Neural Pathways of Visual Face Recognition Immediately After Birth
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli
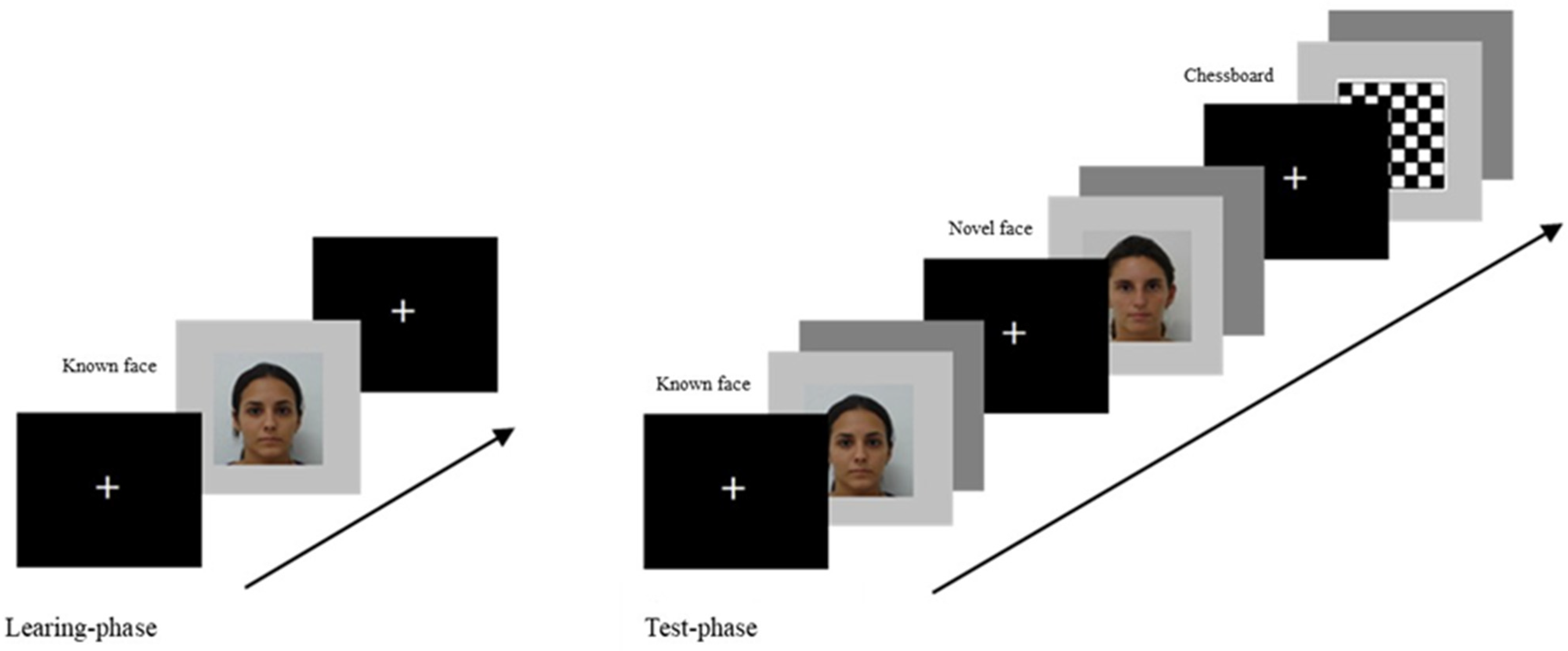
2.3. Procedure
2.4. Video Recordings Encoding
2.5. Electroencephalographic (EEG) Recording and Event-Related Potential (ERP) Analysis
3. Results
ERPs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pascalis, O.; de Martin de Viviés, X.; Anzures, G.; Quinn, P.C.; Slater, A.M.; Tanaka, J.W.; Lee, K. Development of face processing. Wires Cogn. Sci. 2011, 2, 666–675. [Google Scholar] [CrossRef]
- Cecchini, M.; Aceto, P.; Altavilla, D.; Palumbo, L.; Lai, C. The role of the eyes in processing an intact face and its scrambled image: A dense array ERP and low-resolution electromagnetic tomography (sLORETA) study. Soc. Neurosci. 2013, 8, 314–325. [Google Scholar] [CrossRef]
- Simion, F.; Giorgio, D.E. Face perception and processing in early infancy: Inborn predispositions and developmental changes. Front. Psychol. 2015, 6, 969. [Google Scholar] [CrossRef] [PubMed]
- Yovel, G. Neural and cognitive face-selective markers: An integrative review. Neuropsychologia 2016, 83, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bentin, S.; Allison, T.; Puce, A.; Perez, E.; McCarthy, G. Electrophysiological studies of face perception in humans. J. Cogn. Neurosci. 1996, 8, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Itier, R.J.; Taylor, M.J. N170 or N1? Spatiotemporal differences between object and face processing using ERPs. Cereb. Cortex 2004, 14, 132–142. [Google Scholar] [CrossRef]
- Rousselet, G.A.; Macé, M.J.; Fabre-Thorpe, M. Spatiotemporal analyses of the N170 for human faces, animal faces and objects in natural scenes. Neuroreport 2004, 15, 2607–2611. [Google Scholar] [CrossRef]
- Itier, R.J.; Taylor, M.J. Source analysis of the N170 to faces and objects. Neuroreport 2004, 15, 1261–1265. [Google Scholar] [CrossRef]
- Sadeh, B.; Podlipsky, I.; Zhdanov, A.; Yovel, G. Event-related potential and functional MRI measures of face-selectivity are highly correlated: A simultaneous ERP-fMRI investigation. Hum. Brain Mapp. 2010, 31, 1490–1501. [Google Scholar] [CrossRef]
- Kanwisher, N.; McDermott, J.; Chun, M.M. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997, 17, 4302–4311. [Google Scholar] [CrossRef]
- Haxby, J.V.; Hoffman, E.A.; Gobbini, M.I. The distributed human neural system for face perception. Trends Cogn. Sci. 2000, 4, 223–233. [Google Scholar] [CrossRef]
- Pyles, J.A.; Verstynen, T.D.; Schneider, W.; Tarr, M.J. Explicating the face perception network with white matter connectivity. PLoS ONE 2013, 8, e61611. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Fang, H.; Liu, J. The hierarchical brain network for face recognition. PLoS ONE 2013, 8, e59886. [Google Scholar] [CrossRef] [PubMed]
- Craighero, L. An embodied approach to fetal and newborn perceptual and sensorimotor development. Brain Cogn. 2024, 179, 106184. [Google Scholar] [CrossRef] [PubMed]
- Ghio, M.; Cara, C.; Tettamanti, M. The prenatal brain readiness for speech processing: A review on foetal development of auditory and primordial language networks. Neurosci. Biobehav. Rev. 2021, 128, 709–719. [Google Scholar] [CrossRef]
- Reid, V.M.; Dunn, K.; Young, R.J.; Amu, J.; Donovan, T.; Reissland, N. The human fetus preferentially engages with face-like visual stimuli. Curr. Biol. 2017, 27, 1825–1828.e3. [Google Scholar] [CrossRef]
- Bushnell, I.W.R. Mother’s face recognition in newborn infants: Learning and memory. Inf. Child Dev. 2001, 10, 67–74. [Google Scholar] [CrossRef]
- Cecchini, M.; Iannoni, M.E.; Aceto, P.; Baroni, E.; Di Vito, C.; Lai, C. Active sleep is associated with the face preference in newborns who familiarized with a responsive face. Infant Behav. Dev. 2017, 49, 37–45. [Google Scholar] [CrossRef]
- Di Giorgio, E.; Leo, I.; Pascalis, O.; Simion, F. Is the face-perception system human-specific at birth? Dev. Psychol. 2012, 48, 1083. [Google Scholar] [CrossRef]
- Morton, J.; Johnson, M.H. CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychol. Rev. 1991, 98, 164. [Google Scholar] [CrossRef]
- Hoehl, S.; Peykarjou, S. The early development of face processing—What makes faces special? Neurosci. Bull. 2012, 28, 765–788. [Google Scholar] [CrossRef]
- Cecchini, M.; Baroni, E.; Di Vito, C.; Piccolo, F.; Lai, C. Newborn preference for a new face vs. a previously seen communicative or motionless face. Infant Behav. Dev. 2011, 34, 424–433. [Google Scholar] [CrossRef]
- Pascalis, O.; de Schonen, S.; Morton, J.; Deruelle, C.; Fabre-Grenet, M. Mother’s face recognition by neonates: A replication and an extension. Infant Behav. Dev. 1995, 18, 79–85. [Google Scholar] [CrossRef]
- Cecchini, M.; Lai, C.; Langher, V. Communication and crying in newborns. Infant Behav. Dev. 2007, 30, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.; Lai, C.; Langher, V. Dysphonic newborn cries allow prediction of their perceived meaning. Infant Behav. Dev. 2010, 33, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.; Baroni, E.; Di Vito, C.; Lai, C. Smiling in newborns during communicative wake and active sleep. Infant Behav. Dev. 2011, 34, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.; Baroni, E.; Di Vito, C.; Piccolo, F.; Aceto, P.; Lai, C. Effects of different types of contingent tactile stimulation on crying, smiling, and sleep in newborns: An observational study. Dev. Psychobiol. 2013, 55, 508–517. [Google Scholar] [CrossRef]
- Guellai, B.; Streri, A. Cues for early social skills: Direct gaze modulates newborns’ recognition of talking faces. PLoS ONE 2011, 6, e18610. [Google Scholar] [CrossRef]
- Guellai, B.; Mersad, K.; Streri, A. Suprasegmental information affects processing of talking faces at birth. Infant Behav. Dev. 2015, 38, 11–19. [Google Scholar] [CrossRef]
- Pascalis, O.; Scott, L.S.; Kelly, D.J.; Shannon, R.W.; Nicholson, E.; Coleman, M.; Nelson, C.A. Plasticity of face processing in infancy. Proc. Natl Acad. Sci. USA 2005, 102, 5297–5300. [Google Scholar] [CrossRef]
- Pellicano, G.R.; Carola, V.; Bussone, S.; Cecchini, M.; Tambelli, R.; Lai, C. Beyond the dyad: The role of mother and father in newborns’ global DNA methylation during the first month of life—A pilot study. Dev. Psychobiol. 2021, 63, 1345–1357. [Google Scholar] [CrossRef]
- Sai, F.Z. The role of the mother’s voice in developing mother’s face preference: Evidence for intermodal perception at birth. Infant Child Dev. 2005, 14, 29–50. [Google Scholar] [CrossRef]
- Schaal, B.; Saxton, T.K.; Loos, H.; Soussignan, R.; Durand, K. Olfaction scaffolds the developing human from neonate to adolescent and beyond. Philos. T. Roy. Soc. B 2020, 375, 20190261. [Google Scholar] [CrossRef] [PubMed]
- Cassia, V.M.; Kuefner, D.; Westerlund, A.; Nelson, C.A. A behavioural and ERP investigation of 3-month-olds’ face preferences. Neuropsychologia 2006, 44, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Conte, S.; Richards, J.E.; Guy, M.W.; Xie, W.; Roberts, J.E. Face-sensitive brain responses in the first year of life. NeuroImage 2020, 211, 116602. [Google Scholar] [CrossRef] [PubMed]
- Hoehl, S. How do neural responses to eyes contribute to face-sensitive ERP components in young infants? A rapid repetition study. Brain Cogn. 2015, 95, 1–6. [Google Scholar] [CrossRef]
- Halit, H.; Csibra, G.; Volein, A.; Johnson, M.H. Face-sensitive cortical processing in early infancy. J. Child Psychol. Psyc. 2004, 45, 1228–1234. [Google Scholar] [CrossRef]
- Rossion, B.; Jacques, C. Does physical interstimulus variance account for early electrophysiological face-sensitive responses in the human brain? Ten lessons on the N170. Neuroimage 2008, 39, 1959–1979. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; De Schonen, S.; Crivello, F.; Reutter, B.; Aujard, Y.; Mazoyer, B. Neural correlates of woman face processing by 2-month-old infants. Neuroimage 2002, 15, 454–461. [Google Scholar] [CrossRef]
- Farroni, T.; Chiarelli, A.M.; Lloyd-Fox, S.; Massaccesi, S.; Merla, A.; Di Gangi, V.; Mattarello, T.; Faraguna, D.; Johnson, M.H. Infant cortex responds to other humans from shortly after birth. Sci. Rep. 2013, 3, 2851. [Google Scholar] [CrossRef]
- de Haan, M.; Pascalis, O.; Johnson, M.H. Specialization of neural mechanisms underlying face recognition in human infants. J. Cogn. Neurosci. 2002, 14, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.S.; Nelson, C.A. Featural and configural face processing in adults and infants: A behavioral and electrophysiological investigation. Perception 2006, 35, 1107–1128. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.S.; Shannon, R.W.; Nelson, C.A. Neural correlates of human and monkey face processing in 9-month-old infants. Infancy 2006, 10, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Moulson, M.C.; Shannon, R.W.; Nelson, C.A. Neural correlates of visual recognition in 3-month-old infants: The role of experience. Dev. Psychobiol. 2011, 53, 416–424. [Google Scholar] [CrossRef]
- de Haan, M.; Nelson, C.A. Recognition of the mother’s face by six-month-old infants: A neurobehavioral study. Child Dev. 1997, 68, 187–210. [Google Scholar][Green Version]
- Thomaz, C.E.; Giraldi, G.A. A new ranking method for principal components analysis and its application to face image analysis. Image Vis. Comput. 2010, 28, 902–913. [Google Scholar] [CrossRef]
- Picton, T.W.; Bentin, S.; Berg, P.; Donchin, E.; Hillyard, S.A.; Johnson, R.; Miller, G.; Ritter, W.; Ruchkin, D.; Rugg, M.; et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology 2000, 37, 127–152. [Google Scholar] [CrossRef]
- Altavilla, D.; Ciacchella, C.; Pellicano, G.R.; Cecchini, M.; Tambelli, R.; Kalsi, N.; Aceto, P.; Lai, C. Neural correlates of sex-related differences in attachment dimensions. Cogn. Affect. Behav. Neurosci. 2021, 21, 191–211. [Google Scholar] [CrossRef]
- Lai, C.; Pellicano, G.R.; Ciacchella, C.; Guidobaldi, L.; Altavilla, D.; Cecchini, M.; Begotaraj, E.; Aceto, P.; Luciani, M. Neurophysiological correlates of emotional face perception consciousness. Neuropsychologia 2020, 146, 107554. [Google Scholar] [CrossRef]
- Leppänen, J.M.; Moulson, M.C.; Vogel-Farley, V.K.; Nelson, C.A. An ERP study of emotional face processing in the adult and infant brain. Child Dev. 2007, 78, 232–245. [Google Scholar] [CrossRef]
- Jessen, S.; Grossmann, T. Neural signatures of conscious and unconscious emotional face processing in human infants. Cortex 2015, 64, 260–270. [Google Scholar] [CrossRef]
- Key, A.P.; Stone, W.L. Processing of novel and familiar faces in infants at average and high risk for autism. Dev. Cogn. Neurosci. 2012, 2, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Al-Ezzi, A.; Kamel, N.; Faye, I.; Gunaseli, E. Review of EEG, ERP, and brain connectivity estimators as predictive biomarkers of social anxiety disorder. Front. Psychol. 2020, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; McCarthy, G.; Saliba, E.; Degiovanni, E. ERP evidence of developmental changes in processing of faces. Clin. Neurophysiol. 1999, 110, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Edmonds, G.E.; McCarthy, G.; Allison, T. Eyes first! Eye processing develops before face processing in children. Neuroreport 2001, 12, 1671–1676. [Google Scholar] [CrossRef]
- Kosaka, H.; Omori, M.; Iidaka, T.; Murata, T.; Shimoyama, T.; Okada, T.; Wada, Y. Neural substrates participating in acquisition of facial familiarity: An fMRI study. Neuroimage 2003, 20, 1734–1742. [Google Scholar] [CrossRef]
- Leibenluft, E.; Gobbini, M.I.; Harrison, T.; Haxby, J.V. Mothers’ neural activation in response to pictures of their children and other children. Biol. Psychiatry 2004, 56, 225–232. [Google Scholar] [CrossRef]
- Natu, V.; O’Toole, A.J. The neural processing of familiar and unfamiliar faces: A review and synopsis. Br. J. Psychol. 2011, 102, 726–747. [Google Scholar] [CrossRef]
- Pourtois, G.; Schwartz, S.; Seghier, M.L.; Lazeyras, F.; Vuilleumier, P. View-independent coding of face identity in frontal and temporal cortices is modulated by familiarity: An event-related fMRI study. Neuroimage 2005, 24, 1214–1224. [Google Scholar] [CrossRef]
- Sugiura, M.; Kawashima, R.; Nakamura, K.; Sato, N.; Nakamura, A.; Kato, T.; Fukuda, H. Activation reduction in anterior temporal cortices during repeated recognition of faces of personal acquaintances. Neuroimage 2001, 13, 877–890. [Google Scholar] [CrossRef]
- Verosky, S.C.; Turk-Browne, N.B. Representations of facial identity in the left hemisphere require right hemisphere processing. J. Cogn. Neurosci. 2012, 24, 1006–1017. [Google Scholar] [CrossRef]
- Al-Ezzi, A.; Kamel, N.; Faye, I.; Gunaseli, E. Analysis of default mode network in social anxiety disorder: EEG resting-state effective connectivity study. Sensors 2021, 21, 4098. [Google Scholar] [CrossRef] [PubMed]
- Goren, C.C.; Sarty, M.; Wu, P.Y. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics 1975, 56, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, M.J.; Schade, P.F.; Vincent, J.L.; Ponce, C.R.; Livingstone, M.S. Seeing faces is necessary for face-domain formation. Nat. Neurosci. 2017, 20, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Kobylkov, D.; Vallortigara, G. Face detection mechanisms: Nature vs. nurture. Front. Neurosci. 2024, 18, 1404174. [Google Scholar] [CrossRef]
- Ratan Murty, N.A.; Teng, S.; Beeler, D.; Mynick, A.; Oliva, A.; Kanwisher, N. Visual experience is not necessary for the development of face-selectivity in the lateral fusiform gyrus. Proc. Natl. Acad. Sci. USA 2020, 117, 23011–23020. [Google Scholar] [CrossRef]
- Mora, L.; Cowie, D.; Banissy, M.J.; Cocchini, G. My true face: Unmasking one’s own face representation. Acta Psychol. 2018, 191, 63–68. [Google Scholar] [CrossRef]
- Tsakiris, M.; Haggard, P. The rubber hand illusion revisited: Visuotactile integration and self-attribution. J. Exp. Psychol. Human 2005, 31, 80. [Google Scholar] [CrossRef]
- Meltzoff, A.N.; Moore, M.K. Imitation of facial and manual gestures by human neonates. Science 1977, 198, 75–78. [Google Scholar] [CrossRef]
- Dunn, K.; Reissland, N.; Reid, V.M. The functional foetal brain: A systematic preview of methodological factors in reporting foetal visual and auditory capacity. Dev. Cogn. Neurosci. 2015, 13, 43–52. [Google Scholar] [CrossRef]

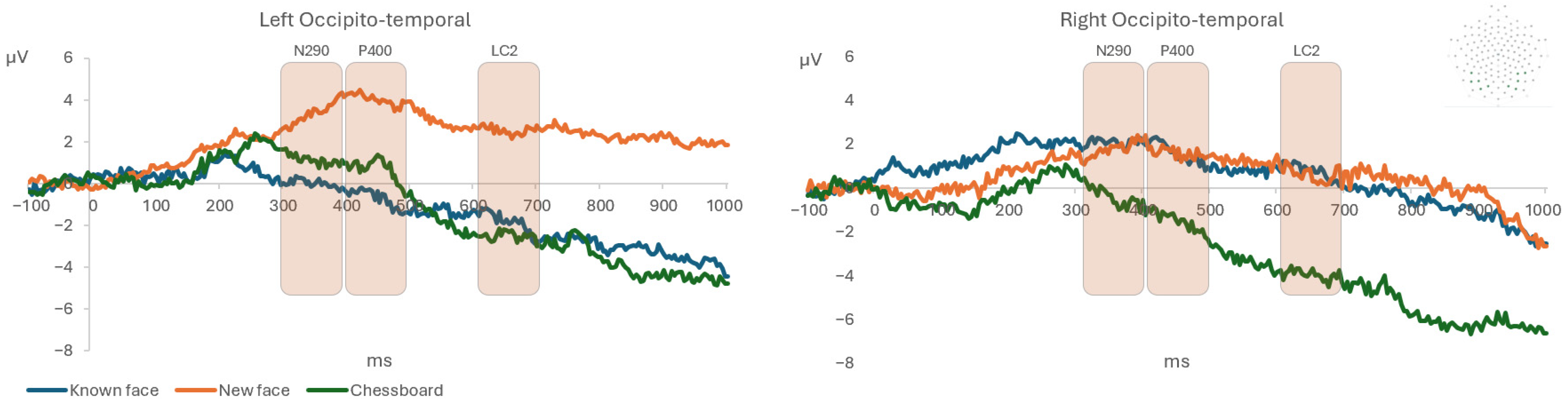

| ERP Component | Effects on Amplitude and Latency | Fisher LSD Post Hoc [M ± SD μV and ms] |
|---|---|---|
| N290 (300–400 ms) | Condition per Hemisphere F(2, 20) = 3.96; p = 0.036, ηp2 = 0.28 (1 − β = 0.99) | Known face (l) [−0.11 ± 8.81] < Novel face (l) [2.67 ± 2.84] * Novel face (l) [2.67 ± 2.84] > Chessboard (r) [0.06 ± 3.34] * |
| Condition F(2, 20) = 5.54; p = 0.012, ηp2 = 0.35 (1 − β = 0.99) | Known face [340.29 ± 32.91] < Novel face [363.85 ± 30.33] ** Chessboard [337.09 ± 31.00] < Novel face [363.85 ± 30.33] ** | |
| P400 (400–500 ms) | Condition per Hemisphere F(2, 20) = 5.24; p = 0.015; ηp2 = 0.34 (1 − β = 0.99) | Known face (l) [−1.18 ± 10.03] < Known face (r) [1.75 ± 7.27] * Known face (l) [−1.18 ± 10.03] < Novel face (l) [3.57 ± 3.22] ** Novel face (l) [3.57 ± 3.22] > Novel face (r) [0.44 ± 4.41] * Novel face (l) [3.57 ± 3.22] > Chessboard (r) [−0.84 ± 3.54] ** |
| LC2 (600–700 ms) | Condition per Hemisphere F(2, 20) = 3.74; p = 0.042; ηp2 = 0.27 (1 − β = 0.99) | Known face (l) [−2.70 ± 10.77] < Novel face (l) [2.07 ± 5.19] ** Known face (r) [0.69 ± 8.31] > Chessboard (r) [−3.61 ± 3.02] * Novel face (l) [2.07 ± 5.19] > Chessboard (l) [−2.07 ± 8.43] * Novel face (l) [2.07 ± 5.19] > Chessboard (r) [−3.61 ± 3.02] ** Novel face (r) [−0.53 ± 5.53] > Chessboard (r) [−3.61 ± 3.02] * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.; Ciacchella, C.; Altavilla, D.; Veneziani, G.; Marano, G.; Pellicano, G.R.; Della Marca, G.; Tonioni, F.; Aceto, P.; Cecchini, M.; et al. Neural Pathways of Visual Face Recognition Immediately After Birth. Life 2025, 15, 1145. https://doi.org/10.3390/life15071145
Lai C, Ciacchella C, Altavilla D, Veneziani G, Marano G, Pellicano GR, Della Marca G, Tonioni F, Aceto P, Cecchini M, et al. Neural Pathways of Visual Face Recognition Immediately After Birth. Life. 2025; 15(7):1145. https://doi.org/10.3390/life15071145
Chicago/Turabian StyleLai, Carlo, Chiara Ciacchella, Daniela Altavilla, Giorgio Veneziani, Giuseppe Marano, Gaia Romana Pellicano, Giacomo Della Marca, Federico Tonioni, Paola Aceto, Marco Cecchini, and et al. 2025. "Neural Pathways of Visual Face Recognition Immediately After Birth" Life 15, no. 7: 1145. https://doi.org/10.3390/life15071145
APA StyleLai, C., Ciacchella, C., Altavilla, D., Veneziani, G., Marano, G., Pellicano, G. R., Della Marca, G., Tonioni, F., Aceto, P., Cecchini, M., Mercuri, E. M., Janiri, L., & Mazza, M. (2025). Neural Pathways of Visual Face Recognition Immediately After Birth. Life, 15(7), 1145. https://doi.org/10.3390/life15071145









