Novel and Potential Photoprotective and Tyrosinase Inhibitory Effects of Tetrastigma erubescens Extracts: Evidence from In Vitro Assays and Computational Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of T. erubescens Extract
2.3. Identification of Phytocompounds of T. erubescens Extract
2.4. Tyrosinase Inhibition and Ultraviolet Radiation Absorption Efficiency Assays
2.5. Computational Study
3. Results and Discussion
3.1. Evaluating Tyrosinase Inhibitory and Photoprotective Effect of T. erubescens Extract
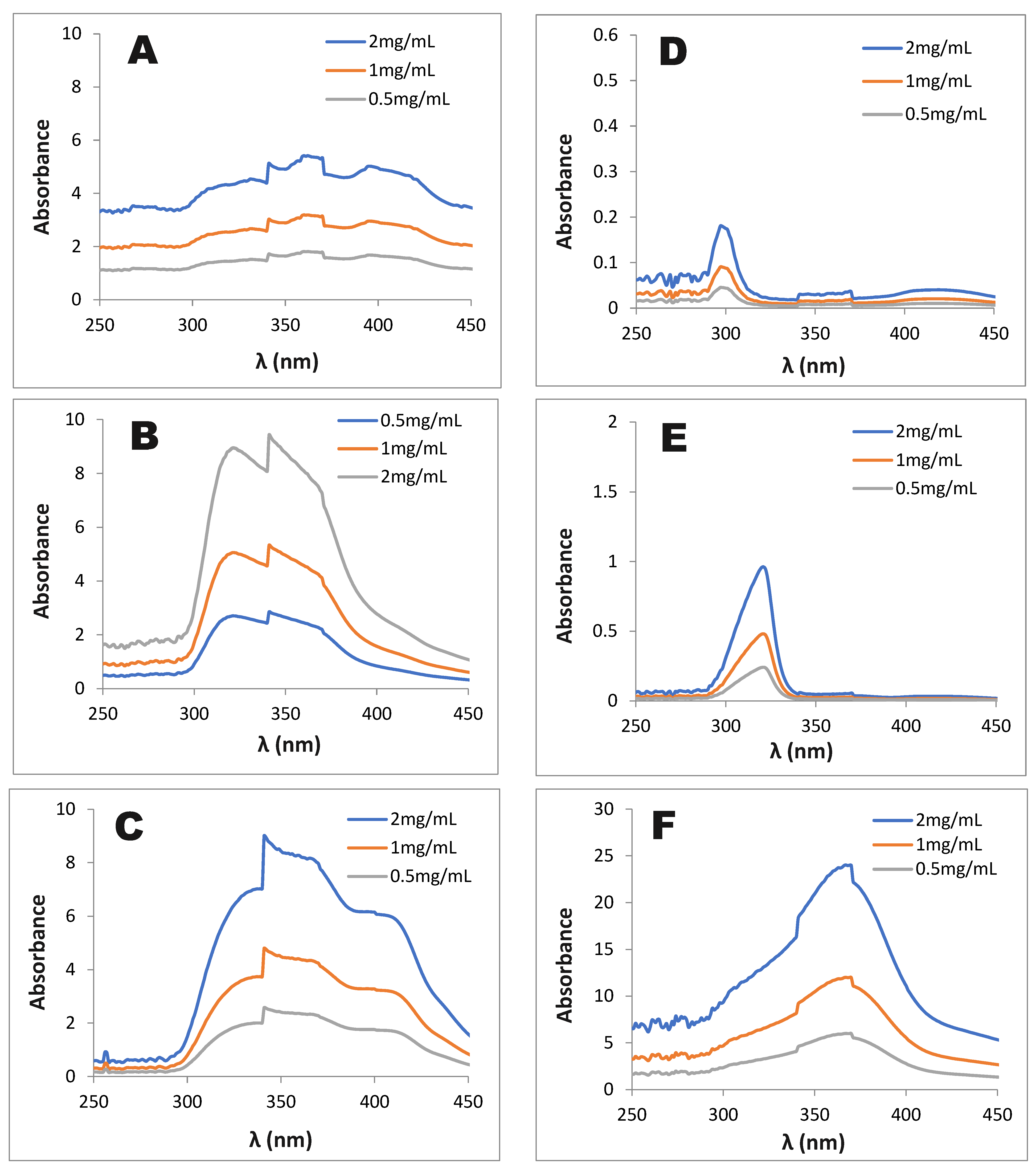
3.1.1. Photoprotective Effect of T. erubescens Extracts
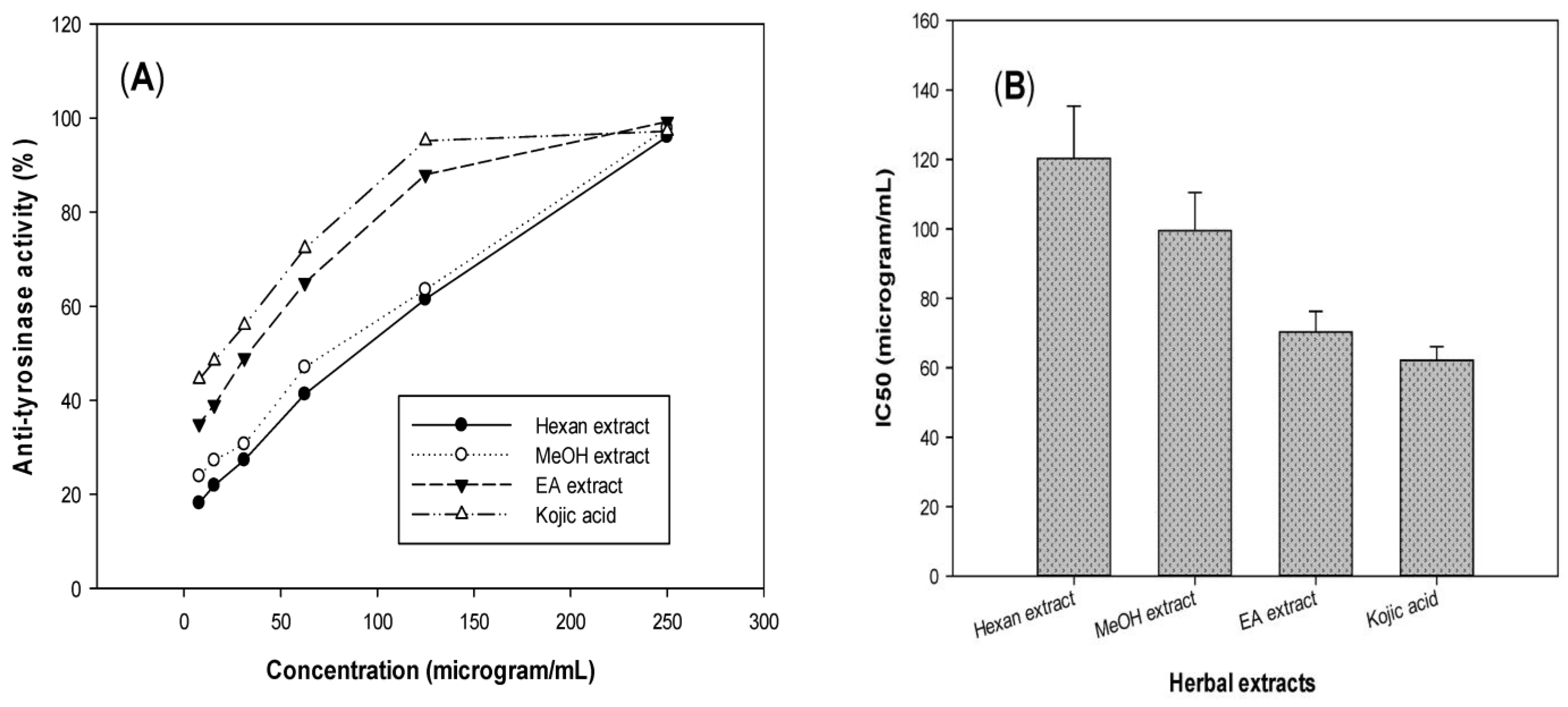
3.1.2. Tyrosinase Inhibitory Activity of T. erubescens Extracts
3.2. The Phytochemical Profile of the T. erubescens Ethyl Acetate Extract
3.3. Molecular Docking, DFT Calculation, Drug-Likeness, and Pharmacokinetic Parameters of Major Phytocompounds
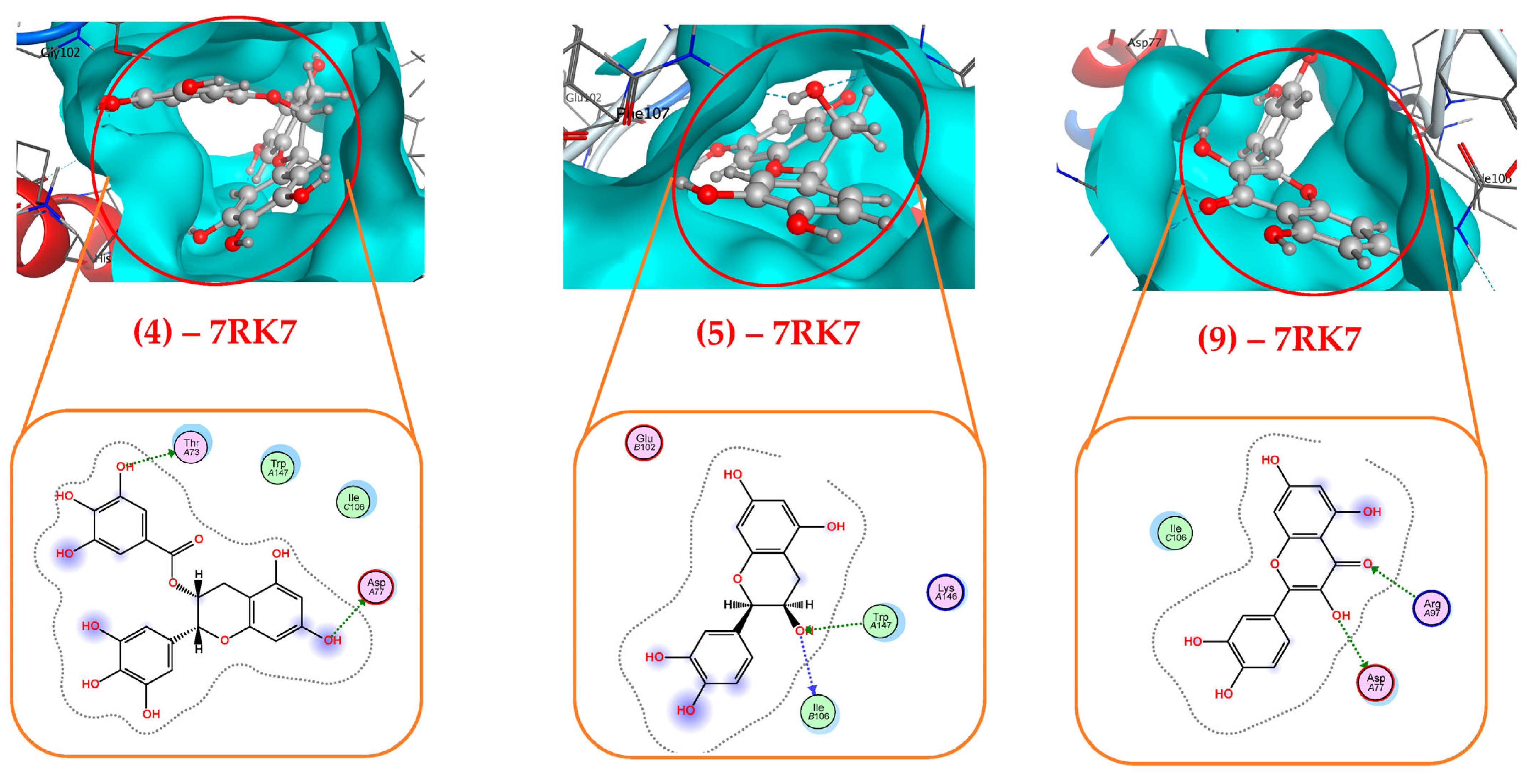
3.3.1. Molecular Docking Study and DFT Calculation
3.3.2. Drug-Likeness and Pharmacokinetic Parameters of Phytocompounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
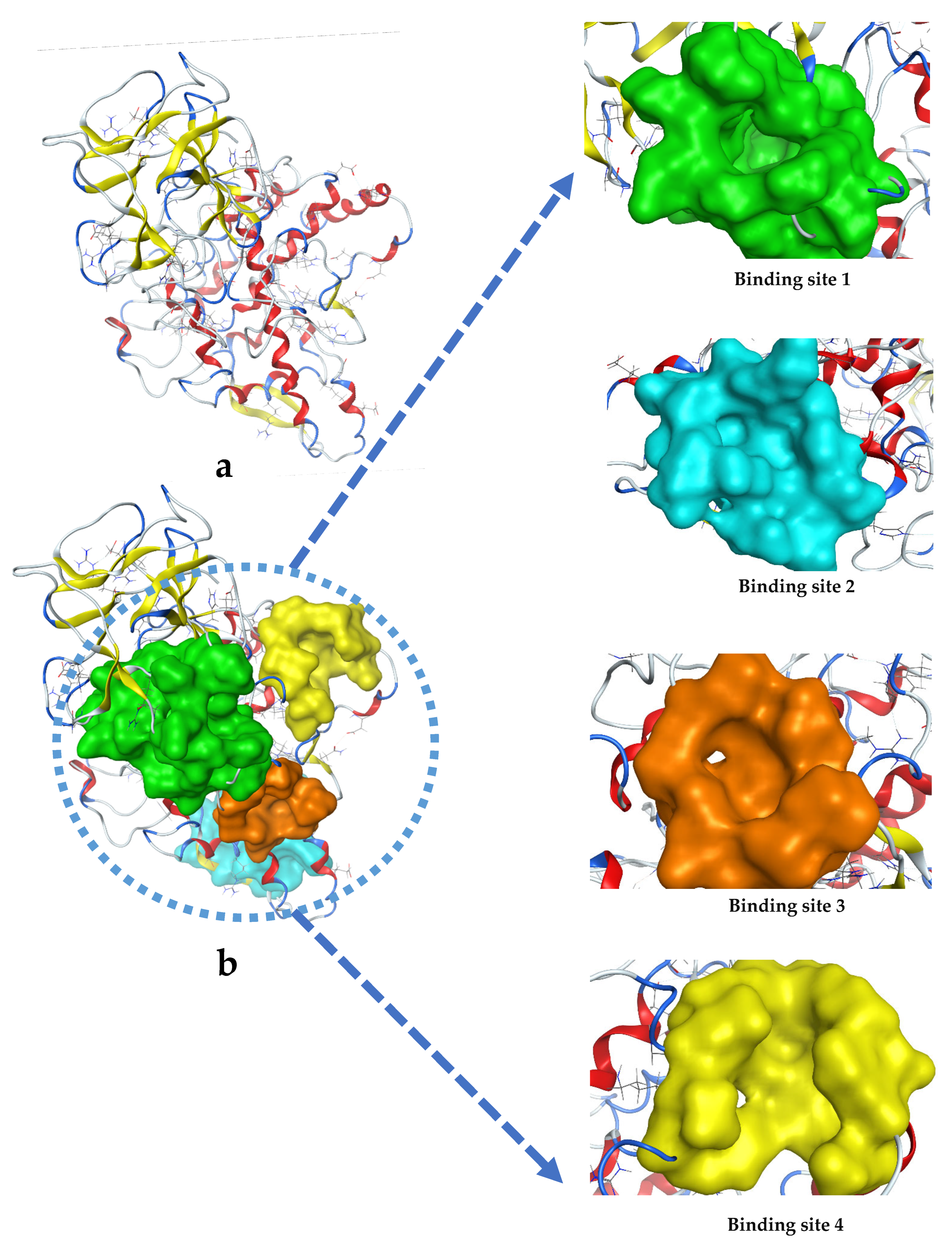
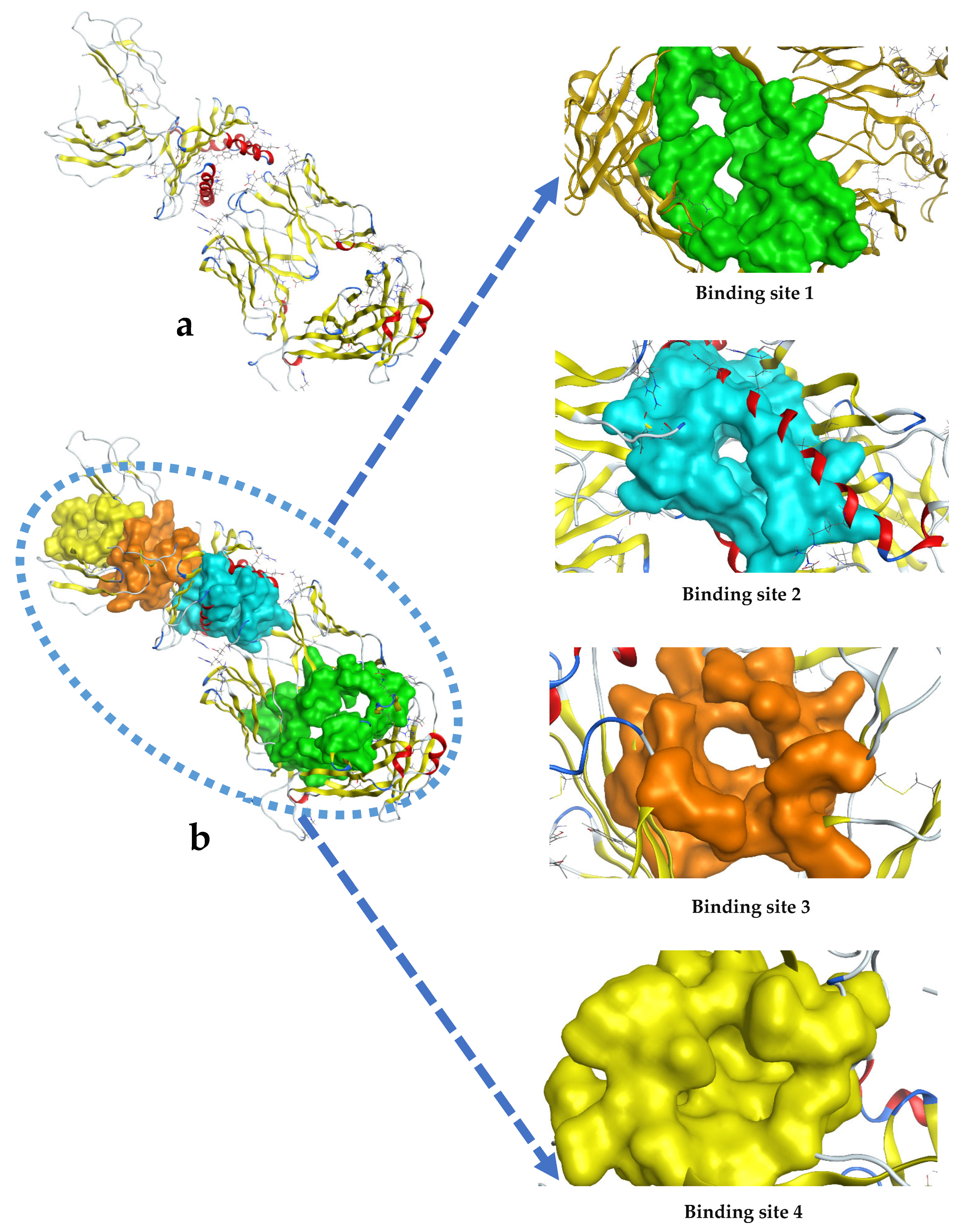
Appendix B
| Color | Site | Size | Residues |
|---|---|---|---|
 | 1 | 184 | 1:(SER2 LYS5 LYS70 GLN72 PRO73 GLN74 LEU75 HIS76 TYR78 TYR82 THR324 MET325 GLY326 LEU327 ILE328 PRO338 GLU340 TYR343 GLN347 ASP348 PRO349)2:(ASP60 GLY61 TYR62 GLN90 LYS93 SER95 ILE96 GLU97 TYR98 PHE105 VAL109 PRO110 ARG111 GLU112 GLY113 GLY114) |
 | 2 | 47 | 1:(VAL27 LYS28 ASN29 ASP30 LYS31 PHE33 THR34 GLN153 ASP157 GLN159 VAL160 GLU161 ILE162 THR163 LYS169 GLU171) |
 | 3 | 22 | 1:(MET8 PRO9 VAL11 GLY12 ILE13 PRO14 GLN103 THR104 TRP106 GLU107) |
 | 4 | 52 | 1:(GLN307 THR308 ASN310 TYR311 ASP312 VAL313 TYR314 GLU356 ASP357 TRP358 LYS376 LYS379 SER380) |
| Color | Site | Size | Residues |
|---|---|---|---|
 | 1 | 331 | 3:(ILE15 GLN45 PHE46 PRO47 SER48 GLN49 LEU87 SER88 ASP89 THR90 VAL92 TYR94 LYS112 LYS115 SER117 VAL118 LYS119 PRO120 SER150 ASP166 LYS167 CYS168 VAL169 ASN180)4:(HIS14 ARG40 GLN41 ASP42 PRO43 GLY44 HIS45 GLY46 ARG48 VAL64 ASP66 GLY67 TYR68 THR86 SER87 SER88 GLN89 THR90 SER91 VAL92 PHE94 TRP119 LEU120 THR121 VAL122 PHE160 ASP162 HIS163 VAL164 GLU165 LEU166 SER167 TRP168 GLU174 VAL175 HIS176 VAL179 CYS180 THR181 ASP182 PRO183 GLN184 PRO185 ASN193 ASP194 TYR197 LEU199 SER201) |
 | 2 | 219 | 1:(MET5 TYR7 PHE9 ALA24 MET45 TYR59 GLU63 LYS66 VAL67 ALA69 HIS70 THR73 ASP77 THR80 LEU81 TYR84 ARG97 TYR99 HIS114 TYR116 TYR123 THR142 THR143 LYS146 TRP147 ALA150 VAL152 GLN155 LEU156 TYR159 THR163 TRP167 TYR171)3:(ASN37 TYR39 LEU99 ASN100 TYR101 GLY102 GLN105)4:(LEU105 ILE106 PHE107 PRO108) |
 | 3 | 151 | 1:(ARG6 PHE8 TYR27 ASP29 ASP30 THR31 GLN32 ARG48 PRO210 ALA211 GLU212 GLU232 THR233 ARG234 PRO235 GLY237 GLY239 PHE241)2:(TYR27 SER29 HIS52 SER53 ASP54 SER56 PHE57 SER58 LYS59 TYR64 LEU65 LEU66 TYR68) |
 | 4 | 59 | 1:(HIS188 TRP204 LEU206 VAL231 ARG234 GLN242 LYS243 TRP244)2:(GLN9 VAL10 TYR11 SER12 HIS14 PRO15 ALA16 GLU17 TRP96 ARG98 ASP99 MET100) |
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow (electronic version). Mol. Asp. Med. 2016, 27, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.V.; Nguyen, T. An Overview of the Use of Plants and Animals in Traditional Medicine Systems in Vietnam; Traffic Southeast Asia: Hanoi, Vietnam, 2008. [Google Scholar]
- Xie, M.; Jiang, Z.; Lin, X.; Wei, X. Application of plant extracts cosmetics in the field of anti-aging. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100014. [Google Scholar] [CrossRef]
- Mansoor, K.; Aburjai, T.; Al-Mamoori, F.; Schmidt, M. Plants with cosmetic uses. Phytother. Res. 2023, 37, 5755–5768. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, R.M.; Chen, S.B.; Li, B. Research progresses of Tetrastigma planicaule (Hook.) Gagnep and same generic plants. Guid. J. Tradit. Chin. Med. Pharm. 2019, 25, 121–126. [Google Scholar]
- Zhang, L.; Li, B.; Wang, M.; Lin, H.; Peng, Y.; Zhou, X.; Peng, C.; Zhan, J.; Wang, W. Genus Tetrastigma: A Review of Its Folk Uses, Phytochemistry and Pharmacology. Chin. Herb. Med. 2022, 14, 210–233. [Google Scholar] [CrossRef]
- Dao, P.T.A.; Quan, T.L.; Mai, N.T.T. Antioxidant constituents from the stem of Tetrastigma erusbescense Planch. (Vitaceae). Nat. Prod. Sci. 2014, 20, 22–28. [Google Scholar]
- Nguyen, V.B.; Nguyen, Q.V.; Nguyen, A.D.; Wang, S.L. Screening and evaluation of α-glucosidase inhibitors from indigenous medicinal plants in Dak Lak province, Vietnam. Res. Chem. Intermed. 2017, 43, 3599–3612. [Google Scholar] [CrossRef]
- Doan, M.D.; Wang, S.L.; Nguyen, V.B.; Phan, T.K.P.; Phan, T.Q.; Nguyen, T.T.; Nguyen, T.H.; Nguyen, Q.V.; Nguyen, A.D. Phytochemical profiles and novel biofunctions of Dillenia ovata Wall. ex Hook.f. et Thomson: A Vietnamese indigenous medicinal plant. Res. Chem. Intermed. 2023, 49, 5567–5593. [Google Scholar] [CrossRef]
- Vu, T.T.L.; Wang, S.L.; Ho, T.T.T.; Luc, T.Q.; Phan, T.Q.; Phan, T.K.T.; Dam, T.B.H.; Phan, T.K.P.; Nguyen, A.D.; Nguyen, V.B. Elucidation of potent mammalian enzymes inhibitors targeting anti-diabetes drug from Castanea mollissima Blume, 1851: An edible herbal collected in Vietnam via experimental and computational approaching. Res. Chem. Intermed. 2024, 50, 6065–6086. [Google Scholar]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Liu, L. Skin-care functions of peptides prepared from Chinese Quince seed protein: Sequences analysis, tyrosinase inhibition and molecular docking study. Ind. Crops Prod. 2020, 148, 112331. [Google Scholar] [CrossRef]
- Seregheti, T.M.Q.; Pinto, A.P.R.; Gonçalves, M.D.C.; Antunes, A.D.S.; Almeida, W.A.D.S.; Machado, R.S.; Silva, J.N.; Ferreira, P.M.P.; Pessoa, C.; Santos, V.M.R.D.; et al. Antiproliferative and Photoprotective Activities of the Extracts and Compounds from Calea fruticosa. Braz. J. Med. Biol. Res. 2020, 53, e9375. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Wang, S.L.; Phan, T.Q.; Nguyen, T.H.; Nguyen, A.D.; Nguyen, V.B. Enhancing nematicidal effect of prodigiosin via micro-encapsulation using chitosan as a novel carrier substance. Res. Chem. Intermed. 2024, 50, 2873–2896. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.L.; Phan, T.Q.; Nguyen, T.H.; Tran, T.H.T.; Doan, M.D.; Ngo, V.A.; Nguyen, A.D.; Nguyen, V.B. New record of reusing brewing by-product for biosynthesis of prodigiosin and its novel anti-pathogen fungi via in vitro tests and molecular docking study. Res. Chem. Intermed. 2024, 50, 925–949. [Google Scholar] [CrossRef]
- Dulsat, J.; López-Nieto, B.; Estrada-Tejedor, R.; Borrell, J.I. Evaluation of free online ADMET tools for academic or small biotech environments. Molecules 2023, 28, 776. [Google Scholar] [CrossRef]
- Mota, M.D.; da Boa Morte, A.; Cerqueira e Silva, L.C.R.; Chinalia, F.A. Sunscreen protection factor enhancement through supplementation with rambutan (Nephelium lappaceum L.) ethanolic extract. J. Photochem. Photobiol. B Biol. 2020, 205, 111837. [Google Scholar] [CrossRef]
- Afaq, F.; Mukhtar, H. Photochemoprevention by botanical antioxidants. Ski. Pharmacol. Physiol. 2002, 15, 297–306. [Google Scholar] [CrossRef]
- Manap, A.S.A.; Lum, Y.K.; Ong, L.H.; Tang, Y.Q.; Gew, L.T.; Chia, A.Y.Y. Perspective approaches on melanogenesis inhibition. Dermatol. Sin. 2021, 39, 1–12. [Google Scholar] [CrossRef]
- Ru, Y.; Chen, X.; Wang, J.; Guo, L.; Lin, Z.; Peng, X.; Qiu, B.; Wong, W.L. Structural characterization, hypoglycemic effects and mechanism of a novel polysaccharide from Tetrastigma hemsleyanum Diels et Gilg. Int. J. Biol. Macromol. 2019, 123, 775–783. [Google Scholar] [CrossRef]
- Wang, C.Y.; Lee, S.; Jang, H.J.; Su, X.D.; Wang, H.S.; Kim, Y.H.; Yang, S.Y. Inhibition potential of phenolic constituents from the aerial parts of Tetrastigma hemsleyanum against soluble epoxide hydrolase and nitric oxide synthase. J. Enzym. Inhib. Med. Chem. 2019, 34, 753–760. [Google Scholar] [CrossRef]
- Karaoğlan, E.S.; Koca, M. Tyrosinase, Cholinesterase Inhibitory Activity and Molecular Docking Studies on Apigenin and Vitexin. Istanb. J. Pharm. 2020, 50, 268–271. [Google Scholar] [CrossRef]
- Ha, T.J.; Ha, Y.W.; Lee, J.H.; Lee, M.W. Apigenin, Tyrosinase Inhibitor Isolated from the Flowers of Hemisteptia lyrata Bunge. Arch. Pharm. Res. 2002, 25, 827–830. [Google Scholar]
- Fan, M.; Zhang, G.; Hu, X.; Xu, X.; Gong, D. Quercetin as a Tyrosinase Inhibitor: Inhibitory Activity, Conformational Change and Mechanism. Food Res. Int. 2017, 100, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Kinst-Hori, I. Kinetics of Mushroom Tyrosinase Inhibition by Quercetin. J. Agric. Food Chem. 1999, 47, 4121–4125. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Xiao, P.; Fujita, K. New Tyrosinase Inhibitors, (+)-Catechin–Aldehyde Polycondensates. Biomacromolecules 2003, 4, 561–567. [Google Scholar] [CrossRef]
- Kang, S.; Lee, H.; Kim, S. Determination of Tyrosinase–Cyanidin-3-O-glucoside and (−/+)-Catechin Interactions Using Computational Simulations and In Vitro Assessment. Sci. Rep. 2021, 11, 3569. [Google Scholar]
- Babu, T.M.C.; Rajesh, S.S.; Bhaskar, B.V.; Devi, S.; Rammohan, A.; Sivaraman, T.; Rajendra, W. Molecular docking, molecular dynamics simulation, biological evaluation and 2D QSAR analysis of flavonoids from Syzygium alternifolium as potent anti-Helicobacter pylori agents. RSC Adv. 2017, 7, 18277–18292. [Google Scholar] [CrossRef]
- Phan, T.Q.; Wang, S.-L.; Vu, T.T.L.; Do, T.L.; Thuy, P.T.; Phan, T.K.P.; Phan, T.K.T.; Nguyen, T.H.; Nguyen, A.D.; Nguyen, V.B. Inside into diverse pharmaceutical properties and phytochemical profiles of Tetrastigma erubescens: A drug discovery via experiments, docking, DFT, ADMET and Lipinski’s rules of five performance. Res. Chem. Intermed. 2025, 51, 3351–3381. [Google Scholar] [CrossRef]
- Kubo, I.; Chen, Q.-X.; Nihei, K. Molecular Design of Antibrowning Agents: Antioxidative Tyrosinase Inhibitors. Food Chem. 2003, 81, 241–247. [Google Scholar] [CrossRef]
- Chen, T.V.; Cuong, T.D.; Quy, P.T.; Anh, N.H.; Tung, B.T.; Minh, N.C.; Nam, N.H. Antioxidant Activity and α-Glucosidase Inhibitability of Distichochlamys citrea M.F. Newman Rhizome Fractionated Extracts: In Vitro and In Silico Screenings. Chem. Pap. 2022, 76, 5655–5675. [Google Scholar] [CrossRef]
- Loan, H.T.P.; Bui, T.Q.; My, T.T.A.; Hai, N.T.T.; Quang, D.T.; Tat, P.V.; Hiep, D.T.; Trung, N.T.; Quy, P.T.; Nhung, N.T.A. In-depth investigation of a Donor–Acceptor Interaction on the Heavy-Group-14@Group-13-Diyls in Transition-Metal Tetrylone complexes: Structure, bonding, and property. ACS Omega 2020, 5, 21271–21287. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Wang, S.L.; Phan, T.Q.; Doan, M.D.; Phan, T.K.P.; Phan, T.K.T.; Pham, T.H.T.; Nguyen, A.D. Novel anti-acetylcholinesterase effect of Euonymus laxiflorus Champ. extracts via experimental and in silico studies. Life 2023, 13, 1281. [Google Scholar] [CrossRef]
- Kwak, T.W.; Park, S.B.; Kim, H.J.; Jeong, Y.I.; Kang, D.H. Anticancer activities of Epigallocatechin-3-gallate against cholangiocarcinoma cells. Onco Targets Ther. 2017, 10, 137–144. [Google Scholar] [CrossRef]
- Zong, P.C.; John, B.S.; Ho, C.T.; Chen, K.Y. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998, 129, 173–179. [Google Scholar]
- Huang, S.; Wang, L.L.; Xue, N.N.; Li, C.; Guo, H.H.; Ren, T.K.; Zhan, Y.; Li, W.B.; Zhang, J.; Chen, X.G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef]
- Li, Z.; Feng, C.; Dong, H.; Jin, W.; Zhang, W.; Zhan, J.; Wang, S. Health promoting activities and corresponding mechanism of (−)-Epicatechin-3-gallate. Food Sci. Hum. Wellness 2022, 11, 568–578. [Google Scholar] [CrossRef]
- Elbaz, H.A.; Lee, I.; Antwih, D.A.; Liu, J.; Hüttemann, M.; Zielske, S.P. Epicatechin stimulates mitochondrial activity and selectively sensitizes cancer cells to radiation. PLoS ONE 2014, 9, e88322. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Q.; Liu, H.; Luo, S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol. Res. 2019, 52, 7. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef]
- Satari, A.; Ghasemi, S.; Habtemariam, S.; Asgharian, S.; Lorigooini, Z. Rutin: A flavonoid as an effective sensitizer for anticancer therapy; insights into multifaceted mechanisms and applicability for combination therapy. Evid.-Based Complement. Altern. Med. 2021, 2021, 9913179. [Google Scholar] [CrossRef] [PubMed]
- Afroze, N.; Pramodh, S.; Hussain, A.; Waleed, M.; Vakharia, K. A review on myricetin as a potential therapeutic candidate for cancer prevention. 3 Biotech 2020, 10, 211. [Google Scholar] [CrossRef] [PubMed]


| No. | Name | Family | Retention Time (min) | Content (μg/mg of EA Extract) |
|---|---|---|---|---|
| 1 | Gallic acid | Non-flavonoid phenolic | 4.135 | 30.837 |
| 2 | Catechin | Flavonoid (flavan-3-ol) | 12.157 | 2.454 |
| 3 | Chlorogenic acid | Non-flavonoid phenolic | 13.798 | 2.793 |
| 4 | Epigallocatechin gallate (EGCG) | Flavonoid (flavan-3-ol) | 14.388 | 0.835 |
| 5 | Epicatechin gallate | Flavonoid (flavan-3-ol) | 16.192 | 1.453 |
| 6 | Vitexin | Flavonoid (C-glycosyl flavone) | 17.643 | 0.168 |
| 7 | Rutin | Flavonoid (glycoside) | 19.883 | 1.284 |
| 8 | Quercetin | Flavonoid (flavonol) | 22.255 | 0.231 |
| 9 | Apigenin | Flavonoid (flavone) | 24.592 | 0.096 |
| No. | Name | Retention Time (min) | EA Extract |
|---|---|---|---|
| 10 | 6β-Hydroxyfluoxymesterone | 4.38 | 0.56 |
| 11 | Paclobutrazol | 5.26 | 0.68 |
| 12 | Iso-olomoucine | 7.98 | 1.4 |
| 13 | α-Methylaminohexanophenone | 19.72 | 1.42 |
| 14 | Hexadecanoic acid, methyl ester (CAS) | 28.42 | 10.39 |
| 15 | Cyclohexane, 1,5-diethenyl-2,3-dimethyl-, (1α,2α,3α,5α)- | 31.29 | 1 |
| 16 | 5-Decene, 4-ethynyl-, (E)- (CAS) | 31.41 | 5.85 |
| 17 | Cyclopentaneethanol, 2-(hydroxymethyl)-β,3-dimethyl- (CAS) | 31.59 | 10.5 |
| 18 | 2H-Pyran-2-carboxylic acid, 3,6-dihydro-6-propoxy-, ethyl | 38.69 | 60.91 |
| 19 | Glafenin | 39.62 | 7.28 |
| The Interaction of Ligands (L) with 2Y9X | The Interaction of Ligands (L) with 7RK7 | ||||||
|---|---|---|---|---|---|---|---|
| Symbol of L-2Y9X | Docking Site | DS (Kcal/mol) | RMSD (Å) | Symbol of L-7RK7 | Docking Site | DS (Kcal/mol) | RMSD (Å) |
| (1)—2Y9X | 1 | −11.3 | 1.128 | (1)—7RK7 | 1 | −9.7 | 1.21 |
| (2)—2Y9X | 1 | −13.1 | 0.93 | (2)—7RK7 | 2 | −11.4 | 1.83 |
| (3)—2Y9X | 1 | −12.6 | 1.30 | (3)—7RK7 | 2 | −10.9 | 0.92 |
| (4)—2Y9X | 1 | −13.9 | 1.90 | (4)—7RK7 | 2 | −14.8 | 1.81 |
| (5)—2Y9X | 1 | −13.5 | 1.51 | (5)—7RK7 | 2 | −12.4 | 1.53 |
| (6)—2Y9X | 1 | −13.5 | 1.27 | (6)—7RK7 | 1 | −11.8 | 1.73 |
| (7)—2Y9X | 1 | −13.7 | 0.98 | (7)—7RK7 | 2 | −10.5 | 1.38 |
| (8)—2Y9X | 1 | −14.1 | 1.04 | (8)—7RK7 | 2 | −11.4 | 1.75 |
| (9)—2Y9X | 1 | −12.1 | 1.01 | (9)—7RK7 | 2 | −12.5 | 1.44 |
| (10)—2Y9X | 1 | −11.3 | 1.24 | (10)—7RK7 | 1 | −10.7 | 1.25 |
| (11)—2Y9X | 1 | −10.9 | 1.78 | (11)—7RK7 | 1 | −8.6 | 1.07 |
| (12)—2Y9X | 1 | −10.1 | 1.17 | (12)—7RK7 | 1 | −8.3 | 1.47 |
| (13)—2Y9X | 1 | −8.9 | 1.51 | (13)—7RK7 | 2 | −10.4 | 1.38 |
| (14)—2Y9X | 1 | −9.8 | 1.85 | (14)—7RK7 | 1 | −8.0 | 0.97 |
| (15)—2Y9X | 1 | −7.5 | 1.17 | (15)—7RK7 | 1 | −8.6 | 2.00 |
| (16)—2Y9X | 1 | −7.6 | 1.59 | (16)—7RK7 | 2 | −7.2 | 1.04 |
| (17)—2Y9X | 2 | −8.5 | 1.79 | (17)—7RK7 | 1 | −6.7 | 1.14 |
| (18)—2Y9X | 1 | −9.5 | 1.78 | (18)—7RK7 | 1 | −7.2 | 1.16 |
| (19)—2Y9X | 1 | −11.1 | 1.66 | (19)—7RK7 | 2 | −8.6 | 1.60 |
| (20)—2Y9X | 1 | −8.7 | 1.30 | (20)—7RK7 | 1 | −8.6 | 1.49 |
| Symbol of Ligand–Protein | Number (Type) of Linkages | Amino Acids of Protein 2Y9X Interacting with the Ligands [Distance (Å)/E (kcal/mol)/Linkage Type] |
|---|---|---|
| Interacting with protein 7RK7 | ||
| (4)—7RK7 | 2 H-donor | Asp77 (2.96/−3.7/H-donor); Thr73 (2.83/−1.2/H-donor) |
| (5)—7RK7 | 1 H-donor, 1 H-acceptor | Ile106 (3.16/−0.7/H-donor); Trp147 (3.19/−1.1/H-acceptor) |
| (9)—7RK7 | 1 H-donor, 2 H-acceptor | Asp77 (2.83/−0.7/H-donor); Arg97 (2.95/−0.6/H-acceptor); Arg97 (2.82/−1.6/H-acceptor) |
| Interacting with protein 2Y9X | ||
| (2)—2Y9X | 1 H-donor, 1 pi-H | Asp60 (3.17/−0.7/H-donor); Glu97 (4.58/−0.5/pi-H) |
| (4)—2Y9X | 2 H-donor | Tyr62 (3.11/−0.9/H-donor); Gln72 (2.92/−1.9/H-donor) |
| (5)—2Y9X | 3 H-donor | Gln72 (2.86/−2.1/H-donor); Glu97 (2.86/−1.2/H-donor); Asp60 (2.76/−5.0/H-donor) |
| (6)—2Y9X | 3 H-donor | Ile96 (3.06/−1.2/H-donor); Gly326 (3.23/−0.6/H-donor); Glu340 (3.19/−0.7/H-donor) |
| (7)—2Y9X | 1 H-donor, 2 H-acceptor, 1 pi-H | Asp60 (3.05/−0.5/H-donor); Lys5 (2.83/−4.3/H-acceptor); Lys5 (2.92/−3.2/H-acceptor); Gln74 (4.50/−0.6/pi-H) |
| (8)—2Y9X | 1 H-donor, 1 H-acceptor | Asp60 (3.05/−1.0/H-donor); Leu75 (3.18/−2.4/H-acceptor) |
| Lipinski’s Rule of Five | |||||
|---|---|---|---|---|---|
| Compd. | Mass | H-Bond Donor | H-Bond Acceptors | logP | Molar Refractivity |
| 1 | 169 | 3 | 5 | −0.8331 | 35.766895 |
| 2 | 290 | 5 | 6 | 1.5461 | 72.622993 |
| 3 | 353 | 5 | 9 | −1.980601 | 79.889977 |
| 4 | 458 | 8 | 11 | 2.233202 | 108.920845 |
| 5 | 442 | 7 | 10 | 2.527601 | 107.256042 |
| 6 | 432 | 7 | 10 | −0.0655 | 103.53405 |
| 7 | 610 | 10 | 16 | −1.878802 | 137.495483 |
| 8 | 302 | 5 | 7 | 2.0109 | 74.050476 |
| 9 | 270 | 3 | 5 | 2.4196 | 70.813889 |
| 10 | 352 | 3 | 4 | 2.303 | 90.462379 |
| 11 | 273 | 1 | 3 | 2.77732 | 79.403778 |
| 12 | 300 | 4 | 7 | 1.3121 | 88.268875 |
| 13 | 206 | 2 | 1 | 1.6213 | 61.934883 |
| 14 | 270 | 0 | 2 | 5.6407 | 82.327972 |
| 15 | 164 | 0 | 0 | 3.656799 | 54.935986 |
| 16 | 164 | 0 | 0 | 3.782299 | 55.895985 |
| 17 | 172 | 2 | 2 | 1.2694 | 48.713585 |
| 18 | 214 | 0 | 4 | 1.6473 | 55.293983 |
| 19 | 352 | 3 | 6 | 2.796819 | 99.579773 |
| 20 | 142 | 2 | 4 | −0.17871 | 32.389095 |
| Lipinski’s rules | ≤500 | ≤5 | ≤10 | ≤5 | 40–130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, T.T.L.; Phan, T.Q.; Do, T.L.; Nguyen, V.B. Novel and Potential Photoprotective and Tyrosinase Inhibitory Effects of Tetrastigma erubescens Extracts: Evidence from In Vitro Assays and Computational Approach. Life 2025, 15, 995. https://doi.org/10.3390/life15070995
Vu TTL, Phan TQ, Do TL, Nguyen VB. Novel and Potential Photoprotective and Tyrosinase Inhibitory Effects of Tetrastigma erubescens Extracts: Evidence from In Vitro Assays and Computational Approach. Life. 2025; 15(7):995. https://doi.org/10.3390/life15070995
Chicago/Turabian StyleVu, Thi Thu Le, Tu Quy Phan, Tien Lam Do, and Van Bon Nguyen. 2025. "Novel and Potential Photoprotective and Tyrosinase Inhibitory Effects of Tetrastigma erubescens Extracts: Evidence from In Vitro Assays and Computational Approach" Life 15, no. 7: 995. https://doi.org/10.3390/life15070995
APA StyleVu, T. T. L., Phan, T. Q., Do, T. L., & Nguyen, V. B. (2025). Novel and Potential Photoprotective and Tyrosinase Inhibitory Effects of Tetrastigma erubescens Extracts: Evidence from In Vitro Assays and Computational Approach. Life, 15(7), 995. https://doi.org/10.3390/life15070995









