electromagnetic field therapy on pain and physical function in patients with non-specific low back pain: A systematic review. Wien. Med. Wochenschr. 2025, 175, 11–19. [Google Scholar] [CrossRef]
- Thapa, N.; Yang, J.G.; Bae, S.; Kim, G.M.; Park, H.J.; Park, H. Effect of Electrical Muscle Stimulation and Resistance Exercise Intervention on Physical and Brain Function in Middle-Aged and Older Women. Int. J. Environ. Res. Public Health 2022, 21, 101. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, Y.J.; Lee, S.Y. Effects of Electrical Muscle Stimulation on Waist Circumference in Adults with Abdominal Obesity: A Randomized, Double-blind, Sham-Controlled Trial. J. Nepal Med. Assoc. 2018, 56, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Šarabon, N.; Kozinc, Ž.; Löfler, S.; Hofer, C. Resistance Exercise, Electrical Muscle Stimulation, and Whole-Body Vibration in Older Adults: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 2902. [Google Scholar] [CrossRef]
- Toma, R.L.; Vassão, P.G.; Antunes, H.K.M.; de Oliveira Assis, L.; Renno, A.C.M. Effect of Low-Level Laser Therapy (808 nm) in Combination with Strength Training on Muscle Performance in Elderly Women: A Randomized Controlled Trial. Lasers Med. Sci. 2016, 31, 1219–1229. [Google Scholar] [CrossRef]
- Clijsen, R.; Brunner, A.; Barbero, M.; Clarys, P.; Taeymans, J. Effects of Low-Level Laser Therapy on Pain in Patients with Musculoskeletal Disorders: A Systematic Review and Meta-Analysis. Eur. J. Phys. Rehabil. Med. 2017, 53, 603–610. Available online: https://pubmed.ncbi.nlm.nih.gov/28145397/ (accessed on 30 January 2025). [CrossRef]
- Almeida, J.N.; Prado, W.L.; Terra, C.M.; Barros, T.L.; Benatti, F.B.; Ugrinowitsch, C.; de Oliveira Assis, L.; Renno, A.C.M. Photobiomodulation Combined with Resistance Training Improves Muscle Strength in Postmenopausal Women: A Randomized Controlled Trial. Lasers Med. Sci. 2021, 36, 853–861. [Google Scholar]
- Hoerner Cubas, I.; Bottaro, M.; Magalhães, F.; Prestes, J.; da Silva, B.V.C.; Brown, L.E.; de Oliveira Assis, L.; Renno, A.C.M. Effect of Pre-Exercise Low-Level Laser Therapy on Muscle Recovery and Performance in Patients with Musculoskeletal Disorders. Sci. Rep. 2023, 13, 1547. [Google Scholar]
- Aliyev, R.M.; Reinhold, J.; Seidov, I.I.; Mikus, E.W.J. Better Functional Results of Conservative Treatment in Fresh Lateral Ligament Injuries of the Ankle with Additional Deep Oscillation. Phys. Med. Rehabil. Kurortmed. 2012, 22, 9–15. [Google Scholar] [CrossRef]
- Koleva, I.B.; Ioshinov, B.R.; Yoshinov, R.R. Complex Analgesia (Infiltrations and Deep Oscillation) in Patients with Stump Pain and Phantom Pain after Lower Limb Amputation: A Double-blind Randomised Controlled Trial of Efficacy. J. Adv. Med. Med. Res. 2017, 22, 1–17. [Google Scholar] [CrossRef]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.I.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain 2019, 20, 1249–1266. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M. Physical Activity, Sedentary Behaviour, and Obesity. In Energy Balance and Obesity; Romieu, I., Dossus, L., Willett, W.C., Eds.; International Agency for Research on Cancer: Lyon, France, 2017; Volume 10, Chapter 6. Available online: https://www.ncbi.nlm.nih.gov/books/NBK565813/ (accessed on 25 February 2025).
- Budui, S.; Bigolin, F.; Giordano, F.; Leoni, S.; Berteotti, M.; Sartori, E.; Franceschini, L.; Taddei, M.; Salvetti, S.; Castiglioni, F.; et al. Effects of an Intensive Inpatient Rehabilitation Program in Elderly Patients with Obesity. Obes. Facts 2019, 12, 199–210. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Morales, J.S.; Pareja-Galeano, H.; Izquierdo, M.; Emanuele, E.; de la Villa, P.; Lucia, A. Physical Strategies to Prevent Disuse-Induced Functional Decline in the Elderly. Ageing Res. Rev. 2018, 47, 80–88. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Dalamaga, M.; Kokkinos, A. Sarcopenic Obesity: Epidemiologic Evidence, Pathophysiology, and Therapeutic Perspectives. Curr. Obes. Rep. 2019, 8, 458. [Google Scholar] [CrossRef]
- Borba, V.Z.C.; Costa, T.M.D.R.L. Sarcopenic Obesity: A Review. Arch. Endocrinol. Metab. 2025, 68, e240084. [Google Scholar] [CrossRef]
- Prado, C.M.; Batsis, J.A.; Donini, L.M.; Gonzales, M.C.; Siervo, M. Sarcopenic obesity in older adults: A clinical over-view. Nat. Rev. Endocrinol. 2024, 20, 261–277. [Google Scholar] [CrossRef]
- de Oliveira, T.R.; Moser, A.D.L.; Paz, L.P.; Ngomo, S.; da Silva, R.A.; de Oliveira, L.V.F.; Brandão, G.S. Sarcopenia, Chronic Pain, and Perceived Health of Older: A Cross-Sectional Study. Fisioter. Mov. 2023, 36, e36106. [Google Scholar] [CrossRef]
- Tsekoura, M.; Billis, E.; Matzaroglou, C.; Tsepis, E.; Gliatis, J. Association between Chronic Pain and Sarcopenia in Greek Community-Dwelling Older Adults: A Cross-Sectional Study. Healthcare 2024, 12, 1303. [Google Scholar] [CrossRef]
- Okifuji, A.; Hare, B.D. The Association between Chronic Pain and Obesity. J. Pain Res. 2015, 8, 399–408. [Google Scholar] [CrossRef]
- Matei, D.; Trăistaru, R.; Amzolini, A.M.; Ianosi, L.S.; Neagoe, C.D.; Mitrea, A.; Clenciu, D.; Avramescu, T.E. A Comparative Study on the Pain Threshold Experienced by Fibromyalgia Patients Following Acute SARS-CoV-2 Infection. Life 2024, 14, 942. [Google Scholar] [CrossRef]
- Kamal, D.; Trăistaru, R.; Alexandru, D.O.; Kamal, K.C.; Pirici, D.N.; Pop, O.T.; Mălăescu, D.G. Morphometric findings in avascular necrosis of the femoral head. Rom. J. Morphol. Embryol. 2012, 53 (Suppl. S3), 763–767. [Google Scholar] [PubMed]
- Chen, J.; Yan, L.; Chu, J.; Wang, X.; Xu, Z. Pain Characteristics and Progression to Sarcopenia in Chinese Middle-Aged and Older Adults: A 4-Year Longitudinal Study. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae080. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Koyanagi, A.; Barbagallo, M.; Dominguez, L.J.; Maggi, S.; Soysal, P.; Bolzetta, F.; Ruotolo, G.; Castagna, A.; Smith, L. Pain Increases the Risk for Sarcopenia in Community-Dwelling Adults: Results from the English Longitudinal Study of Ageing. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2023, 78, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Flynn, D.M. Chronic Musculoskeletal Pain: Nonpharmacologic, Noninvasive Treatments. Am. Fam. Physician 2020, 102, 465–477. [Google Scholar]
- Kikuchi, Y.; Nakano, H.; Goda, A.; Mori, K.; Abiko, T.; Mitsumaru, N.; Murata, S. The Influence of Physical, Mental, and Cognitive Factors on Health-Related Quality of Life among Community Dwelling Older Adults: A Focus on Central Sensitization-Related Symptoms. Geriatrics 2024, 9, 11. [Google Scholar] [CrossRef]
- Park, G.; Kim, C.W.; Park, S.B.; Kim, M.J.; Jang, S.H. Reliability and usefulness of the pressure pain threshold meas-urement in patients with myofascial pain. Ann. Rehabil. Med. 2011, 35, 412–417. [Google Scholar] [CrossRef]
- Melchior, M.; Poisbeau, P.; Gaumond, I.; Marchand, S. Insights into the Mechanisms and the Emergence of Sex-Differences in Pain. Neuroscience 2016, 338, 63–80. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful Change and Responsiveness in Common Physical Performance Measures in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Beaudart, C.; Alcazar, J.; Aprahamian, I.; Bauer, J.; Bruyère, O.; Cesari, M.; Cruz-Jentoft, A.J.; Landi, F.; Rolland, Y.; Woo, J.; et al. Health Outcomes of Sarcopenia: A Consensus Report by the Outcome Working Group of the Global Leadership Initiative in Sarcopenia (GLIS). Aging Clin. Exp. Res. 2025, 37, 100. [Google Scholar] [CrossRef] [PubMed]
- Ghiotto, L.; Muollo, V.; Tatangelo, T.; Schena, F.; Rossi, A.P. Exercise and Physical Performance in Older Adults with Sarcopenic Obesity: A Systematic Review. Front. Endocrinol. 2022, 13, 913953. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Phu, S.; Kirk, B.; Bani Hassan, E.; Vogrin, S.; Zanker, J.; Duque, G. The Diagnostic Value of the Short Physical Performance Battery for Sarcopenia. BMC Geriatr. 2020, 20, 242. [Google Scholar] [CrossRef]
- Geerinck, A.; Alekna, V.; Beaudart, C.; Buckinx, F.; Bruyère, O.; Cruz-Jentoft, A.J.; Dent, E.; Gasparik, A.I.; Landi, F.; Reginster, J.Y.; et al. Standard Error of Measurement and Smallest Detectable Change of the Sarcopenia Quality of Life (SarQoL) Questionnaire: An Analysis of Subjects from 9 Validation Studies. PLoS ONE 2019, 14, e0216065. [Google Scholar] [CrossRef]
- Pap, Z.; Kalabiska, I.; Balogh, Á.; Pintér, S.; Tóth, J.; Mészáros, L.; Kökény, Z.; Tóth, E.; Nagy, G.; Bender, T. Evaluation of the Sarcopenia Quality of Life (SarQoL) Questionnaire in Community-Dwelling Outpatient Postmenopausal Hungarian Women. BMC Musculoskelet. Disord. 2023, 24, 331. [Google Scholar] [CrossRef]
- Martini, S.; Held, C.; Schluessel, S.; Weber, T.; Swart, E.; Kirchner, M.; Beaudart, C.; Freiberger, E. Validation of the German Version of the SarQoL® Questionnaire in Sarcopenic and Probable Sarcopenic Patients. Aging Clin. Exp. Res. 2024, 36, 217. [Google Scholar] [CrossRef]
- Goulard, I.; Mihai, G.; Beaudart, C.; Reginster, J.Y.; Bruyère, O. Psychometric Performance of the Romanian Version of the SarQoL®, a Health-Related Quality of Life Questionnaire for Sarcopenia. Arch. Osteoporos. 2017, 12, 103. [Google Scholar] [CrossRef]
- den Uijl, I.; van den Berg-Emons, R.J.G.; Sunamura, M.; Lenzen, M.J.; Stam, H.J.; Boersma, E.; Tenbült-van Limpt, N.C.C.W.; Kemps, H.M.C.; Geleijnse, M.L.; ter Hoeve, N. Effects of a Dedicated Cardiac Rehabilitation Program for Patients with Obesity on Body Weight, Physical Activity, Sedentary Behavior, and Physical Fitness: The OPTICARE XL Randomized Controlled Trial. Phys. Ther. 2023, 103, pzad055. [Google Scholar] [CrossRef]
- Paolucci, T.; Vetrano, M.; Iosa, M.; Capobianco, S.V.; Saraceni, V.M.; Mangone, M. The Role of Electromagnetic Therapy in Pain and Muscle Function. Int. J. Environ. Res. Public Health 2020, 17, 9390. [Google Scholar] [CrossRef]
- Clijsen, R.; Baeyens, J.P.; Foidart-Dessalle, M.; Duquet, W.; Demoulin, C. Low-Level Laser Therapy in Musculoskeletal Disorders: A Systematic Review. Lasers Med. Sci. 2017, 32, 229–239. [Google Scholar]
- Rogan, S.; Taeymans, J.; Radlinger, L. Effect of Whole-Body Vibration on Balance and Functional Mobility in Elderly: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Physiother. Res. Int. 2017, 22, e1669. [Google Scholar] [CrossRef]
- Tache-Codreanu, D.L.; Trăistaru, M.R. The Effectiveness of High Intensity Laser in Improving Motor Deficits in Patients with Lumbar Disc Herniation. Life 2024, 14, 1302. [Google Scholar] [CrossRef]
- Dong, H.J.; Dragioti, E.; Rivano Fischer, M.; Gerdle, B. Lose Pain, Lose Weight, and Lose Both: A Cohort Study of Patients with Chronic Pain and Obesity Using a National Quality Registry. J. Pain Res. 2021, 14, 1863–1873. [Google Scholar] [CrossRef]
- Pudalov, L.R.; Krause, S.J.; Heinberg, L.J.; Hogue, O. Refractory Chronic Pain and Obesity: Promising Implications for Multidisciplinary Pain Rehabilitation. Pain Med. 2021, 22, 2290–2297. [Google Scholar] [CrossRef]
- Pereira, A.P.; Janela, D.; Areias, A.C.; Molinos, M.; Tong, X.; Bento, V.; Yanamadala, V.; Cohen, S.P.; Correia, F.D.; Costa, F. Evaluating Digital Rehabilitation Outcomes in Chronic Musculoskeletal Conditions Across Non-Obesity, Obesity, and Severe Obesity. J. Pain Res. 2025, 18, 73–87. [Google Scholar] [CrossRef]


| Study Group (SG) 35 Patients | Control Group (CG) 36 Patients | p-Value | ||
|---|---|---|---|---|
| Age (years) | 72.65 ± 3.94 | 72.91 ± 3.84 | t-test for independent samples | 0.7798 |
| Weight (kg) | 81.11 ± 6.42 | 84.13 ± 4.65 | 0.0259 | |
| Height (m) | 1.62 ± 0.07 | 1.63 ± 0.06 | 0.5488 | |
| BMI (Kg/m2) | 30.78 ± 2.34 | 31.53 ± 1.40 | 0.1044 | |
| BF % | 45.08 ± 4.62 | 46.71 ± 3.95 | 0.1162 | |
| SMMI (kg/m2) | 7.07 ± 0.91 | 7.00 ± 0.38 | 0.6908 | |
| SPPB | 7.11 ± 0.75 | 7.11 ± 0.70 | 0.9855 | |
| SarQoL | 57.02 ± 5.94 | 59.79 ± 4.02 | 0.0242 | |
| PPT (N/m2) | 69.31 ± 8.55 | 72.36 ± 9.5 | 0.1582 | |
| NRS | 6.94 ± 0.63 | 8.72 ± 0.95 | 0.2730 | |
| Urban (n, %) | 18 (52%) | 18 (50%) | Chi-square statistic: 2.3034 p-value: 0.1291 | |
| Rural (n, %) | 17 (48%) | 18 (50%) | ||
| Women (n, %) | 23 (66%) | 25 (70%) | Chi-square statistic: 1.7582 p-value: 0.1849 | |
| Men (n, %) | 12 (34%) | 11 (30%) | ||
| Parameters | Mean Value | SD | Min Value | 25th Percentile | Median Value | 75th Percentile | Max Value | |
|---|---|---|---|---|---|---|---|---|
| Total SarQoL | T1 | 57.02 | 5.94 | 41.2 | 53.45 | 56.6 | 59.15 | 68.9 |
| T2 | 63.98 | 5.12 | 49.4 | 62.85 | 65.7 | 66.8 | 74.1 | |
| SPPB | T1 | 7.14 | 0.73 | 6 | 7 | 7 | 8 | 8 |
| T2 | 8.4 | 0.55 | 7 | 8 | 8 | 9 | 9 | |
| NRS | T1 | 6.94 | 0.63 | 6 | 7 | 7 | 7 | 8 |
| T2 | 4.65 | 0.63 | 4 | 4 | 5 | 5 | 6 | |
| PPT | T1 | 69.31 | 8.55 | 51 | 62.5 | 70 | 75 | 85 |
| T2 | 78.05 | 10.96 | 54 | 71.5 | 80 | 86 | 98 | |
| Sex | Environment | SarQoL_diff | SPPB_diff | NRS_diff | PPT_diff |
|---|---|---|---|---|---|
| Women | Urban | 6.24 (p = 0.03) | 1.27 (p = 0.006) | −2.36 (p < 0.001) | 7.36 (p < 0.001) |
| Women | Rural | 6.67 (p = 0.02) | 1.33 (p = 0.002) | −2.25 (p < 0.001) | 5.83 (p = 0.006) |
| Men | Urban | 9.98 (p = 0.01) | 1.28 (p = 0.01) | −2.28 (p = 0.01) | 11 (p = 0.01) |
| Men | Rural | 4.94 (p = 0.18) | 1 (p = 0.05) | −2.2 (p = 0.06) | 15.6 (p = 0.06) |
| Parameters | Mean Value | SD | Min Value | 25th Percentile | Median Value | 75th Percentile | Max Value | |
|---|---|---|---|---|---|---|---|---|
| Total SarQoL | T1 | 59.79 | 4.02 | 51.4 | 58.15 | 59.7 | 61.5 | 68.7 |
| T2 | 59.69 | 3.57 | 52.8 | 57.67 | 59.05 | 61.86 | 68.9 | |
| SPPB | T1 | 7.11 | 0.74 | 6 | 7 | 7 | 8 | 8 |
| T2 | 7.36 | 0.59 | 6 | 7 | 7 | 8 | 8 | |
| NRS | T1 | 8.72 | 9.5 | 6 | 7 | 7 | 8 | 8 |
| T2 | 6.61 | 0.59 | 5 | 6 | 7 | 7 | 8 | |
| PPT | T1 | 72.16 | 9.41 | 53 | 68 | 73.5 | 78 | 96 |
| T2 | 72.36 | 9.05 | 54 | 67 | 73.5 | 77 | 94 | |
| Sex | Environment | SarQoL_diff | SPPB_diff | NRS_diff | PPT_diff |
|---|---|---|---|---|---|
| Women | Urban | 0.40 (p = 0.40) | 0.27 (p = 0.08) | −0.45 (p = 0.09) | 0.36 (p = 0.36) |
| Women | Rural | −0.78 (p = 0.40) | 0.21 (p = 0.08) | −0.64 (p = 0.01) | −0.5 (p = 0.07) |
| Men | Urban | 0.97 (p = 0.93) | 0.28 (p = 0.15) | 0 (p = 1) | 0.57 (p = 0.37) |
| Men | Rural | −0.97 (p = 0.28) | 0.25 (p = 0.31) | −1 (p = 0.12) | −0.25 (p = 0.87) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladutu, B.M.; Matei, D.; Genunche-Dumitrescu, A.; Kamal, C.; Traistaru, M.R. The Role of Rehabilitation Program in Managing the Triad of Sarcopenia, Obesity, and Chronic Pain. Life 2025, 15, 1174. https://doi.org/10.3390/life15081174
Vladutu BM, Matei D, Genunche-Dumitrescu A, Kamal C, Traistaru MR. The Role of Rehabilitation Program in Managing the Triad of Sarcopenia, Obesity, and Chronic Pain. Life. 2025; 15(8):1174. https://doi.org/10.3390/life15081174
Chicago/Turabian StyleVladutu, Bianca Maria, Daniela Matei, Amelia Genunche-Dumitrescu, Constantin Kamal, and Magdalena Rodica Traistaru. 2025. "The Role of Rehabilitation Program in Managing the Triad of Sarcopenia, Obesity, and Chronic Pain" Life 15, no. 8: 1174. https://doi.org/10.3390/life15081174
APA StyleVladutu, B. M., Matei, D., Genunche-Dumitrescu, A., Kamal, C., & Traistaru, M. R. (2025). The Role of Rehabilitation Program in Managing the Triad of Sarcopenia, Obesity, and Chronic Pain. Life, 15(8), 1174. https://doi.org/10.3390/life15081174






