Gastrointestinal Cancers with Consideration of DPD and UGT1A1 Plasma Levels: Chemotherapy-Related Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Information
2.1.1. Study Design
2.1.2. Patient Recruiting Procedure
2.1.3. Key Parameters of the Studied Groups of Patients
2.2. Dihydropyrimidine Dehydrogenase (DPD) and UDP-Glucuronosyltransferase (UGT1A1) Enzyme Level Determination
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALAT | Alanine aminotransferase |
| ASAT | Aspartate aminotransferase |
| DPD | Dihydropyrimidine dehydrogenase |
| FOLFOX | Folinic acid, fluorouracil and oxaliplatin |
| FOLFIRI | Folinic acid, fluorouracil and irinotecan |
| GR | Granulocytes, absolute |
| ChT | Chemotherapy |
| Cr | Creatinine |
| HCT | Hematocrit |
| HGB | Hemoglobin |
| LY | Lymphocytes, absolute |
| MO | Monocytes, absolute |
| PLT | Platelets |
| RBC | Red blood cells |
| TBIL | Total bilirubin |
| UGT1A1 | UDP-glucuronosyltransferase |
| WBC | White blood cells |
| 5-FU | Fluorouracil |
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349. [Google Scholar] [CrossRef]
- Jardim, S.R.; de Souza, L.M.P.; de Souza, H.S.P. The Rise of Gastrointestinal Cancers as a Global Phenomenon: Unhealthy Behavior or Progress? Int. J. Environ. Res. Public Health 2023, 20, 3640. [Google Scholar] [CrossRef]
- Vishwanath, A.; Krishna, S.; Manudhane, A.P.; Hart, P.A.; Krishna, S.G. Early-Onset Gastrointestinal Malignancies: An Investigation into a Rising Concern. Cancers 2024, 16, 1553. [Google Scholar] [CrossRef]
- Kayali, S.; Marabotto, E.; Giannini, E. Gastrointestinal Tract Cancers, an Increasing Burden of the Modern Era: Epidemiology and Prevention. Cancers 2023, 15, 4634. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2022, 10, 1367–1401. [Google Scholar] [CrossRef]
- Parker, W.B.; Cheng, Y.C. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol. Ther. 1990, 48, 381–395. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef]
- Mathijssen, R.H.; van Alphen, R.J.; Verweij, J.; Loos, W.J.; Nooter, K.; Stoter, G.; Sparreboom, A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin. Cancer Res. 2001, 7, 2182–2194. [Google Scholar] [PubMed]
- Kciuk, M.; Marciniak, B.; Kontek, R. Irinotecan—Still an Important Player in Cancer Chemotherapy: A Comprehensive Overview. Int. J. Mol. Sci. 2020, 21, 4919. [Google Scholar] [CrossRef]
- Lee, J.J.; Beumer, J.H.; Chu, E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother. Pharmacol. 2016, 78, 447–464. [Google Scholar] [CrossRef]
- Garg, M.B.; Lincz, L.F.; Adler, K.; Scorgie, F.E.; Ackland, S.P.; Sakoff, J.A. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: A multivariate analysis. Br. J. Cancer 2012, 107, 1525–1533. [Google Scholar] [CrossRef]
- Tentoni, N.; Combs, R.; Hwang, M.; Ward, S.; McCracken, A.; Lowe, J.; Howard, S.C. Long-Term Outcomes of 5-Fluorouracil-Related Early-Onset Toxicities: A Retrospective Cohort Study. Cancers 2024, 16, 4050. [Google Scholar] [CrossRef]
- Tam, V.C.; Rask, S.; Koru–Sengul, T.; Dhesy–Thind, S. Generalizability of Toxicity Data from Oncology Clinical Trials to Clinical Practice: Toxicity of Irinotecan-Based Regimens in Patients with Metastatic Colorectal Cancer. Curr. Oncol. 2009, 16, 13–20. [Google Scholar] [CrossRef][Green Version]
- Petrelli, N.; Herrera, L.; Rustum, Y.; Burke, P.; Creaven, P.; Stulc, J.; Emrich, L.J.; Mittelman, A. A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J. Clin. Oncol. 1987, 5, 1559–1565. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Peeters, M. Irinotecan monotherapy in the treatment of colorectal cancers: Results of phase II trials. Bull. Cancer 1998, 85 (SPEC. ISS. DEC.), 33–37. [Google Scholar] [PubMed]
- Kano, Y.; Suenaga, M.; Uetake, H. Strategic Insight into the Combination Therapies for Metastatic Colorectal Cancer. Curr. Oncol. 2023, 30, 6546–6558. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Z. Advances in targeted therapy and biomarker research for endocrine-related cancers. Front. Endocrinol. 2024, 15, 1533623. [Google Scholar] [CrossRef]
- Koirala, M.; DiPaola, M. Overcoming Cancer Resistance: Strategies and Modalities for Effective Treatment. Biomedicines 2024, 12, 1801. [Google Scholar] [CrossRef]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef]
- Etienne, M.C.; Chatelut, E.; Pivot, X.; Lavit, M.; Pujol, A.; Canal, P.; Milano, G. Co-variables influencing 5-fluorouracil clearance during continuous venous infusion. A NONMEM analysis. Eur. J. Cancer 1998, 34, 92–97. [Google Scholar] [CrossRef]
- Bhushan, S.; McLeod, H.; Walk, C.M. Role of pharmacogenetics as predictive biomarkers of response and/or toxicity in the treatment of colorectal cancer. Clin. Color. Cancer 2009, 8, 15–21. [Google Scholar] [CrossRef]
- Kralovánszky, J.; Adleff, V.; Hitre, E.; Pap, E.; Réti, A.; Komlósi, V.; Budai, B. Pharmacogenetic studies on the prediction of efficacy and toxicity of fluoropyrimidine-based adjuvant therapy in colorectal cancer. Magy. Onkológia 2007, 51, 113–125. [Google Scholar] [PubMed]
- Oyaga-Iriarte, E.; Insausti, A.; Sayar, O.; Aldaz, A. Population Pharmacokinetic Model of Irinotecan and Its Metabolites in Patients with Metastatic Colorectal Cancer. Eur. J. Clin. Pharmacol. 2019, 75, 529–542. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- National Council on Pricing and Reimbursement of Medicinal Products (NCPRMP). Pharmacotherapeutic Guideline on Medical Oncology; Adopted at a Meeting of the NCPRMP Held on 19 April 2018, Protocol No. 274/19.04.2018. Available online: https://www.ncpr.bg/bg/component/content/article/33-%D0%BD%D0%BE%D1%80%D0%BC%D0%B0%D1%82%D0%B8%D0%B2%D0%BD%D0%B8-%D0%B0%D0%BA%D1%82%D0%BE%D0%B2%D0%B5/345-%D1%84%D0%B0%D1%80%D0%BC%D0%B0%D0%BA%D0%BE-%D1%82%D0%B5%D1%80%D0%B0%D0%BF%D0%B5%D0%B2%D1%82%D0%B8%D1%87%D0%BD%D0%B8-%D1%80%D1%8A%D0%BA%D0%BE%D0%B2%D0%BE%D0%B4%D1%81%D1%82%D0%B2%D0%B0.html?Itemid=101 (accessed on 7 June 2025).
- Chen, L.; Hao, Y.; Zhu, L.; Li, S.; Zuo, Y.; Zhang, Y.; Song, H.; Xue, Y. Monocyte to lymphocyte ratio predicts survival in patients with advanced gastric cancer undergoing neoadjuvant chemotherapy. OncoTargets Ther. 2017, 10, 4007–4016. [Google Scholar] [CrossRef]
- Kanbur, B.; Unek, I.T.; Uzun, M.; Ozturk, C.; Yarol, R.C.; Balci, A. Association of Systemic Inflammatory Response Index and Prognostic Nutritional Index Scores with Sarcopenia in Patients with Metastatic Gastric Cancer. Medicina 2025, 61, 785. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Minchev, V.; Hristova-Avakumova, N.; Kamenova, K.; Atanasova, L.; Angelov, M.; Todorov, L.; Surcheva, S.; Nikolov, R. Does dihydropyrimidine dehydrogenase level modify plasma antioxidant capacity in colorectal cancer patients treated with fluoropyrimidines? Folia Medica 2021, 63, 760–767. [Google Scholar] [CrossRef]
- Hristova-Avakumova, N.G.; Minchev, V.T.; Kamenova, K.V.; Todorov, L.T.; Angelov, M.P.; Atanasova, L.A.; Surcheva, S.K.; Nikolov, R.P. Dihydropyrimidine dehydrogenase level and the redox status in patients with colorectal cancer are prognostic for adverse effects of fluoropyrimidines. Biotechnol. Biotechnol. Equip. 2021, 35, 1247–1255. [Google Scholar] [CrossRef]
- Neugut, A.I.; Lin, A.; Raab, G.T.; Hillyer, G.C.; Keller, D.; O’Neil, D.S.; Accordino, M.K.; Kiran, R.P.; Wright, J.; Hershman, D.L. FOLFOX and FOLFIRI Use in Stage IV Colon Cancer: Analysis of SEER-Medicare Data. Clin. Color. Cancer 2019, 18, 133–140. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, R.; Wang, Y.; Liu, X.; Zhang, W.; Zhu, X.; Chen, Z.; Shen, W.; He, Y.; Wang, H.Q.; et al. FOLFIRI (folinic acid, fluorouracil, and irinotecan) increases not efficacy but toxicity compared with single-agent irinotecan as a second-line treatment in metastatic colorectal cancer patients: A randomized clinical trial. Ther. Adv. Med. Oncol. 2022, 14, 17588359211068737. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, Z.; Wang, Q.; Wang, B.; Huang, R.; Xu, W.; Shang, C.; Chen, Y. Epidemiological Trends in Gastrointestinal Cancers and Risk Factors across U.S. States from 2000 to 2021: A Systematic Analysis for the Global Burden of Disease Study 2021. BMC Public Health 2025, 25, 43. [Google Scholar] [CrossRef]
- Khan, M.; Alharbi, S.; Aljuhani, S.; Tunkar, M.; Morya, A.; Alnatsheh, A.; Alshamrani, M.; Felemban, R. The incidence of hematological toxicities in colorectal cancer patients treated with fluoropyrimidine-based regimens at princess noorah oncology center. Cureus 2023, 15, e44267. [Google Scholar] [CrossRef]
- Kogan, L.G.; Davis, S.L.; Brooks, G.A. Treatment delays during FOLFOX chemotherapy in patients with colorectal cancer: A multicenter retrospective analysis. J. Gastrointest. Oncol. 2019, 10, 841–846. [Google Scholar] [CrossRef]
- Liu, X.; Ou, K.; Ma, X.; Gao, L.; Wang, Q.; Zhang, H.; Yang, L. Safety and efficacy of irinotecan, oxaliplatin, and capecitabine (XELOXIRI) regimen with or without targeted drugs in patients with metastatic colorectal cancer: A retrospective cohort study. BMC Cancer 2022, 22, 807. [Google Scholar] [CrossRef]
- Varughese, L.A.; Lau-Min, K.S.; Cambareri, C.; Damjanov, N.; Massa, R.; Reddy, N.; Oyer, R.; Teitelbaum, U.; Tuteja, S. DPYD and UGT1A1 Pharmacogenetic Testing in Patients with Gastrointestinal Malignancies: An Overview of the Evidence and Considerations for Clinical Implementation. Pharmacotherapy 2020, 40, 1108–1129. [Google Scholar] [CrossRef]
- Deac, A.L.; Burz, C.C.; Bocşe, H.F.; Bocşan, I.C.; Buzoianu, A.D. A Review on the Importance of Genotyping and Phenotyping in Fluoropyrimidine Treatment. Med. Pharm. Rep. 2020, 93, 223–230. [Google Scholar] [CrossRef]
- Nave, O. A Mathematical Model for Treatment Using Chemo-Immunotherapy. Heliyon 2022, 8, e09288. [Google Scholar] [CrossRef]
- Martin, J.H.; Galettis, P.; Flynn, A.; Schneider, J. Phenotype versus Genotype to Optimize Cancer Dosing in the Clinical Setting—Focus on 5-Fluorouracil and Tyrosine Kinase Inhibitors. Pharmacol. Res. Perspect. 2024, 12, e1182. [Google Scholar] [CrossRef]
- van Kuilenburg, A.B. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur. J. Cancer 2004, 40, 939–950. [Google Scholar] [CrossRef]
- Diasio, R.B.; Offer, S.M. Testing for Dihydropyrimidine Dehydrogenase Deficiency to Individualize 5-Fluorouracil Therapy. Cancers 2022, 14, 3207. [Google Scholar] [CrossRef]
- Offer, S.M.; Wegner, N.J.; Fossum, C.; Wang, K.; Diasio, R.B. Phenotypic profiling of DPYD variations relevant to 5-fluorouracil sensitivity using real-time cellular analysis and in vitro measurement of enzyme activity. Cancer Res. 2013, 73, 1958–1968. [Google Scholar] [CrossRef]
- van Kuilenburg, A.B.; Haasjes, J.; Richel, D.J.; Zoetekouw, L.; Van Lenthe, H.; De Abreu, R.A.; Maring, J.G.; Vreken, P.; van Gennip, A.H. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: Identification of new mutations in the DPD gene. Clin. Cancer Res. 2000, 6, 4705–4712. [Google Scholar] [PubMed]
- Salonga, D.; Danenberg, K.D.; Johnson, M.; Metzger, R.; Groshen, S.; Tsao-Wei, D.D.; Lenz, H.J.; Leichman, C.G.; Leichman, L.; Diasio, R.B.; et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin. Cancer Res. 2000, 6, 1322–1327. [Google Scholar] [PubMed]
- Tsuji, T.; Sawai, T.; Takeshita, H.; Nakagoe, T.; Hidaka, S.; Nanashima, A.; Yamaguchi, H.; Yasutake, T.; Nagayasu, T.; Tagawa, Y. Tumor dihydropyrimidine dehydrogenase in stage II and III colorectal cancer: Low level expression is a beneficial marker in oral-adjuvant chemotherapy, but is also a predictor for poor prognosis in patients treated with curative surgery alone. Cancer Lett. 2004, 204, 97–104. [Google Scholar] [CrossRef]
- Kobayashi, H.; Koike, T.; Nakatsuka, A.; Kurita, H.; Sagara, J.; Taniguchi, S.; Kurashina, K. Dihydropyrimidine dehydrogenase expression predicts survival outcome and chemosensitivity to 5-fluorouracil in patients with oral squamous cell carcinoma. Oral Oncol. 2005, 41, 38–47. [Google Scholar] [CrossRef]
- Baba, H.; Watanabe, M.; Okabe, H.; Miyamoto, Y.; Sakamoto, Y.; Baba, Y.; Iwatsuki, M.; Chikamoto, A.; Beppu, T. Upregulation of ERCC1 and DPD Expressions after Oxaliplatin-Based First-Line Chemotherapy for Metastatic Colorectal Cancer. Br. J. Cancer 2012, 107, 1950–1955. [Google Scholar] [CrossRef][Green Version]
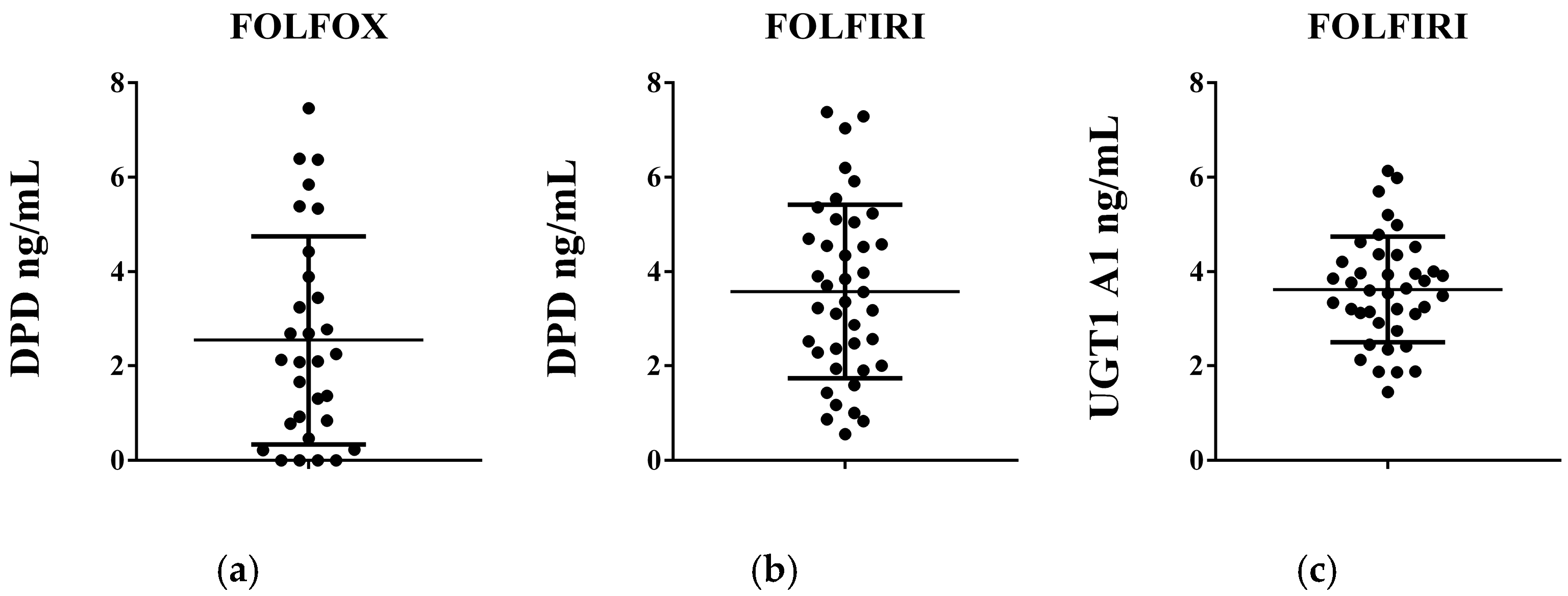
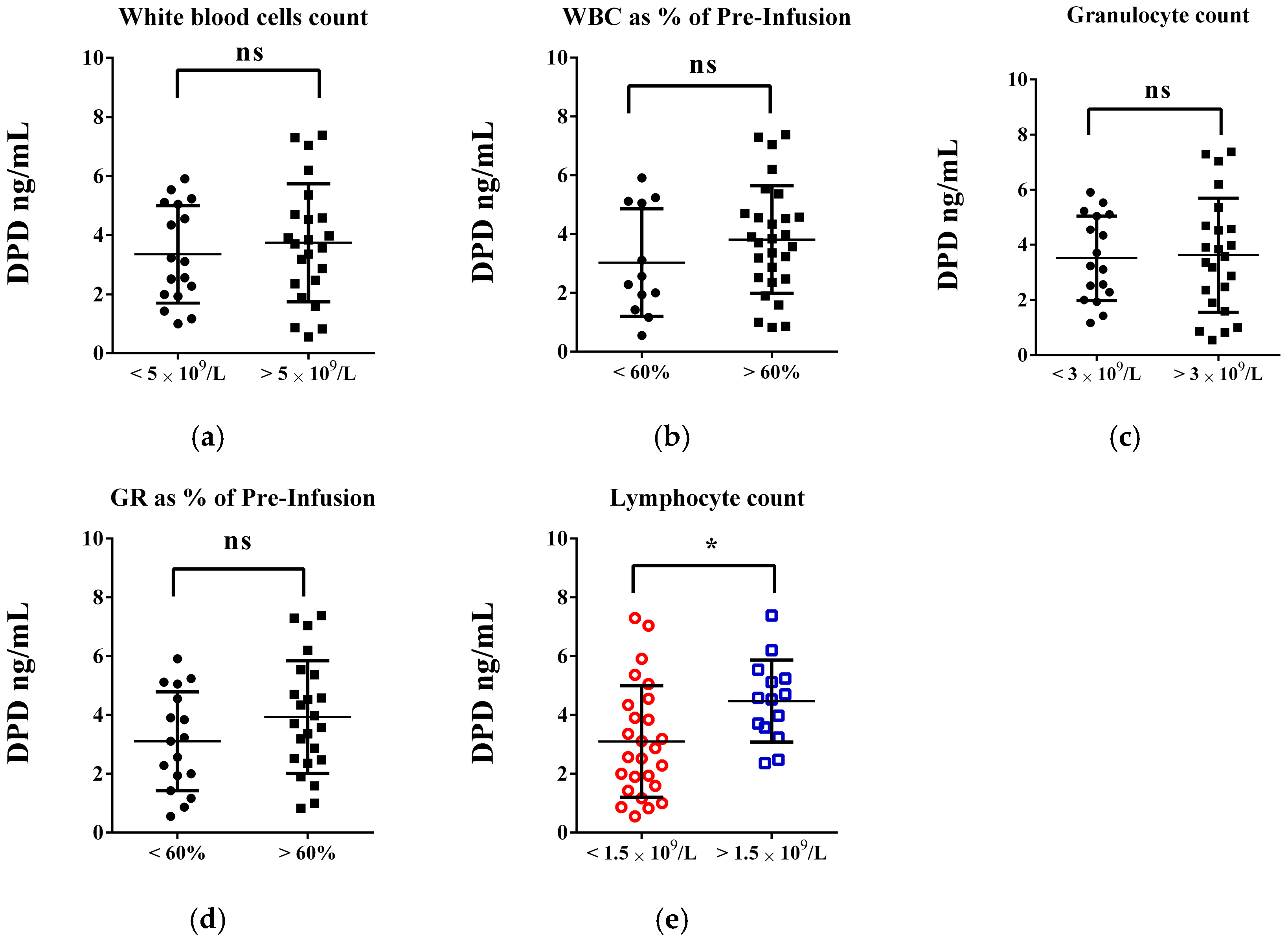
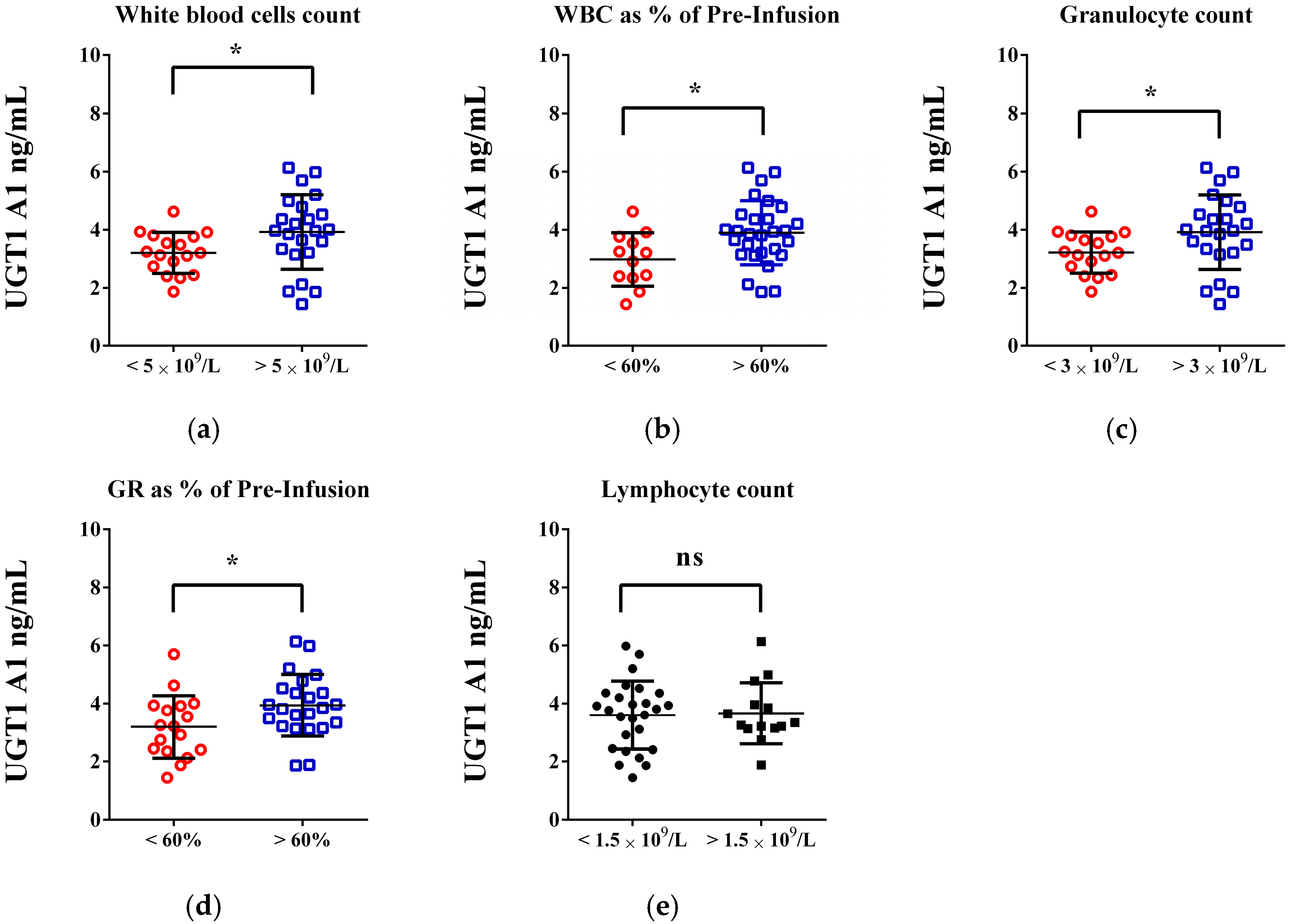
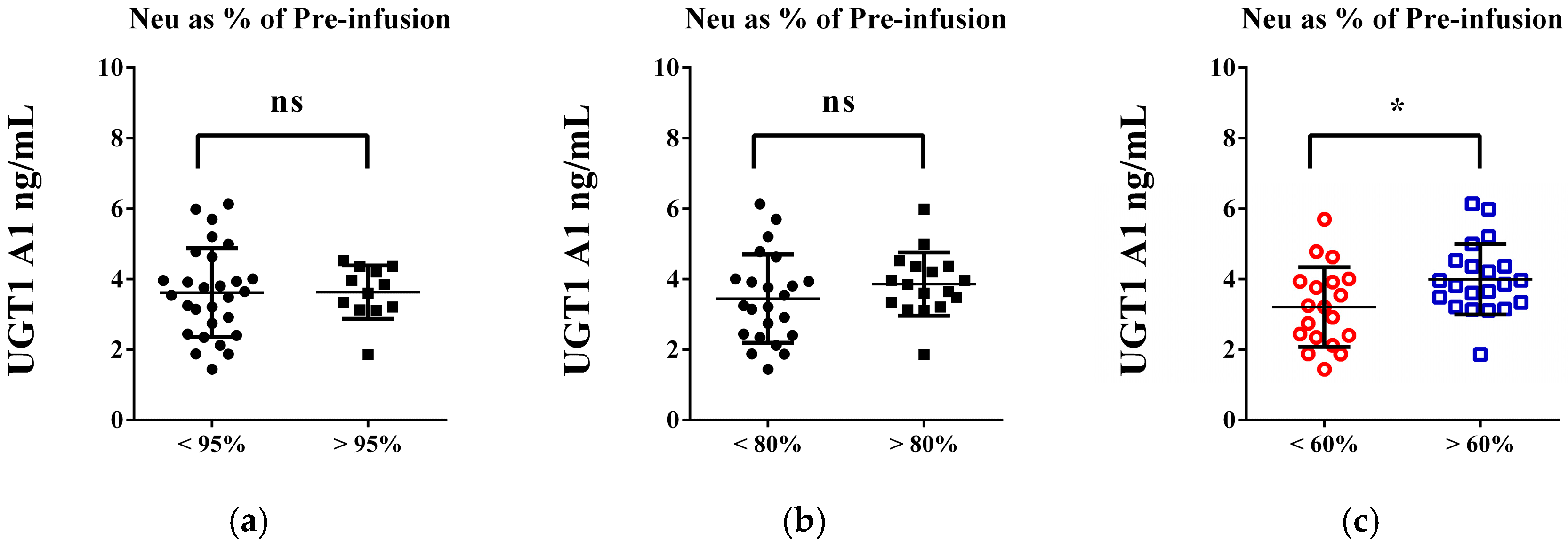
| FOLFOX n = 30, (%) | FOLFIRI n = 40, (%) | p-Values | |
|---|---|---|---|
| Gender | ns | ||
| Male | 16 (53.3%) | 22 (55.0%) | |
| Female | 14 (46.7%) | 18 (45.0%) | |
| Age at diagnosis | ns | ||
| Average | 63 y, 9 mo. | 63 y, 6 mo. | |
| Range | 43 ↔ 80 | 34 ↔ 83 | |
| Age-Related Distribution | ns | ||
| Below 50 years | 4 (13.3%) | 5 (12.5%) | |
| Between 50 and 59 | 6 (20.0%) | 5 (12.5%) | |
| Between 60 and 69 | 8 (26.7%) | 13 (32.5%) | |
| Between 70 and 79 | 11 (36.7%) | 14 (35.0%) | |
| Above 80 years | 1 (3.3%) | 3 (7.5%) | |
| Primary Tumor Location | ns | ||
| Ca. oesophagi | 2 (6.7%) | 2 (5.0%) | |
| Ca. ventriculi | 1 (3.3%) | 9 (22.5%) | |
| Ca. glandulae pancreaticae | 0 (0.0%) | 4 (10.0%) | |
| Ca. recti | 9 (30.0%) | 6 (15.0%) | |
| Ca. rectosigmoidei | 1 (3.3%) | 2 (5.0%) | |
| Ca. sigmoidei | 9 (30.0%) | 6 (15.0%) | |
| Ca. flexurae hepatis | 0 (0.0%) | 4 (10.0%) | |
| Ca. coli | 5 (16.7%) | 3 (7.5%) | |
| Ca. cecum | 3 (10.0%) | 3 (7.5%) | |
| Ca. duodeni | 0 (0.0%) | 1 (2.5%) | |
| Grading | ** | ||
| Well differentiated (G1) | 7 (23.3%) | 2 (5.0%) | |
| Moderately differentiated (G2) | 22 (73.3%) | 27 (67.5%) | |
| Poor/undifferentiated (G3) | 1 (3.3%) | 11 (27.5%) | |
| Disease Stage | **** | ||
| Stage I | 0 (0%) | 0 (0%) | |
| Stage II | 7 (23.3%) | 0 (0%) | |
| Stage III | 10 (33.3%) | 0 (0%) | |
| Stage IV | 13 (43.3%) | 40 (100.0%) | |
| ECOG performance status | ns | ||
| 0 | 2 (6.7%) | 0 (0.0%) | |
| 1 | 20 (66.7%) | 26 (65.0%) | |
| 2 | 8 (26.6%) | 13 (32.5%) | |
| 3 | 0 (0%) | 1 (2.5%) |
| Parameter | Before ChT (Median; IQR) | After ChT (Median; IQR) | p-Value (Wilcoxon Test) | |
|---|---|---|---|---|
| Peripheral blood routine | ||||
| WBC | 109/L | 7.15; (5.50; 8.40) | 6.35; (4.23; 7.60) | ** |
| LY | 109/L | 1.64; (1.36; 1.99) | 1.66; (1.25; 1.99) | ns |
| MO | 109/L | 0.48; (0.36; 0.63) | 0.41; (0.33; 0.60) | ns |
| GR | 109/L | 5.30; (3.52; 6.09) | 4.39; (2.30; 4.94) | ** |
| RBC | 1012/L | 4.35; (3.83; 4.59) | 4.26; (3.97; 4.69) | ns |
| HGB | g/dL | 121.0; (111.5; 130.3) | 124.0; (108.8; 135.5) | ns |
| HCT | L/L | 37.40; (33.68; 39.25) | 37.10; (33.63; 40.00) | ns |
| PLT | 109/L | 280.0; (243.5; 347.5) | 244.0; (183.5; 324.5) | ** |
| Liver functions | ||||
| ALAT | U/L | 19.70; (13.20; 31.63) | 16.80; (11.45; 25.33) | ns |
| ASAT | U/L | 20.05; (17.53; 31.48) | 19.20; (15.93; 25.15) | ns |
| TBIL | µmol/L | 8.95; (7.00; 15.18) | 10.80; (7.83; 15.00) | ns |
| Renal functions | ||||
| Cr | µmol/L | 81.50; (69.75; 89.25) | 80.80; (72.00; 92.50) | ns |
| Parameter | Before ChT (Median; IQR) | After ChT (Median; IQR) | p-Value (Wilcoxon Test) | |
|---|---|---|---|---|
| Peripheral blood routine | ||||
| WBC | 109/L | 8.30; (5.69; 9.82) | 5.24; (3.74; 9.51) | * |
| LY | 109/L | 1.39; (1.07; 1.86) | 1.28; (0.98; 1.68) | ns |
| MO | 109/L | 0.58; (0.42; 0.76) | 0.43; (0.33; 0.66) | * |
| GR | 109/L | 6.00; (3.74; 7.83) | 3.51; (2.01; 7.21) | ** |
| RBC | 1012/L | 4.00; (3.60; 4.39) | 3.96; (3.56; 4.31) | ns |
| HGB | g/dL | 116.0; (105.0; 129.8) | 116.5; (102.0; 128.0) | ns |
| HCT | L/L | 35.25; (32.23; 39.00) | 36.10; (32.03; 38.23) | ns |
| PLT | 109/L | 243.5; (178.0; 350.8) | 238.5; (160.5; 346.5) | ns |
| Liver functions | ||||
| ALAT | U/L | 14.40; (11.13; 25.40) | 15.24; (11.25; 28.40) | ns |
| ASAT | U/L | 24.80; (18.75; 35.53) | 27.05; (18.05; 33.68) | ns |
| TBIL | µmol/L | 10.05; (7.50; 17.55) | 10.20; (7.18; 17.90) | ns |
| Renal functions | ||||
| Cr | µmol/L | 80.00; (73.00; 90.75) | 75.00; (69.25; 93.25) | ns |
| Parameter | FOLFOX (Median; IQR) | FOLFIRY (Median; IQR) | p-Value (Mann–Whitney U Test) |
|---|---|---|---|
| Platelet-to-lymphocyte ratio | |||
| PLR Before ChT | 181.0; (135.7; 210.4) | 168.7; (123.6; 256.4) | ns |
| PLR After ChT | 153.7; (108.6; 223.1) | 186.4; (120.5; 270.4) | ns |
| Lymphocyte-to-monocyte ratio | |||
| LMR Before ChT | 3.643; (2.772; 4.265) | 2.548; (2.070; 3.759) | ns |
| LMR After ChT | 3.738; (3.106; 5.190) | 2.998; (2.328; 4.607) | ns |
| Parameter | b* | Std. Error of b* | b | Std. Error of b | t | p-Value |
|---|---|---|---|---|---|---|
| Regression Summary for Dependent Variables: DPD (age, gender, disease stage); | ||||||
| R = 0.252; R2 = 0.064; Adjusted R2 = 0.021 | ||||||
| F(3, 66) = 1.492; p < 0.225; STD. Error of estimate: 2.037 | ||||||
| N = 70; t(66) | ||||||
| Intercept | 0.428 | 1.907 | 0.224 | 0.823 | ||
| Age | 0.074 | 0.121 | 0.013 | 0.021 | 0.609 | 0.545 |
| Gender | −0.0900 | 0.119 | −0.369 | 0.489 | −0.756 | 0.455 |
| Cancer stage | 0.211 | 0.121 | 0.662 | 0.379 | 0.745 | 0.086 |
| Regression Summary for Dependent Variables: DPD (age, gender, disease progression); | ||||||
| R = 0.292; R2 = 0.085; Adjusted R2 = 0.044 | ||||||
| F(3, 66) = 2.057; p < 0.114; STD. Error of estimate: 2.013 | ||||||
| N = 70; t(66) | ||||||
| Intercept | 0.673 | 1.709 | 0.394 | 0.694 | ||
| Age | 0.075 | 0.119 | 0.013 | 0.021 | 0.634 | 0.528 |
| Gender | −0.091 | 0.118 | −0.375 | 0.483 | −0.776 | 0.441 |
| Disease progression | 0.258 | 0.119 | 0.513 | 0.237 | 2.169 | 0.034 |
| Regression Summary for Dependent Variables: DPD (disease progression); | ||||||
| R = 0.262; R2 = 0.069; Adjusted R2 = 0.055 | ||||||
| F(1, 68) = 5.029; p < 0.282; STD. Error of estimate: 2.001 | ||||||
| N = 70; t(68) | ||||||
| Intercept | - | - | 0.935 | 1.010 | 0.925 | 0.358 |
| Disease stage | 0.262 | 0.117 | 0.520 | 0.232 | 2.243 | 0.028 |
| Regression Summary for Dependent Variables: UGT1A1 (age, gender) | ||||||
| R = 0.328; R2 = 0.107; Adjusted R2 = 0.059 | ||||||
| F(2, 37) = 2.224; p < 0.1222; Std. Error of estimate: 1.089 | ||||||
| N = 40; t(36) | ||||||
| Intercept | 2.257 | 1.1170 | 2.021 | 0.051 | ||
| Age | 0.293 | 0.156 | 0.027 | 0.014 | 1.879 | 0.068 |
| Gender | −0.126 | 0.156 | −0.280 | 0.347 | −0.806 | 0.426 |
| Parameter | b* | Std. Error of b* | b | Std. Error of b | t | p-Value |
|---|---|---|---|---|---|---|
| Regression Summary for Dependent Variables: WBC After (DPD, UGT1A1); | ||||||
| R = 0.340; R2 = 0.116; Adjusted R2 = 0.068 | ||||||
| F(2, 37) = 2.421; p < 0.103; STD. Error of estimate: 3.743 | ||||||
| N = 40; t(37) | ||||||
| Intercept | 2.386 | 2.058 | 1.159 | 0.254 | ||
| DPD | −0.053 | 0.171 | −0.111 | 0.359 | −0.308 | 0.760 |
| UGT1A1 | 0.359 | 0.171 | 1.240 | 0.589 | 2.105 | 0.042 |
| Regression Summary for Dependent Variables: Gran after (DPD, UGT1A1); | ||||||
| R = 0.357; R2 = 0.128; Adjusted R2 = 0.081 | ||||||
| F(2, 37) = 2.708; p < 0.0800; STD. Error of estimate: 3.293 | ||||||
| N = 40; t(37) | ||||||
| Intercept | 0.779 | 1.811 | 0.430 | 0.669 | ||
| DPD | −0.076 | 0.169 | −0.141 | 0.316 | −0.446 | 0.658 |
| UGT1A1 | 0.383 | 0.169 | 1.171 | 0.518 | 2.259 | 0.030 |
| Regression Summary for Dependent Variables: Neu count after (DPD, UGT1A1); | ||||||
| R = 0.357; R2 = 0.128; Adjusted R2 = 0.081 | ||||||
| F(2, 37) = 0.271; p < 0.080; STD. Error of estimate: 3.287 | ||||||
| N = 40; t(37) | ||||||
| Intercept | 0.656 | 1.807 | 0.363 | 0.719 | ||
| DPD | −0.076 | 0.169 | −0.142 | 0.315 | −0.449 | 0.656 |
| UGT1A1 | 0.383 | 0.169 | 1.169 | 0.517 | 2.260 | 0.030 |
| Ly count after (DPD, UGT1A1); | ||||||
| R = 0.089; R2 = 0.008; Adjusted R2 = _0.005 | ||||||
| F(2, 37) = 0.149; p < 0.862; STD. Error of estimate: 0.975 | ||||||
| N = 40; t(37) | ||||||
| Intercept | 1.416 | 0.536 | 2.642 | 0.012 | ||
| DPD | 0.098 | 0.181 | 0.051 | 0.093 | 0.544 | 0.590 |
| UGT1A1 | −0.035 | 0.181 | −0.030 | 0.153 | −0.194 | 0.847 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minchev, V.; Tsankov, H.; Robev, B.; Takov, M.; Federchev, S.; Kamenova, K.; Todorov, L.; Atanasova, L.; Hristova-Avakumova, N.; Nikolov, R.; et al. Gastrointestinal Cancers with Consideration of DPD and UGT1A1 Plasma Levels: Chemotherapy-Related Toxicity. Life 2025, 15, 1071. https://doi.org/10.3390/life15071071
Minchev V, Tsankov H, Robev B, Takov M, Federchev S, Kamenova K, Todorov L, Atanasova L, Hristova-Avakumova N, Nikolov R, et al. Gastrointestinal Cancers with Consideration of DPD and UGT1A1 Plasma Levels: Chemotherapy-Related Toxicity. Life. 2025; 15(7):1071. https://doi.org/10.3390/life15071071
Chicago/Turabian StyleMinchev, Velko, Hristo Tsankov, Bozil Robev, Martin Takov, Stefan Federchev, Kalina Kamenova, Lozan Todorov, Liliya Atanasova, Nadya Hristova-Avakumova, Rumen Nikolov, and et al. 2025. "Gastrointestinal Cancers with Consideration of DPD and UGT1A1 Plasma Levels: Chemotherapy-Related Toxicity" Life 15, no. 7: 1071. https://doi.org/10.3390/life15071071
APA StyleMinchev, V., Tsankov, H., Robev, B., Takov, M., Federchev, S., Kamenova, K., Todorov, L., Atanasova, L., Hristova-Avakumova, N., Nikolov, R., Gateva, P., & Mitev, V. (2025). Gastrointestinal Cancers with Consideration of DPD and UGT1A1 Plasma Levels: Chemotherapy-Related Toxicity. Life, 15(7), 1071. https://doi.org/10.3390/life15071071







