Relationship Between Subclinical Renal Damage and Maximum Rate of Blood Pressure Variation Assessed by Fourier Analysis of 24-h Blood Pressure Curve in Patients with Essential Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- History of renovascular, parenchymal, endocrine, or malignant hypertension;
- Presence of hematuria or overt proteinuria;
- Personal history of glomerulonephritis or hereditary kidney disease;
- Errors in 24-h urine collection, defined as:
- ○
- Under-collection: urinary creatinine < 10 mg/kg for women or <15 mg/kg for men;
- ○
- Over-collection: urinary creatinine > 25 mg/kg for women or >30 mg/kg for men;
- Inability to obtain at least 80% valid BP readings during 24-h ambulatory blood pressure monitoring (ABPM);
- History or clinical signs of heart failure, ischemic heart disease, or cerebrovascular disease;
- Presence of major non-cardiovascular comorbidities;
- Estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2;
- Inability to suspend antihypertensive medications for at least one week prior to ABPM and biochemical assessment.
2.2. Definition of Subclinical Kidney Damage
2.3. Laboratory Methods
2.4. Ambulatory Blood Pressure Monitoring (ABPM)
- SBP > 260 mmHg or <70 mmHg;
- DBP > 150 mmHg or <40 mmHg;
- Pulse pressure > 150 mmHg or <20 mmHg.
2.5. Fourier-Derived Parameters
- The maximum slope of SBP and DBP (Slope max SBP and DBP), calculated as the first derivative of the Fourier-fitted curve in mmHg/hour.
- The maximum and minimum BP values (SBP max/min and DBP max/min), and their absolute differences.
- The weighted standard deviation (wSD) of 24-h SBP and DBP, derived from day and night SDs.
2.6. Circadian BP Variability
- Reverse dippers: nighttime BP > daytime BP
- Non-dippers: night–day difference 0–10%
- Dippers: night–day difference 10–20%
- Extreme dippers: reduction > 20%
2.7. Fourier Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mancia Chairperson, G.; Kreutz Co-Chair, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.C.; Conell, C.; Ren, X.; Banki, N.M.; Chan, S.L.; Rao, V.A.; Melles, R.B.; Bhatt, D.L. Effect of Systolic and Diastolic Blood Pressure on Cardiovascular Outcomes. N. Engl. J. Med. 2019, 381, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Wei, W.; Pu, Y.; Zhang, L.; Cui, T.; Ma, L.; Wang, B.; Zhao, Y.; Fu, P. Blood Pressure Variability and the Progression of Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2023, 38, 1272–1281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanevold, C.D.; Seo, J.D.; Daniels, S.R.; Falkner, B.E.; Ferguson, M.A.; Flynn, J.T.; Ingelfinger, J.R.; Khoury, P.R.; Lande, M.B.; Meyers, K.E.; et al. Ambulatory blood pressure variability in prediction of target organ injury: The SHIP AHOY study. Pediatr. Nephrol. 2025; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Ohishi, M.; Kamide, K.; Onishi, M.; Takeya, Y.; Tatara, Y.; Oguro, R.; Yamamoto, K.; Sugimoto, K.; Rakugi, H. The impact of visit-to-visit variability in blood pressure on renal function. Hypertens. Res. 2012, 35, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Viazzi, F.; Leoncini, G.; Conti, N.; Tomolillo, C.; Giachero, G.; Vercelli, M.; Deferrari, G.; Pontremoli, R. Combined effect of albuminuria and estimated glomerular filtration rate on cardiovascular events and all-cause mortality in uncomplicated hypertensive patients. J. Hypertens. 2010, 28, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Liu, G.; Shi, Y.; Zhang, Y. Impact of microalbuminuria on incident coronary heart disease, cardiovascular and all-cause mortality: A meta-analysis of prospective studies. Int. J. Clin. Exp. Med. 2015, 8, 1–9. [Google Scholar] [PubMed] [PubMed Central]
- Chronic Kidney Disease Prognosis Consortium; Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Bidani, A.K.; Polichnowski, A.J.; Loutzenhiser, R.; Griffin, K.A. Renal microvascular dysfunction, hypertension and CKD progression. Curr. Opin. Nephrol. Hypertens. 2013, 22, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krishnan, S.; Suarez-Martinez, A.D.; Bagher, P.; Gonzalez, A.; Liu, R.; Murfee, W.L.; Mohandas, R. Microvascular dysfunction and kidney disease: Challenges and opportunities? Microcirculation 2021, 28, e12661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Somes, G.W.; Harshfield, G.A.; Arheart, K.L.; Miller, S.T. A Fourier series approach for comparing groups of subjects on ambulatory blood pressure patterns. Stat. Med. 1994, 13, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Mulè’, G.; Sorce, A.; Vario, M.G.; Giambrone, M.; Cottone, S. Should reduction of increased short-term blood pressure variability be a target of antihypertensive therapy? J. Clin. Hypertens. 2021, 23, 1162–1164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schutte, A.E.; Kollias, A.; Stergiou, G.S. Blood pressure and its variability: Classic and novel measurement techniques. Nat. Rev. Cardiol. 2022, 19, 643–654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giuseppe, M.; Sorce, A.; Carollo, C.; Geraci, G.; Cottone, S. Self-blood pressure monitoring as a tool to increase hypertension awareness, adherence to antihypertensive therapy, and blood pressure control. J. Clin. Hypertens. 2019, 21, 1305. [Google Scholar]
- Tatasciore, A.; Di Nicola, M.; Tommasi, R.; Santarelli, F.; Palombo, C.; Parati, G.; De Caterina, R. From short-term blood pressure variability to atherosclerosis: Relative roles of vascular stiffness and endothelial dysfunction. J. Clin. Hypertens. 2020, 22, 1218–1227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ko, Y.E.; Jhee, J.H. Short-term blood pressure variability as a potential therapeutic target for kidney disease. Clin. Hypertens. 2023, 29, 23. [Google Scholar] [CrossRef] [PubMed]
- Gaciong, Z.; Siński, M.; Lewandowski, J. Blood Pressure Control and Primary Prevention of Stroke: Summary of the Recent Clinical Trial Data and Meta-Analyses. Curr. Hypertens. Rep. 2013, 15, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, C.; Kim, S.; Kim, J.S.; Lim, S.Y.; Seo, H.S.; Lim, H.E.; Sung, K.C.; Cho, G.Y.; Lee, S.K.; et al. Prevalence of Isolated Nocturnal Hypertension and Development of Arterial Stiffness, Left Ventricular Hypertrophy, and Silent Cerebrovascular Lesions: The KoGES (Korean Genome and Epidemiology Study). J. Am. Heart Assoc. 2022, 11, e025641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loutzenhiser, R.; Griffin, K.; Williamson, G.; Bidani, A. Renal autoregulation: New perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1153–R1167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, W.; Remedies, C.E.; Obi, I.E.; Aldous, S.R.; Meera, S.I.; Sanders, P.W.; Inscho, E.W.; Guan, Z. Restoration of afferent arteriolar autoregulatory behavior in ischemia-reperfusion injury in rat kidneys. Am. J. Physiol. Ren. Physiol. 2021, 320, F429–F441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mizdrak, M.; Kumrić, M.; Kurir, T.T.; Božić, J. Emerging Biomarkers for Early Detection of Chronic Kidney Disease. J. Pers. Med. 2022, 12, 548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function--measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Nwabuo, C.C.; Yano, Y.; Moreira, H.T.; Appiah, D.; Vasconcellos, H.D.; Aghaji, Q.N.; Viera, A.J.; Rana, J.S.; Shah, R.V.; Murthy, V.L.; et al. Long-Term Blood Pressure Variability in Young Adulthood and Coronary Artery Calcium and Carotid Intima-Media Thickness in Midlife: The CARDIA Study. Hypertension 2020, 76, 404–409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mancia, G.; Parati, G.; Pomidossi, G.; Grassi, G.; Casadei, R.; Zanchetti, A. Relationship between blood pressure variability and carotid artery damage in hypertension. Hypertension 1994, 24, 739–745. [Google Scholar]
- Kikuya, M.; Hozawa, A.; Ohokubo, T.; Tsuji, I.; Michimata, M.; Matsubara, M.; Ota, M.; Nagai, K.; Araki, T.; Satoh, H.; et al. Prognostic significance of blood pressure variability in individuals with and without hypertension: The Ohasama study. Hypertens. Res. 2000, 27, 273–279. [Google Scholar] [CrossRef]
- Mulè, G.; Castiglia, A.; Cusumano, C.; Scaduto, E.; Geraci, G.; Altieri, D.; Di Natale, E.; Cacciatore, O.; Cerasola, G. Subclinical kidney damage in hypertensive patients: A renal window opened on the cardiovascular system. Focus on microalbuminuria. Adv. Exp. Med. Biol. 2017, 956, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Massierer, D.; Leiria, L.F.; Severo, M.D.; Ledur, P.; Dos, S.; Becker, A.D.; Aguiar, F.M.; Lima, E.; Freitas, V.C.; Schaan, B.D.; et al. Blood pressure variability and its association with echocardiographic parameters in hypertensive diabetic patients. BMC Cardiovasc. Disord. 2016, 16, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kario, K.; Hoshide, S.; Shimada, K. Impact of visit-to-visit variability of blood pressure on deterioration of renal function in patients with non-diabetic chronic kidney disease. Hypertens. Res. 2013, 36, 151–157. [Google Scholar] [CrossRef]
- Mena, L.; Parati, G.; Bilo, G. Visit-to-visit blood pressure variability: A review of the literature. J. Clin. Hypertens. 2017, 19, 607–613. [Google Scholar] [CrossRef]
- Mena, L.; Pintos, S.; Queipo, N.V.; Aizpurua, J.M.; Maestre, G.; Sulbarán, T. A reliable index for the prognostic significance of blood pressure variability. J. Hypertens. 2005, 23, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Ochoa, J.E.; Lombardi, C.; Bilo, G. Assessment and management of blood-pressure variability. Nat. Rev. Cardiol. 2013, 10, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Hoshide, S.; Pickering, T.G. Blood pressure variability and cardiovascular risk: Current evidence and clinical implications. Hypertens. Res. 2013, 36, 393–404. [Google Scholar]
- Tonneijck, L.; Muskiet, M.H.A.; Smits, M.M.; van Bommel, E.J.; Heerspink, H.J.L.; van Raalte, D.H. Glomerular hemodynamics and albuminuria: Mechanisms and therapeutic implications. Nat. Rev. Nephrol. 2017, 13, 720–731. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Mojón, A.; Fernández, J.R. Ambulatory blood pressure monitoring: Clinical relevance of blood pressure variability and circadian rhythm. Hypertens. Res. 2013, 36, 375–385. [Google Scholar]
- Parati, G.; Stergiou, G.S. Ambulatory blood pressure monitoring: Indications and interpretation. J. Clin. Hypertens. 2018, 20, 1249–1257. [Google Scholar]

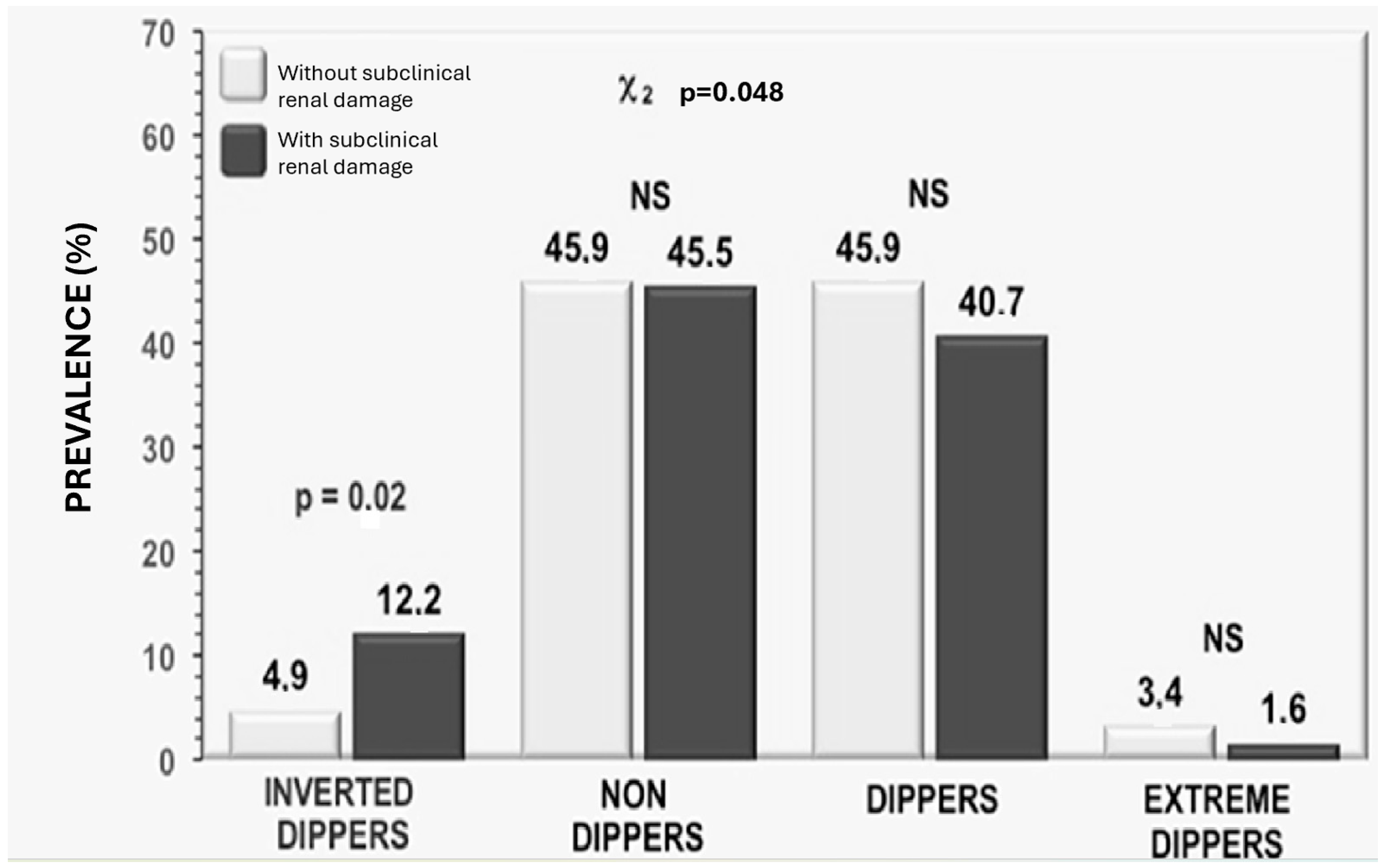
| Total Patients | GFR > 90 mL/min/1.73 m2 | eGFR < 90 mL/min/1.73 m2 | p | |
|---|---|---|---|---|
| N = 389 | N = 266 | N = 123 | ||
| Age (years) | 48.8 ± 12.4 | 47.9 ± 11.8 | 50.7 ± 13.6 | 0.04 |
| Sex (Males) (%) | 57.8 | 58 | 57.7 | 0.51 |
| Smokers (%) | 36.7 | 33.5 | 40.2 | 0.16 |
| Diabetes (%) | 11.2 | 9.1 | 13.4 | 0.12 |
| Glycemia (mg/dL) | 99.4 ± 24.4 | 98.2 ± 20.7 | 102.2 ± 31.2 | 0.15 |
| BMI (kg/m2) | 28.4 ± 4.1 | 28.2 ± 4.2 | 28.8 ± 4.1 | 0.16 |
| Waist circumference (cm) | 96.7 ± 12.4 | 95.8 ± 11.1 | 98.6 ± 14.7 | 0.04 |
| Total cholesterol (mg/dL) | 211.6 ± 40.7 | 211.5 ± 41.2 | 211.8 ± 39.7 | 0.95 |
| HDL cholesterol (mg/dL) | 45.4 ± 9.8 | 46.3 ± 9.5 | 43.5 ± 10.5 | 0.34 |
| Triglycerides (mg/dL) | 134 (94–189) | 130 (88–180) | 140 (107–202.5) | 0.0549 |
| Uricaemia (mg/dL) | 4.9 ± 1.5 | 4.7 ± 1.4 | 5.2 ± 1.5 | 0.001 |
| Albuminuria (mg/min) | 9 (5–22) | 8 (5–17) | 10 (5–31) | <0.001 |
| Previous antihypertensive therapy (%) | 72.6 | 69.6 | 75.9 | 0.11 |
| Office SBP (mmHg) | 154 ± 19 | 130 ± 12.5 | 136 ± 13.4 | <0.001 |
| Office DBP (mmHg) | 93 ± 18 | 80 ± 11.7 | 84 ± 11.1 | 0.09 |
| Total Patients | eGFR > 90 mL/min | eGFR < 90 mL/min | p | |
|---|---|---|---|---|
| N = 389 | N = 266 | N = 123 | ||
| 24 h SBP (mmHg) | 132 ± 13.2 | 130 ± 13.5 | 136 ± 13.2 | <0.001 |
| 24 h DBP (mmHg) | 81.7 ± 11.6 | 81 ± 11.7 | 84 ± 11.1 | 0.06 |
| daytime SBP(mmHg) | 136 ± 13.5 | 134 ± 13.03 | 140 ± 13.8 | <0.001 |
| daytime DBP (mmHg) | 86 ± 11 | 85 ± 10.8 | 87 ± 11.5 | 0.27 |
| nocturnal SBP (mmHg) | 123 ± 14.3 | 120 ± 15 | 129 ± 14.3 | <0.001 |
| nocturnal DBP (mmHg) | 74 ± 11 | 73 ± 10.5 | 76 ± 11.5 | 0.001 |
| SBP max (mmHg) | 150 (140–162) | 148 (139–161) | 153 (141–165) | 0.14 |
| DBP max (mmHg) | 98 (89–105) | 98 (90–106) | 97 (88–105) | 0.58 |
| SBP max–min (mmHg) | 37 (28–46) | 36 (27–44) | 39 (28–48) | 0.102 |
| DBP max–min (mmHg) | 32 (25–39) | 32 (25–39) | 31 (25–37) | 0.487 |
| Slope max SBP | 11.7 (7.9–16.5) | 10.8 (7.6–15.1) | 12.8 (8.9–17.6) | 0.028 |
| Slope max DBP | 10.6 (7.9–14.3) | 10.7 (7.9–14.2) | 10.5 (7.8–14.3) | 0.736 |
| Patients Without Subclinical Renal Damage N = 272 | Patients with Subclinical Renal Damage N = 117 | p | |
|---|---|---|---|
| Age (years) | 48 ± 11.7 | 51 ± 13.8 | 0.03 |
| Sex (Males) (%) | 53.7 | 68.3 | 0.008 |
| Smokers (%) | 32.8 | 46.4 | 0.019 |
| Diabetes (%) | 10.3 | 13.3 | 0.512 |
| Glycemia (mg/dL) | 99 ± 22.1 | 101 ± 30 | 0.41 |
| BMI (kg/m2) | 28.2 ± 4.2 | 28.8 ± 4.1 | 0.23 |
| Waist circumference (cm) | 95.9 ± 11.2 | 98.4 ± 14.9 | 0.08 |
| Total cholesterol (mg/dL) | 212 ± 41 | 210.5 ± 49.7 | 0.73 |
| HDL cholesterol (mg/dL) | 46.1 ± 9.4 | 43.7 ± 10.8 | 0.07 |
| Triglycerides (mg/dL) | 130 (93–182) | 152 (99–200) | 0.08 |
| Uricaemia (mg/dL) | 4.7 ± 1.4 | 5.3 ± 1.6 | 0.001 |
| AER (μg/min) | 6 (4–10) | 23 (37–57) | <0.001 |
| Previous antihypertensive therapy (%) | 70.5 | 77.8 | 0.167 |
| Clinical SBP (mmHg) | 152 ± 18 | 159 ± 19 | 0.001 |
| Clinical DBP (mmHg) | 92 ± 17 | 96 ± 19 | 0.043 |
| Patients Without Subclinical Renal Damage | Patients With Subclinical Renal Damage | p | |
|---|---|---|---|
| N = 272 | N = 117 | ||
| 24 h SBP (mmHg) | 130 ± 13 | 137 ± 13 | <0.001 |
| 24 h DBP (mmHg) | 81 ± 12 | 84 ± 11 | 0.008 |
| daytime SBP(mmHg) | 134 ± 13 | 140 ± 14 | <0.001 |
| daytime DBP (mmHg) | 85 ± 11 | 87 ± 11 | 0.037 |
| nocturnal SBP (mmHg) | 121 ± 13 | 129 ± 15 | 0.001 |
| nocturnal DBP (mmHg) | 73 ± 11 | 77 ± 12 | 0.002 |
| SBP max (mmHg) | 147 (138–160) | 157 (144–165) | 0.01 |
| DBP max (mmHg) | 96 (89–105) | 100 (91–108) | 0.054 |
| SBP max–min (mmHg) | 11 (7–15) | 14 (10–18) | 0.014 |
| DBP max–min (mmHg) | 10 (8–14) | 11 (8–15) | 0.164 |
| Slope max SBP (mmHg/h) | 10.7 (7.6–15.5) | 14.2 (10.2–18.2) | 0.007 |
| Slope max DBP (mmHg/h) | 10.3 (7.0–13.9) | 11.4 (7.9–14.8) | 0.92 |
| GFR | Log MA | |||
|---|---|---|---|---|
| r | p | r | p | |
| 24 h SBP | −0.198 | <0.001 | 0.279 | <0.001 |
| 24 h DBP | −0.091 | ns | 0.189 | <0.001 |
| daytime SBP | −0.179 | <0.001 | 0.249 | <0.001 |
| daytime DBP | −0.09 | ns | 0.158 | 0.002 |
| nocturnal SBP | −0.21 | <0.001 | 0.298 | <0.001 |
| nocturnal DBP | −0.118 | 0.02 | 0.187 | <0.001 |
| (Log) Slope max SBP | −0.153 | 0.002 | 0.215 | <0.001 |
| (Log) Slope max DBP | −0.008 | ns | 0.138 | 0.007 |
| (Log) SBP max | −0.145 | 0.005 | 0.259 | <0.001 |
| (Log) DBP max | −0.005 | ns | 0.192 | <0.001 |
| (Log) SBP max–min | −0.127 | 0.008 | 0.156 | 0.002 |
| (Log) DBP max–min | 0.025 | ns | 0.152 | 0.003 |
| Δ% day–night SBP | 0.09 | 0.075 | −0.114 | 0.025 |
| (a) | (Log) Urinary Albumin Excretion (R2 = 0.154) | |
| β | p | |
| 24 h average SBP | 0.231 | <0.001 |
| (Log) Slope max SBP | 0.220 | <0.001 |
| Sex (M: 1; F = 0) | 0.152 | 0.001 |
| Δ% day–night SBP | −0.142 | 0.003 |
| (b) | eGFR (R2 = 0.243) | |
| β | p | |
| Age | −0.453 | <0.001 |
| 24 h average SBP | −0.152 | 0.001 |
| Subclinical Renal Damage (R2 = 0.154) | |||
|---|---|---|---|
| Odds ratio | 95% CI | p | |
| 24 h average SBP * | 1.600 | 1.236–2.035 | <0.001 |
| (Log) Slope max SBP * | 1.536 | 1.241–2.004 | 0.001 |
| Δ% day–night SBP * | 0.683 | 0.535–0.872 | 0.002 |
| Sex (M: 1; F = 0) | 0.567 | 0.345–0.932 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carollo, C.; Sorce, A.; Vario, M.G.; Cirafici, E.; Bologna, D.; Ciuppa, M.E.; Evola, S.; Mulè, G.; Geraci, G. Relationship Between Subclinical Renal Damage and Maximum Rate of Blood Pressure Variation Assessed by Fourier Analysis of 24-h Blood Pressure Curve in Patients with Essential Hypertension. Life 2025, 15, 1149. https://doi.org/10.3390/life15071149
Carollo C, Sorce A, Vario MG, Cirafici E, Bologna D, Ciuppa ME, Evola S, Mulè G, Geraci G. Relationship Between Subclinical Renal Damage and Maximum Rate of Blood Pressure Variation Assessed by Fourier Analysis of 24-h Blood Pressure Curve in Patients with Essential Hypertension. Life. 2025; 15(7):1149. https://doi.org/10.3390/life15071149
Chicago/Turabian StyleCarollo, Caterina, Alessandra Sorce, Maria Giovanna Vario, Emanuele Cirafici, Davide Bologna, Maria Elena Ciuppa, Salvatore Evola, Guseppe Mulè, and Giulio Geraci. 2025. "Relationship Between Subclinical Renal Damage and Maximum Rate of Blood Pressure Variation Assessed by Fourier Analysis of 24-h Blood Pressure Curve in Patients with Essential Hypertension" Life 15, no. 7: 1149. https://doi.org/10.3390/life15071149
APA StyleCarollo, C., Sorce, A., Vario, M. G., Cirafici, E., Bologna, D., Ciuppa, M. E., Evola, S., Mulè, G., & Geraci, G. (2025). Relationship Between Subclinical Renal Damage and Maximum Rate of Blood Pressure Variation Assessed by Fourier Analysis of 24-h Blood Pressure Curve in Patients with Essential Hypertension. Life, 15(7), 1149. https://doi.org/10.3390/life15071149







