Neural Mechanisms and Alterations of Sweet Sensing: Insights from Functional Magnetic Resonance Imaging Studies
Abstract
1. Introduction
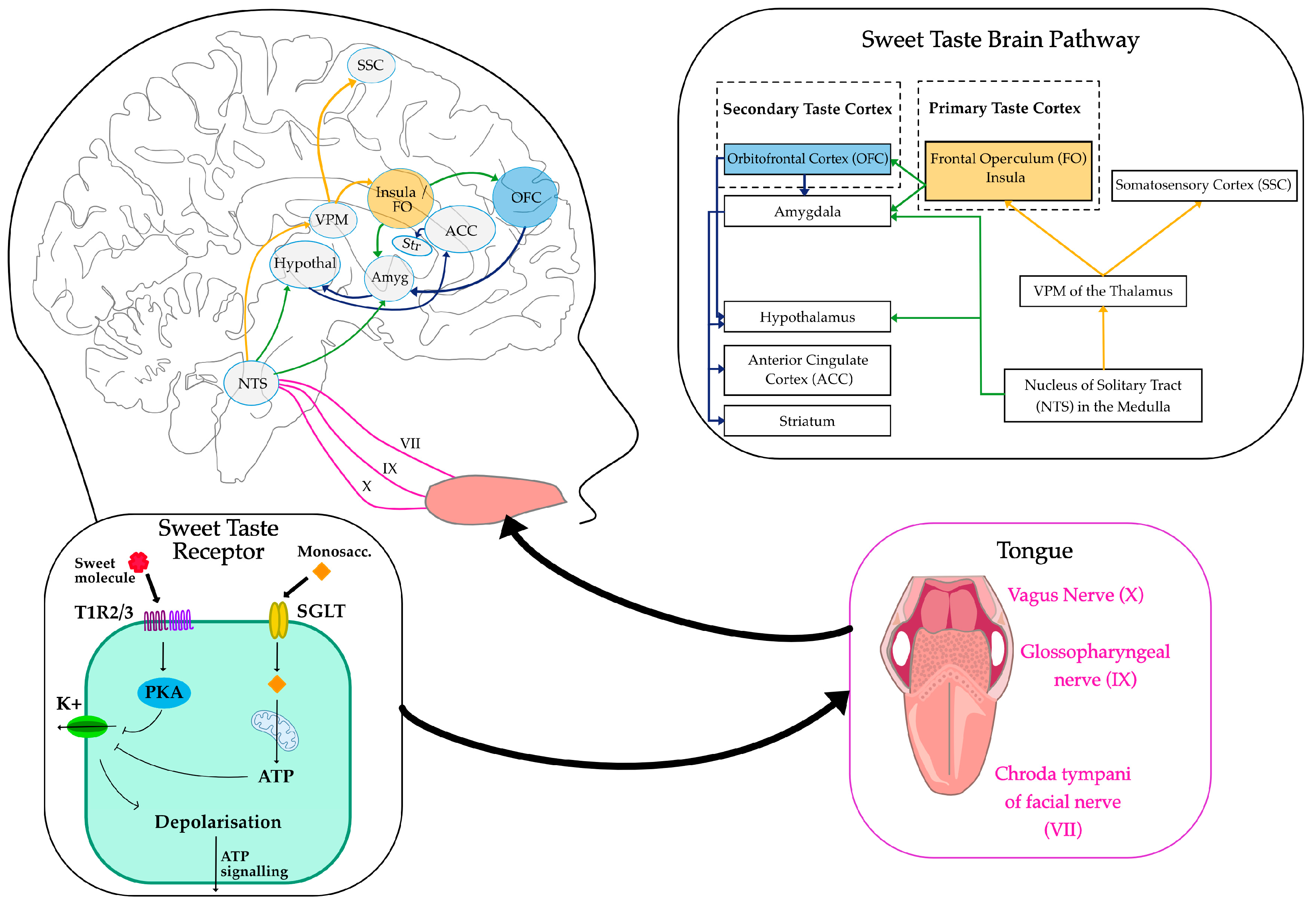
2. Sweet Sensing Mechanisms
2.1. Sweet Taste Receptors
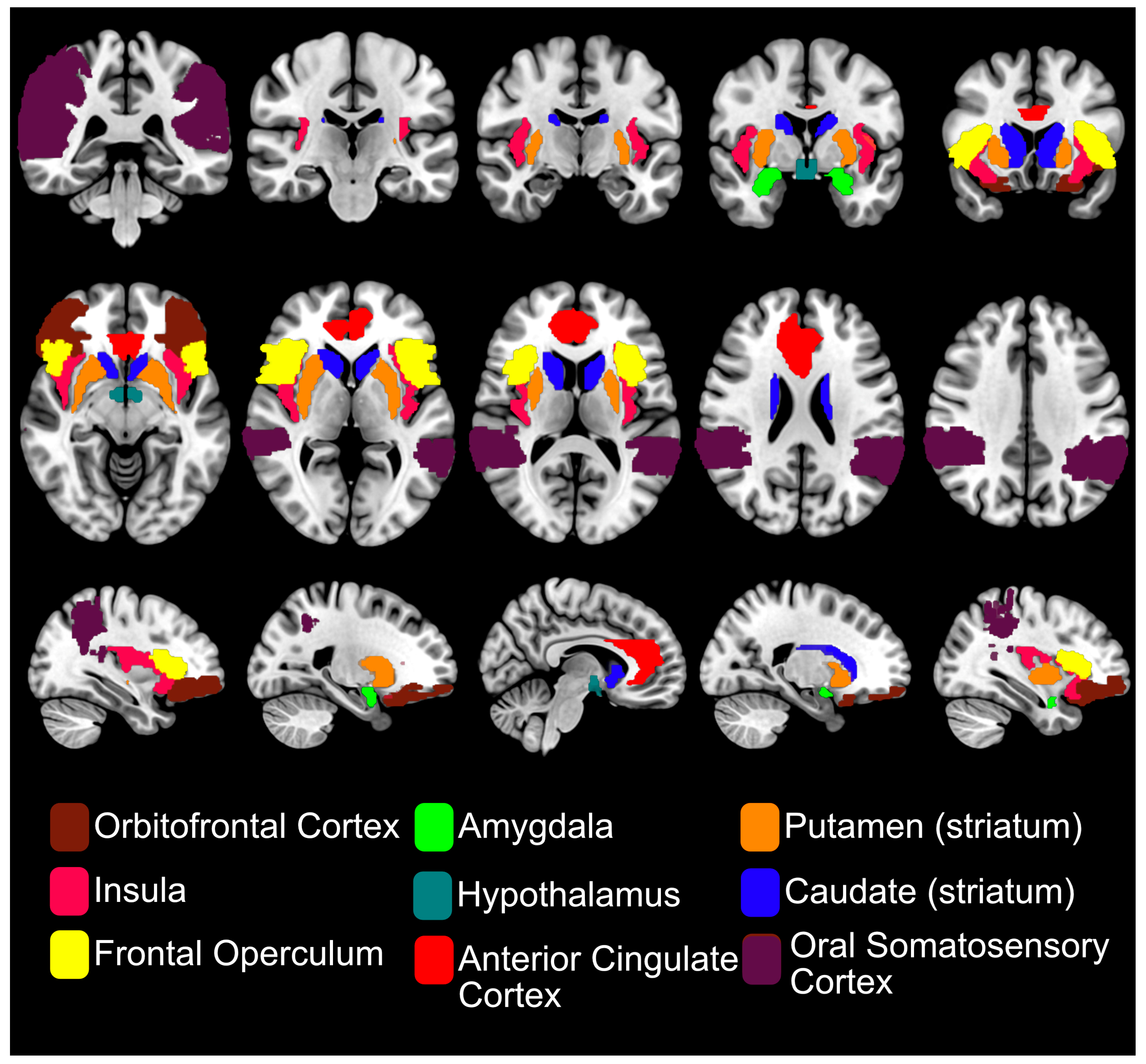
2.2. Sweet Taste Pathways
2.3. Sweet-Tasting Molecules
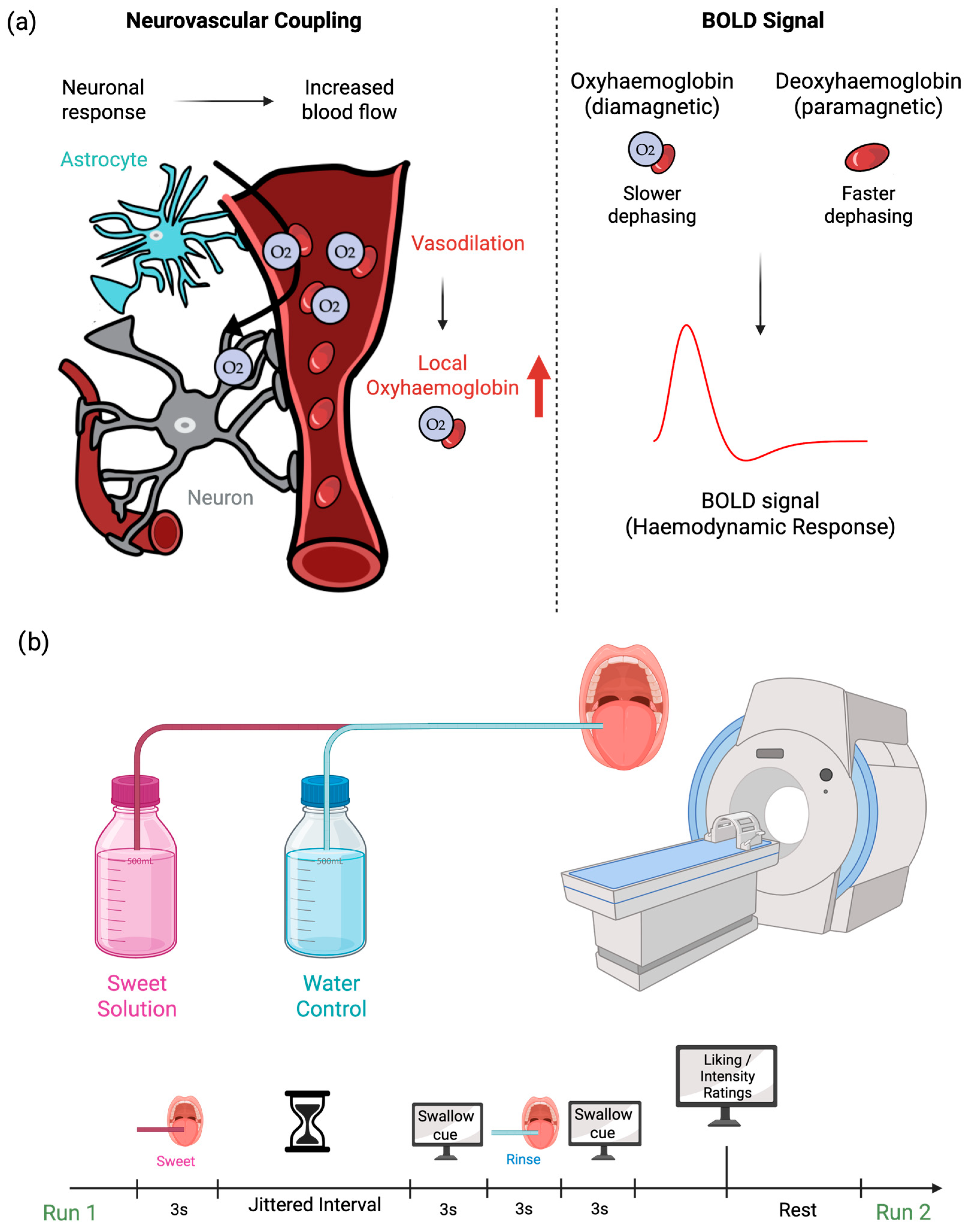
3. fMRI of Sweet Sensing in the Human Brain
Principles of fMRI
4. fMRI of Oral Sweet Sensing
4.1. fMRI of Individual Variations in Sweet Perception
4.2. Brain Activation of Caloric Sweeteners vs. NNS
4.3. Alterations in Brain Responses to Sweet Taste Following Habitual NNS Consumption
5. fMRI of Post-Oral Ingestive Sweet Responses
6. fMRI Studies of Gastrointestinal Sweet Sensing
7. Alterations in Sweet Sensing Responses in Metabolic Disorders
7.1. fMRI of Alteration in Oral Sweet Sensing
7.2. fMRI of Alteration in Post-Oral Ingestive Glucose/Sweet Sensing
7.3. fMRI of Alteration in Intestinal Sweet Sensing
8. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | anterior cingulate cortex |
| ATP | adenosine triphosphate |
| BMI | body mass index |
| BOLD | blood oxygen level-dependent |
| CBF | cerebral blood flow |
| fALFF | fractional amplitude of low-frequency fluctuation |
| fMRI | functional magnetic resonance imaging |
| GLP-1 | glucagon-like peptide-1 |
| GPCR | G-protein-coupled receptor |
| IG | intragastric |
| IV | intravenous |
| LPH | lower posterior hypothalamus |
| MHL | metabolically healthy lean |
| MHO | metabolically healthy obesity |
| MUO | metabolically unhealthy obesity |
| NNS | non-nutritive sweetener |
| NTS | nucleus of the solitary tract |
| OGTT | oral glucose tolerance test |
| OFC | orbitofrontal cortex |
| PKA | protein kinase A |
| PNS | parasympathetic nervous system |
| ROI | region of interest |
| rsFC | resting-state functional connectivity |
| SGLT | sodium-glucose co-transporter |
| SNS | sympathetic nervous system |
| STR | sweet taste receptor |
| T1R2 | taste 1 receptor member 2 |
| T1R3 | taste 1 receptor member 3 |
| T2D | type 2 diabetes |
| TRC | taste receptor cell |
| UAH | upper anterior hypothalamus |
| vmPFC | ventromedial prefrontal cortex |
| VPM | ventroposterior medial nucleus |
References
- Booth, D.A. Learned Ingestive Motivation and the Pleasures of the Palate. In Hedonics of Taste; Psychology Press: Hillsdale, NJ, USA, 1991. [Google Scholar]
- Sclafani, A. The Hedonics of Sugar and Starch. In Hedonics of Taste; Psychology Press: Hillsdale, NJ, USA, 1991. [Google Scholar]
- Lutter, M.; Nestler, E.J. Homeostatic and Hedonic Signals Interact in the Regulation of Food Intake. J. Nutr. 2009, 139, 629–632. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Roger, C.; Lasbleiz, A.; Guye, M.; Dutour, A.; Gaborit, B.; Ranjeva, J.-P. The Role of the Human Hypothalamus in Food Intake Networks: An MRI Perspective. Front. Nutr. 2022, 8, 760914. [Google Scholar] [CrossRef] [PubMed]
- Osada, T.; Suzuki, R.; Ogawa, A.; Tanaka, M.; Hori, M.; Aoki, S.; Tamura, Y.; Watada, H.; Kawamori, R.; Konishi, S. Functional subdivisions of the hypothalamus using areal parcellation and their signal changes related to glucose metabolism. NeuroImage 2017, 162, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.P. Role of the hypothalamus in the regulation of food and water intake. Psychol. Rev. 1975, 82, 200–224. [Google Scholar] [CrossRef]
- Rolls, E.T. Functions of the anterior insula in taste, autonomic, and related functions. Brain Cogn. 2016, 110, 4–19. [Google Scholar] [CrossRef]
- Tulloch, A.J.; Murray, S.; Vaicekonyte, R.; Avena, N.M. Neural Responses to Macronutrients: Hedonic and Homeostatic Mechanisms. Gastroenterology 2015, 148, 6. [Google Scholar] [CrossRef]
- Nieh, E.H.; Matthews, G.A.; Allsop, S.A.; Presbrey, K.N.; Leppla, C.A.; Wichmann, R.; Neve, R.; Wildes, C.P.; Tye, K.M. Decoding Neural Circuits that Control Compulsive Sucrose Seeking. Cell 2015, 160, 528–541. [Google Scholar] [CrossRef]
- Morenga, L.T.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2013, 346, e7492. [Google Scholar] [CrossRef]
- Ward, Z.J.; Bleich, S.N.; Long, M.W.; Gortmaker, S.L. Association of body mass index with health care expenditures in the United States by age and sex. PLoS ONE 2021, 16, e0247307. [Google Scholar] [CrossRef]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for eight countries. BMJ Glob. Health 2021, 6, e006351. [Google Scholar] [CrossRef] [PubMed]
- Running, C.A.; Craig, B.A.; Mattes, R.D. Oleogustus: The Unique Taste of Fat. Chem. Senses 2015, 40, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Pirkwieser, P.; Behrens, M.; Somoza, V. Metallic Sensation—Just an Off-Flavor or a Biologically Relevant Sensing Pathway? J. Agric. Food Chem. 2021, 69, 1775–1780. [Google Scholar] [CrossRef]
- Iwata, S.; Yoshida, R.; Ninomiya, Y. Taste Transductions in Taste Receptor Cells: Basic Tastes and Moreover. Curr. Pharm. Des. 2014, 20, 2684–2692. [Google Scholar] [CrossRef]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.P.; Zuker, C.S. Mammalian Sweet Taste Receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Treesukosol, Y.; Smith, K.R.; Spector, A.C. The Functional Role of the T1R Family of Receptors in Sweet Taste and Feeding. Physiol. Behav. 2011, 105, 14–26. [Google Scholar] [CrossRef]
- Sukumaran, S.K.; Palayyan, S.R. Sweet Taste Signaling: The Core Pathways and Regulatory Mechanisms. Int. J. Mol. Sci. 2022, 23, 15. [Google Scholar] [CrossRef]
- Depoortere, I. Taste receptors of the gut: Emerging roles in health and disease. Gut 2014, 63, 179–190. [Google Scholar] [CrossRef]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497. [Google Scholar] [CrossRef]
- von Molitor, E.; Riedel, K.; Krohn, M.; Hafner, M.; Rudolf, R.; Cesetti, T. Sweet Taste Is Complex: Signaling Cascades and Circuits Involved in Sweet Sensation. Front. Hum. Neurosci. 2021, 15, 667709. [Google Scholar] [CrossRef]
- Raka, F.; Farr, S.; Kelly, J.; Stoianov, A.; Adeli, K. Metabolic control via nutrient-sensing mechanisms: Role of taste receptors and the gut-brain neuroendocrine axis. Am. J. Physiol.-Endocrinol. Metab. 2019, 317, E559–E572. [Google Scholar] [CrossRef] [PubMed]
- Sternini, C.; Rozengurt, E. Bitter taste receptors as sensors of gut luminal contents. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Norgren, R.; Leonard, C.M. Ascending central gustatory pathways. J. Comp. Neurol. 1973, 150, 217–237. [Google Scholar] [CrossRef]
- Small, D.M. Taste representation in the human insula. Brain Struct. Funct. 2010, 214, 551–561. [Google Scholar] [CrossRef]
- Ohla, K.; Yoshida, R.; Roper, S.D.; Di Lorenzo, P.M.; Victor, J.D.; Boughter, J.D.; Fletcher, M.; Katz, D.B.; Chaudhari, N. Recognizing Taste: Coding Patterns Along the Neural Axis in Mammals. Chem. Senses 2019, 44, 237–247. [Google Scholar] [CrossRef]
- Chikazoe, J.; Lee, D.H.; Kriegeskorte, N.; Anderson, A.K. Distinct representations of basic taste qualities in human gustatory cortex. Nat. Commun. 2019, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Carleton, A.; Accolla, R.; Simon, S.A. Coding in the mammalian gustatory system. Trends Neurosci. 2010, 33, 326–334. [Google Scholar] [CrossRef]
- Rolls, E.T. The functions of the orbitofrontal cortex. Brain Cogn. 2004, 55, 11–29. [Google Scholar] [CrossRef]
- Del Parigi, A.; Gautier, J.-F.; Chen, K.; Salbe, A.D.; Ravussin, E.; Reiman, E.; Tataranni, P.A. Neuroimaging and Obesity. Ann. N. Y. Acad. Sci. 2002, 967, 389–397. [Google Scholar] [CrossRef]
- Luo, L.; Han, P. Assessing food-evoked emotions using functional magnetic resonance imaging: A systematic review. Food Qual. Prefer. 2023, 108, 104877. [Google Scholar] [CrossRef]
- Zocchi, D.; Wennemuth, G.; Oka, Y. The cellular mechanism for water detection in the mammalian taste system. Nat. Neurosci. 2017, 20, 927–933. [Google Scholar] [CrossRef]
- Fu, O.; Minokoshi, Y.; Nakajima, K. Recent Advances in Neural Circuits for Taste Perception in Hunger. Front. Neural Circuits 2021, 15, 609824. [Google Scholar] [CrossRef] [PubMed]
- Hartig, R.; Karimi, A.; Evrard, H.C. Interconnected sub-networks of the macaque monkey gustatory connectome. Front. Neurosci. 2023, 16, 818800. [Google Scholar] [CrossRef]
- Fitch, C.; Keim, K.S. Position of the Academy of Nutrition and Dietetics: Use of Nutritive and Nonnutritive Sweeteners. J. Acad. Nutr. Diet. 2012, 112, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Picone, D.; Temussi, P.A. Dissimilar sweet proteins from plants: Oddities or normal components? Plant Sci. 2012, 195, 135–142. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners—A review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. ESPEN 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Schiano, C.; Grimaldi, V.; Scognamiglio, M.; Costa, D.; Soricelli, A.; Nicoletti, G.F.; Napoli, C. Soft drinks and sweeteners intake: Possible contribution to the development of metabolic syndrome and cardiovascular diseases. Beneficial or detrimental action of alternative sweeteners? Food Res. Int. 2021, 142, 110220. [Google Scholar] [CrossRef]
- Gauthier, E.; Milagro, F.I.; Navas-Carretero, S. Effect of low-and non-calorie sweeteners on the gut microbiota: A review of clinical trials and cross-sectional studies. Nutrition 2024, 117, 112237. [Google Scholar] [CrossRef]
- World Health Organization. Use of Non-Sugar Sweeteners: WHO Guideline Summary; World Health Organization: Geneva, Switzerland, 2023; Available online: https://iris.who.int/handle/10665/375565 (accessed on 1 July 2025).
- Ahmed, A.; Khan, T.A.; Ramdath, D.D.; Kendall, C.W.C.; Sievenpiper, J.L. Rare sugars and their health effects in humans: A systematic review and narrative synthesis of the evidence from human trials. Nutr. Rev. 2022, 80, 255–270. [Google Scholar] [CrossRef]
- Kim, S.-G.; Ogawa, S. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J. Cereb. Blood Flow Metab. 2012, 32, 1188–1206. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef]
- Vinod, M. 20 years of the default mode network: A review and synthesis. Neuron 2023, 111, 2469–2487. [Google Scholar] [CrossRef]
- Lindner, T.; Bolar, D.S.; Achten, E.; Barkhof, F.; Bastos-Leite, A.J.; Detre, J.A.; Golay, X.; Günther, M.; Wang, D.J.J.; Haller, S.; et al. Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging. Magn. Reson. Med. 2023, 89, 2024–2047. [Google Scholar] [CrossRef]
- Avery, J.A.; Liu, A.G.; Ingeholm, J.E.; Riddell, C.D.; Gotts, S.J.; Martin, A. Taste Quality Representation in the Human Brain. J. Neurosci. 2020, 40, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.A.; Giesbrecht, T.; Fallon, N.; Thomas, A.; Mela, D.J.; Kirkham, T.C. A Systematic Review and Activation Likelihood Estimation Meta-Analysis of fMRI Studies on Sweet Taste in Humans. J. Nutr. 2020, 150, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Zeffiro, T.A. Hunger and BMI modulate neural responses to sweet stimuli: fMRI meta-analysis. Int. J. Obes. 2020, 44, 8. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Wong, N.S.M. How Does Our Brain Process Sugars and Non-Nutritive Sweeteners Differently: A Systematic Review on Functional Magnetic Resonance Imaging Studies. Nutrients 2020, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.; Rolls, E.T.; Francis, S.; Bowtell, R.; McGlone, F. Representation of Pleasant and Aversive Taste in the Human Brain. J. Neurophysiol. 2001, 85, 1315–1321. [Google Scholar] [CrossRef]
- Veldhuizen, M.G.; Albrecht, J.; Zelano, C.; Boesveldt, S.; Breslin, P.; Lundström, J.N. Identification of human gustatory cortex by activation likelihood estimation. Hum. Brain Mapp. 2011, 32, 2256–2266. [Google Scholar] [CrossRef]
- Rudenga, K.J.; Small, D.M. Ventromedial Prefrontal Cortex Response to Concentrated Sucrose Reflects Liking Rather Than Sweet Quality Coding. Chem. Senses 2013, 38, 7. [Google Scholar] [CrossRef]
- Kavaliauskaite, G.; Thibodeau, M.; Ford, R.; Yang, Q. Using correlation matrices to standardise sweet liking status classification. Food Qual. Prefer. 2023, 104, 104759. [Google Scholar] [CrossRef]
- Spetter, M.S.; Smeets, P.A.M.; de Graaf, C.; Viergever, M.A. Representation of Sweet and Salty Taste Intensity in the Brain. Chem. Senses 2010, 35, 9. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Gregory, M.D.; Mak, Y.E.; Gitelman, D.; Mesulam, M.M.; Parrish, T. Dissociation of Neural Representation of Intensity and Affective Valuation in Human Gustation. Neuron 2003, 39, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Eiler, W.J.A.; Dzemidzic, M.; Soeurt, C.M.; Carron, C.R.; Oberlin, B.G.; Considine, R.V.; Harezlak, J.; Kareken, D.A. Family history of alcoholism and the human brain response to oral sucrose. NeuroImage Clin. 2018, 17, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Hort, J.; Ford, R.A.; Eldeghaidy, S.; Francis, S.T. Thermal taster status: Evidence of cross-modal integration. Hum. Brain Mapp. 2016, 37, 2263–2275. [Google Scholar] [CrossRef]
- Yang, Q.; Kraft, M.; Shen, Y.; MacFie, H.; Ford, R. Sweet Liking Status and PROP Taster Status impact emotional response to sweetened beverage. Food Qual. Prefer. 2019, 75, 133–144. [Google Scholar] [CrossRef]
- Yeomans, M.R.; Vi, C.; Mohammed, N.; Armitage, R.M. Re-evaluating how sweet-liking and PROP-tasting are related. Physiol. Behav. 2022, 246, 113702. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Oberndorfer, T.A.; Simmons, A.N.; Paulus, M.P.; Fudge, J.L.; Yang, T.T.; Kaye, W.H. Sucrose activates human taste pathways differently from artificial sweetener. NeuroImage 2008, 39, 4. [Google Scholar] [CrossRef]
- Smeets, P.A.M.; Weijzen, P.; De Graaf, C.; Viergever, M.A. Consumption of caloric and non-caloric versions of a soft drink differentially affects brain activation during tasting. NeuroImage 2011, 54, 2. [Google Scholar] [CrossRef]
- Chambers, E.S.; Bridge, M.W.; Jones, D.A. Carbohydrate sensing in the human mouth: Effects on exercise performance and brain activity. J. Physiol. 2009, 587, 8. [Google Scholar] [CrossRef] [PubMed]
- Van Opstal, A.M.; Hafkemeijer, A.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Mulder, T.P.J.; Pijl, H.; Rombouts, S.A.R.B.; van der Grond, J. Brain activity and connectivity changes in response to nutritive natural sugars, non-nutritive natural sugar replacements and artificial sweeteners. Nutr. Neurosci. 2021, 24, 5. [Google Scholar] [CrossRef]
- Budzinska, A.; Teysseire, F.; Flad, E.; Dupont, P.; Wölnerhanssen, B.; Meyer-Gerspach, A.C.; Van Oudenhove, L.; Weltens, N. Neural responses to oral administration of erythritol vs sucrose and sucralose explain differences in subjective liking ratings. Appetite 2024, 200, 107422. [Google Scholar] [CrossRef]
- Overduin, J.; Collet, T.-H.; Medic, N.; Henning, E.; Keogh, J.M.; Forsyth, F.; Stephenson, C.; Kanning, M.W.; Ruijschop, R.M.A.J.; Farooqi, I.S.; et al. Failure of sucrose replacement with the non-nutritive sweetener erythritol to alter GLP-1 or PYY release or test meal size in lean or obese people. Appetite 2016, 107, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, Z.A.; Smith, G.; Palm, L.; Motwani, K.; Butterfield, J.; Archer, C.; Henderson, R.; Heldermon, C.; Gautam, S.; Brantly, M.L. An Erythritol-Sweetened Beverage Induces Satiety and Suppresses Ghrelin Compared to Aspartame in Healthy Non-Obese Subjects: A Pilot Study. Cureus 2020, 12, e11409. [Google Scholar] [CrossRef]
- Green, E.; Murphy, C. Altered processing of sweet taste in the brain of diet soda drinkers. Physiol. Behav. 2012, 107, 4. [Google Scholar] [CrossRef]
- Griffioen-Roose, S.; Smeets, P.A.M.; Weijzen, P.L.G.; van Rijn, I.; van den Bosch, I.; de Graaf, C. Effect of Replacing Sugar with Non-Caloric Sweeteners in Beverages on the Reward Value after Repeated Exposure. PLoS ONE 2013, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Dalenberg, J.R.; Patel, B.P.; Denis, R.; Veldhuizen, M.G.; Nakamura, Y.; Vinke, P.C.; Luquet, S.; Small, D.M. Short-Term Consumption of Sucralose with, but Not without, Carbohydrate Impairs Neural and Metabolic Sensitivity to Sugar in Humans. Cell Metab. 2020, 31, 3. [Google Scholar] [CrossRef]
- Foletto, K.C.; Melo Batista, B.A.; Neves, A.M.; de Matos Feijó, F.; Ballard, C.R.; Marques Ribeiro, M.F.; Bertoluci, M.C. Sweet taste of saccharin induces weight gain without increasing caloric intake, not related to insulin-resistance in Wistar rats. Appetite 2016, 96, 604–610. [Google Scholar] [CrossRef]
- Davidson, T.L.; Swithers, S.E. A Pavlovian approach to the problem of obesity. Int. J. Obes. 2004, 28, 933–935. [Google Scholar] [CrossRef]
- Wright, H.; Li, X.; Fallon, N.B.; Crookall, R.; Giesbrecht, T.; Thomas, A.; Halford, J.C.G.; Harrold, J.; Stancak, A. Differential effects of hunger and satiety on insular cortex and hypothalamic functional connectivity. Eur. J. Neurosci. 2016, 43, 1181–1189. [Google Scholar] [CrossRef]
- Al-Zubaidi, A.; Heldmann, M.; Mertins, A.; Jauch-Chara, K.; Münte, T.F. Influences of Hunger, Satiety and Oral Glucose on Functional Brain Connectivity: A Multimethod Resting-State fMRI Study. Neuroscience 2018, 382, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Al-Zubaidi, A.; Heldmann, M.; Mertins, A.; Brabant, G.; Nolde, J.M.; Jauch-Chara, K.; Münte, T.F. Impact of Hunger, Satiety, and Oral Glucose on the Association Between Insulin and Resting-State Human Brain Activity. Front. Hum. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef]
- Wölnerhanssen, B.K.; Meyer-Gerspach, A.C.; Schmidt, A.; Zimak, N.; Peterli, R.; Beglinger, C.; Borgwardt, S. Dissociable Behavioral, Physiological and Neural Effects of Acute Glucose and Fructose Ingestion: A Pilot Study. PLoS ONE 2015, 10, e0130280. [Google Scholar] [CrossRef]
- Luo, S.; Monterosso, J.R.; Sarpelleh, K.; Page, K.A. Differential effects of fructose versus glucose on brain and appetitive responses to food cues and decisions for food rewards. Proc. Natl. Acad. Sci. USA 2015, 112, 20. [Google Scholar] [CrossRef] [PubMed]
- Smeets, P.A.M.; De Graaf, C.; Stafleu, A.; Van Osch, M.J.P.; Van Der Grond, J. Functional MRI of human hypothalamic responses following glucose ingestion. NeuroImage 2005, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, J.-H.; Liu, H.-L.; Fox, P.T. The temporal response of the brain after eating revealed by functional MRI. Nature 2000, 405, 6790. [Google Scholar] [CrossRef]
- van Opstal, A.M.; Hafkemeijer, A.; van den Berg-Huysmans, A.A.; Hoeksma, M.; Blonk, C.; Pijl, H.; Rombouts, S.A.R.B.; van der Grond, J. Brain activity and connectivity changes in response to glucose ingestion. Nutr. Neurosci. 2020, 23, 2. [Google Scholar] [CrossRef]
- Matsuda, M.; Liu, Y.; Mahankali, S.; Pu, Y.; Mahankali, A.; Wang, J.; DeFronzo, R.A.; Fox, P.T.; Gao, J.H. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999, 48, 9. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Carakostas, M.C.; Moore, N.H.; Poulos, S.P.; Renwick, A.G. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016, 74, 670–689. [Google Scholar] [CrossRef]
- Tack, J.; Verbeure, W.; Mori, H.; Schol, J.; Van den Houte, K.; Huang, I.-H.; Balsiger, L.; Broeders, B.; Colomier, E.; Scarpellini, E.; et al. The gastrointestinal tract in hunger and satiety signalling. United Eur. Gastroenterol. J. 2021, 9, 727–734. [Google Scholar] [CrossRef]
- Smeets, P.A.M.; Vidarsdottir, S.; de Graaf, C.; Stafleu, A.; van Osch, M.J.P.; Viergever, M.A.; Pijl, H.; van der Grond, J. Oral glucose intake inhibits hypothalamic neuronal activity more effectively than glucose infusion. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, 3. [Google Scholar] [CrossRef]
- Little, T.J.; McKie, S.; Jones, R.B.; D’Amato, M.; Smith, C.; Kiss, O.; Thompson, D.G.; McLaughlin, J.T. Mapping glucose-mediated gut-to-brain signalling pathways in humans. NeuroImage 2014, 96, 1–11. [Google Scholar] [CrossRef]
- Iven, J.; Biesiekierski, J.R.; Zhao, D.; Tack, J.; Van Oudenhove, L. Intragastric fructose administration interacts with emotional state in homeostatic and hedonic brain regions. Nutr. Neurosci. 2022, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Stopyra, M.A.; Friederich, H.-C.; Sailer, S.; Pauen, S.; Bendszus, M.; Herzog, W.; Simon, J.J. The effect of intestinal glucose load on neural regulation of food craving. Nutr. Neurosci. 2021, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Spetter, M.S.; de Graaf, C.; Mars, M.; Viergever, M.A.; Smeets, P.A.M. The Sum of Its Parts—Effects of Gastric Distention, Nutrient Content and Sensory Stimulation on Brain Activation. PLoS ONE 2014, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Peinado, B.R.R.; Frazão, D.R.; Bittencourt, L.O.; Souza-Rodrigues, R.D.d.; Vidigal, M.T.C.; da Silva, D.T.; Paranhos, L.R.; Magno, M.B.; Fagundes, N.C.F.; Maia, L.C.; et al. Is obesity associated with taste alterations? a systematic review. Front. Endocrinol. 2023, 14, 1167119. [Google Scholar] [CrossRef]
- Tan, S.-Y.; Hack, C.; Yu, C.; Rennick, I.; Ohanian, J.; Dezan, M.; Mott, N.; Manibo, R.; Tucker, R.M. Alterations in sweet taste function in adults with diabetes mellitus: A systematic review and potential implications. Crit. Rev. Food Sci. Nutr. 2023, 63, 2613–2625. [Google Scholar] [CrossRef]
- Dunn, J.P.; Lamichhane, B.; Smith, G.I.; Garner, A.; Wallendorf, M.; Hershey, T.; Klein, S. Dorsal striatal response to taste is modified by obesity and insulin resistance. Obesity 2023, 31, 2065–2075. [Google Scholar] [CrossRef]
- Vidarsdottir, S.; Smeets, P.A.M.; Eichelsheim, D.L.; van Osch, M.J.P.; Viergever, M.A.; Romijn, J.A.; van der Grond, J.; Pijl, H. Glucose Ingestion Fails to Inhibit Hypothalamic Neuronal Activity in Patients with Type 2 Diabetes. Diabetes 2007, 56, 10. [Google Scholar] [CrossRef]
- Yousefvand, S.; Hamidi, F. The Role of Ventromedial Hypothalamus Receptors in the Central Regulation of Food Intake. Int. J. Pept. Res. Ther. 2021, 27, 689–702. [Google Scholar] [CrossRef]
- Chakravartti, S.P.; Jann, K.; Veit, R.; Liu, H.; Yunker, A.G.; Angelo, B.; Monterosso, J.R.; Xiang, A.H.; Kullmann, S.; Page, K.A. Non-caloric sweetener effects on brain appetite regulation in individuals across varying body weights. Nat. Metab. 2025, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.J.; Stopyra, M.A.; Mönning, E.; Sailer, S.; Lavandier, N.; Kihm, L.P.; Bendszus, M.; Preissl, H.; Herzog, W.; Friederich, H.-C. Neuroimaging of hypothalamic mechanisms related to glucose metabolism in anorexia nervosa and obesity. J. Clin. Investig. 2020, 130, 8. [Google Scholar] [CrossRef]
- Fazal, Z.; Gomez, D.E.P.; Llera, A.; Marques, J.P.R.F.; Beck, T.; Poser, B.A.; Norris, D.G. A comparison of multiband and multiband multiecho gradient-echo EPI for task fMRI at 3 T. Hum. Brain Mapp. 2023, 44, 82–93. [Google Scholar] [CrossRef]
- Okada, T.; Fujimoto, K.; Fushimi, Y.; Akasaka, T.; Thuy, D.H.D.; Shima, A.; Sawamoto, N.; Oishi, N.; Zhang, Z.; Funaki, T.; et al. Neuroimaging at 7 Tesla: A pictorial narrative review. Quant. Imaging Med. Surg. 2022, 12, 3406–3435. [Google Scholar] [CrossRef] [PubMed]
- Barisano, G.; Sepehrband, F.; Ma, S.; Jann, K.; Cabeen, R.; Wang, D.J.; Toga, A.W.; Law, M. Clinical 7 T MRI: Are we there yet? A review about magnetic resonance imaging at ultra-high field. Br. J. Radiol. 2019, 92, 20180492. [Google Scholar] [CrossRef]
- Parsons, N.; Steward, T.; Clohesy, R.; Almgren, H.; Duehlmeyer, L. A systematic review of resting-state functional connectivity in obesity: Refining current neurobiological frameworks and methodological considerations moving forward. Rev. Endocr. Metab. Disord. 2022, 23, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Rashid, B.; Poole, V.N.; Fortenbaugh, F.C.; Esterman, M.; Milberg, W.P.; McGlinchey, R.E.; Salat, D.H.; Leritz, E.C. Association between metabolic syndrome and resting-state functional brain connectivity. Neurobiol. Aging 2021, 104, 1–9. [Google Scholar] [CrossRef]
- Wachsmuth, H.R.; Weninger, S.N.; Duca, F.A. Role of the gut–brain axis in energy and glucose metabolism. Exp. Mol. Med. 2022, 54, 377–392. [Google Scholar] [CrossRef]
- Belelli, D.; Lambert, J.J.; Wan, M.L.Y.; Monteiro, A.R.; Nutt, D.J.; Swinny, J.D. From bugs to brain: Unravelling the GABA signalling networks in the brain–gut–microbiome axis. Brain 2025, 148, 1479–1506. [Google Scholar] [CrossRef]
- Smith, A.; Avery, A.; Ford, R.; Yang, Q.; Goux, A.; Mukherjee, I.; Neville, D.C.A.; Jethwa, P. Rare sugars: Metabolic impacts and mechanisms of action: A scoping review. Br. J. Nutr. 2022, 128, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, E.; Sadowska, A.; Sawicka, D.; Kotulska-Bąblińska, I.; Car, H. A head-to-head comparison review of biological and toxicological studies of isomaltulose, d-tagatose, and trehalose on glycemic control. Crit. Rev. Food Sci. Nutr. 2022, 62, 5679–5704. [Google Scholar] [CrossRef] [PubMed]
- Donner, T.W.; Wilber, J.F.; Ostrowski, D. D-tagatose, a novel hexose: Acute effects on carbohydrate tolerance in subjects with and without type 2 diabetes. Diabetes Obes. Metab. 1999, 1, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Hira, T.; Nakamura, M.; Iida, T.; Kishimoto, Y.; Hara, H. Secretion of GLP-1 but not GIP is potently stimulated by luminal d-Allulose (d-Psicose) in rats. Biochem. Biophys. Res. Commun. 2018, 496, 898–903. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Sendo, M.; Dezaki, K.; Hira, T.; Sato, T.; Nakata, M.; Goswami, C.; Aoki, R.; Arai, T.; Kumari, P.; et al. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat. Commun. 2018, 9, 113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, T.; Milbourn, C.C.; Smith, A.; Khin, K.L.S.; Page, A.J.; Idris, I.; Yang, Q.; Young, R.L.; Eldeghaidy, S. Neural Mechanisms and Alterations of Sweet Sensing: Insights from Functional Magnetic Resonance Imaging Studies. Life 2025, 15, 1075. https://doi.org/10.3390/life15071075
Long T, Milbourn CC, Smith A, Khin KLS, Page AJ, Idris I, Yang Q, Young RL, Eldeghaidy S. Neural Mechanisms and Alterations of Sweet Sensing: Insights from Functional Magnetic Resonance Imaging Studies. Life. 2025; 15(7):1075. https://doi.org/10.3390/life15071075
Chicago/Turabian StyleLong, Tobias, Colette C. Milbourn, Alison Smith, Kyaw Linn Su Khin, Amanda J. Page, Iskandar Idris, Qian Yang, Richard L. Young, and Sally Eldeghaidy. 2025. "Neural Mechanisms and Alterations of Sweet Sensing: Insights from Functional Magnetic Resonance Imaging Studies" Life 15, no. 7: 1075. https://doi.org/10.3390/life15071075
APA StyleLong, T., Milbourn, C. C., Smith, A., Khin, K. L. S., Page, A. J., Idris, I., Yang, Q., Young, R. L., & Eldeghaidy, S. (2025). Neural Mechanisms and Alterations of Sweet Sensing: Insights from Functional Magnetic Resonance Imaging Studies. Life, 15(7), 1075. https://doi.org/10.3390/life15071075









