Comparing the Effects of Cranial Electrotherapy Stimulation and Cognitive Behavioral Therapy for Insomnia on Daily Mood and Physiological Sleep Parameters in Athletes with Poor Pre-Competition Sleep Quality
Abstract
1. Introduction
2. Methods
2.1. Study Procedures
2.2. Interventions
2.2.1. Cranial Electrotherapy Stimulation
2.2.2. Cognitive Behavioral Therapy
2.3. Assessments
2.3.1. Pittsburgh Sleep Quality Index
2.3.2. Epworth Sleepiness Scale
2.3.3. Profile of Mood States
2.3.4. Cardiopulmonary Coupling Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fullagar, H.H.; Duffield, R.; Skorski, S.; Coutts, A.J.; Julian, R.; Meyer, T. Sleep and recovery in team sport: Current sleep-related issues facing professional team-sport athletes. Int. J. Sports Physiol. Perform. 2015, 10, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Li, X. Sleep patterns during pre-competition training phase: A comparison between male and female collegiate swimmers. Nat. Sci. Sleep 2024, 16, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Samuels, C. Sleep, recovery, and performance: The new frontier in high-performance athletics. Neurol. Clin. 2008, 26, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.M. Sleep and athletic performance. Curr. Sports Med. Rep. 2017, 16, 413–418. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Liebetanz, D.; Lang, N.; Antal, A.; Tergau, F.; Paulus, W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin. Neurophysiol. 2003, 114, 2220–2222. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Yin, J.; Cao, G.; Qiu, J. The effect of short-term cranial electrotherapy stimulation on sleep quality in athletes: A pilot study. Medicine 2023, 102, e34725. [Google Scholar] [CrossRef]
- Kirsch, D.L.; Price, L.R.; Nichols, F.; Marksberry, J.A.; Platoni, K.T. Military service member and veteran self reports of efficacy of cranial electrotherapy stimulation for anxiety, posttraumatic stress disorder, insomnia, and depression. US Army Med. Dep. J. 2014, 46–54. [Google Scholar]
- Aseem, A.; Hussain, M.E. Impact of cranial electrostimulation on sleep: A systematic review. Sleep Vigil. 2019, 3, 101–112. [Google Scholar] [CrossRef]
- Edinger, J.D.; Arnedt, J.T.; Bertisch, S.M.; Carney, C.E.; Harrington, J.J.; Lichstein, K.L.; Sateia, M.J.; Troxel, W.M.; Zhou, E.S.; Kazmi, U.; et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2021, 17, 255–262. [Google Scholar] [CrossRef]
- Ho, F.Y.; Chung, K.F.; Yeung, W.F.; Ng, T.H.; Kwan, K.S.; Yung, K.P.; Cheng, S.K. Self-help cognitive-behavioral therapy for insomnia: A meta-analysis of randomized controlled trials. Sleep Med. Rev. 2015, 19, 17–28. [Google Scholar] [CrossRef]
- Gwyther, K.; Rice, S.; Purcell, R.; Pilkington, V.; Santesteban-Echarri, O.; Bailey, A.; Walton, C.C. Sleep interventions for performance, mood and sleep outcomes in athletes: A systematic review and meta-analysis. Psychol. Sport Exerc. 2022, 58, 102094. [Google Scholar] [CrossRef]
- Badahdah, A.; Khamis, F.; Aloud, N. Validation and cutoff score for the single-item sleep quality scale. Sleep Breath. 2024, 29, 24. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.G.; Gragnani, C. Efficacy of cranial electric stimulation for the treatment of insomnia: A randomized pilot study. Complement. Ther. Med. 2013, 21, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Muench, A.; Perlis, M.L.; Vargas, I. Cognitive behavioraltherapy for insomnia (CBT-I): A primer. Klin. Spec. Psihol. 2022, 11, 123–137. [Google Scholar]
- Tsai, P.S.; Wang, S.Y.; Wang, M.Y.; Su, C.T.; Yang, T.T.; Huang, C.J.; Fang, S.C. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual. Life Res. 2005, 14, 1943–1952. [Google Scholar] [CrossRef]
- Chen, N.H.; Johns, M.W.; Li, H.Y.; Chu, C.C.; Liang, S.C.; Shu, Y.H.; Chuang, M.L.; Wang, P.C. Validation of a Chinese version of the Epworth sleepiness scale. Qual. Life Res. 2002, 11, 817–821. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Chang, Y.C.; Lu, J.H. The revision of Profile of Mood State Questionnaire. Sports Exerc. Res. 2003, 5, 85–95. [Google Scholar]
- Cabiddu, R.; Cerutti, S.; Viardot, G.; Werner, S.; Bianchi, A.M. Modulation of the sympatho-vagal balance during sleep: Frequency domain study of heart rate variability and respiration. Front. Physiol. 2012, 3, 45. [Google Scholar] [CrossRef]
- Thomas, R.J.; Mietus, J.E.; Peng, C.K.; Goldberger, A.L. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep 2005, 28, 1151–1161. [Google Scholar] [CrossRef]
- Sacha, J. Interaction between heart rate and heart rate variability. Ann. Noninvasive Electrocardiol. 2014, 19, 207–216. [Google Scholar] [CrossRef]
- Edinger, J.D.; Means, M.K. Cognitive-behavioral therapy for primary insomnia. Clin. Psychol. Rev. 2005, 25, 539–558. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Kim, H.J.; Kim, W.Y.; Min, W.K.; Min, T.J.; Lee, Y.S.; Kim, J.H. Effects of cranial electrotherapy stimulation on preoperative anxiety and blood pressure during anesthetic induction in patients with essential hypertension. J. Int. Med. Res. 2020, 48, 300060520939370. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.R.; Jeon, H.J.; Yoon, I.Y. Modest effects of low-frequency electrical stimulation on patients with chronic insomnia in an open trial. Sleep Med. Res. 2019, 10, 17–24. [Google Scholar] [CrossRef]
- Bystritsky, A.; Kerwin, L.; Feusner, J. A pilot study of cranial electrotherapy stimulation for generalized anxiety disorder. J. Clin. Psychiatry 2008, 69, 412–417. [Google Scholar] [CrossRef]
- Weiss, M.F. The treatment of insomnia through the use of electrosleep: An EEG study. J. Nerv. Ment. Dis. 1973, 157, 108–120. [Google Scholar] [CrossRef]
- Halson, S.L. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med. 2014, 44, 13–23. [Google Scholar] [CrossRef]
- Chang, W.D.; Tsou, Y.A.; Chen, Y.Y.; Hung, B.L. Cranial electrotherapy stimulation to improve the physiology and psychology response, response-ability, and sleep efficiency in athletes with poor sleep quality. Int. J. Environ. Res. Public. Health 2022, 19, 1946. [Google Scholar] [CrossRef]
- Park, K.S.; Choi, S.H.; Yoon, H. Modulation of sleep using noninvasive stimulations during sleep. Biomed. Eng. Lett. 2023, 13, 329–341. [Google Scholar] [CrossRef]
- Morin, C.M.; Bootzin, R.R.; Buysse, D.J.; Edinger, J.D.; Espie, C.A.; Lichstein, K.L. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004). Sleep 2006, 29, 1398–1414. [Google Scholar] [CrossRef]
- Lastella, M.; Lovell, G.P.; Sargent, C. Athletes’ precompetitive sleep behaviour and its relationship with subsequent precompetitive mood and performance. Eur. J. Sport Sci. 2014, 14, 123–130. [Google Scholar] [CrossRef]
- Haskell, B.; Eiler, A.; Essien, H. Sleep quality and cognitive skills impact neurocognitive function and reduce sports-related injury risk. Arthrosc. Sports Med. Rehabil. 2025, 7, 101077. [Google Scholar] [CrossRef] [PubMed]
- Koffel, E.A.; Koffel, J.B.; Gehrman, P.R. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep. Med. Rev. 2015, 19, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Day, C.; Nishino, N.; Tsukahara, Y. Sleep in the athlete. Clin. Sports Med. 2024, 43, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Juliff, L.E.; Halson, S.L.; Peiffer, J.J. Understanding sleep disturbance in athletes prior to important competitions. J. Sci. Med. Sport. 2015, 18, 13–18. [Google Scholar] [CrossRef]
- Seyffert, M.; Lagisetty, P.; Landgraf, J.; Chopra, V.; Pfeiffer, P.N.; Conte, M.L.; Rogers, M.A. Internet-delivered cognitive behavioral therapy to treat insomnia: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0149139. [Google Scholar] [CrossRef]
- De Niet, G.J.; Tiemens, B.G.; Kloos, M.W.; Hutschemaekers, G.J. Review of systematic reviews about the efficacy of non-pharmacological interventions to improve sleep quality in insomnia. Int. J. Evid. Based Healthc. 2009, 7, 233–242. [Google Scholar] [CrossRef]
- Morriss, R.; Xydopoulos, G.; Craven, M.; Price, L.; Fordham, R. Clinical effectiveness and cost minimisation model of Alpha-Stim cranial electrotherapy stimulation in treatment seeking patients with moderate to severe generalised anxiety disorder. J. Affect. Disord. 2019, 253, 426–437. [Google Scholar] [CrossRef]
| CBT-I Group (n = 12) | CES Group (n = 12) | p | |
|---|---|---|---|
| Age (years) | 20.92 ± 3.37 | 20.33 ± 0.89 | 0.57 |
| Height (cm) | 169.79 ± 8.76 | 169.66 ± 8.71 | 0.97 |
| Weight (kg) | 66.93 ± 14.13 | 60.02 ± 6.78 | 0.15 |
| BMI (kg/m2) | 23.11 ± 3.90 | 20.85 ± 1.84 | 0.09 |
| SISQ | 2.33 ± 0.78 | 2.58 ± 0.51 | 0.37 |
| Insomnia frequency (times/a week) | 2.83 ± 1.35 | 2.96 ± 1.39 | 0.83 |
| CBT-I Group (n = 12) | CES Group (n = 12) | |||||
|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |
| Confusion | 15.33 ± 5.07 | 11.58 ± 4.85 | 0.01 * | 18.83 ± 7.38 | 17.33 ± 8.75 | 0.11 |
| Fatigue | 10.83 ± 2.66 | 10.42 ± 3.18 | 0.69 | 15.67 ± 7.63 | 13.33 ± 5.99 | 0.14 |
| Anger | 7.08 ± 2.31 | 6.50 ± 0.67 | 0.29 | 10.01 ± 5.72 | 9.83 ± 4.78 # | 0.80 |
| Tension | 10.75 ± 2.56 | 8.02 ± 2.66 | 0.01 * | 12.08 ± 3.37 | 11.01 ± 3.93 # | 0.11 |
| Depression | 3.83 ± 1.40 | 3.58 ± 1.24 | 0.27 | 5.58 ± 2.47 | 5.50 ± 2.50 # | 0.88 |
| Vigor | 20.50 ± 6.84 | 22.50 ± 7.40 | 0.10 | 18.01 ± 5.36 | 18.75 ± 5.46 | 0.35 |
| Esteem | 12.50 ± 3.37 | 13.17 ± 4.15 | 0.34 | 11.25 ± 3.89 | 11.67 ± 3.75 | 0.49 |
| CBT-I Group (n = 12) | CES Group (n = 12) | |||||
|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |
| PSQI | 9.33 ± 2.74 | 6.58 ± 2.54 | 0.01 * | 10.69 ± 3.31 | 6.92 ± 3.34 | 0.01 * |
| ESS | 14.75 ± 4.71 | 10.92 ± 4.76 | 0.01 * | 14.58 ± 3.96 | 12.00 ± 5.41 | 0.04 * |
| Sleep architecture | ||||||
| TST (min) | 407.58 ± 99.92 | 375.58 ± 99.01 | 0.42 | 372.33 ± 60.55 | 407.17 ± 77.11 | 0.19 |
| Sleep efficiency (%) | 86.71 ± 8.76 | 91.15 ± 5.15 | 0.03 * | 89.06 ± 0.06 | 89.08 ± 6.50 | 0.91 |
| S1 and S2 (min) | 156.01 ± 61.97 | 159.92 ± 83.72 | 0.85 | 181.08 ± 53.59 | 128.08 ± 49.94 | 0.02 * |
| Percentage of S1 and S2 (%) | 38.21 ± 15.33 | 39.86 ± 16.09 | 0.65 | 42.19 ± 7.45 | 32.45 ± 10.82 | 0.01 * |
| S3 and S4 (min) | 155.75 ± 99.47 | 131.58 ± 57.14 | 0.33 | 127.42 ± 45.14 | 169.92 ± 64.71 | 0.09 |
| Percentage of S3 and S4 (%) | 34.83 ± 17.89 | 34.18 ± 14.74 | 0.85 | 30.63 ± 11.62 | 44.05 ± 15.95 | 0.02 * |
| REM (min) | 84.08 ± 42.59 | 95.50 ± 37.84 | 0.39 | 74.33 ± 43.83 | 98.67 ± 26.68 | 0.13 |
| Percentage of REM (%) | 21.02 ± 7.89 | 22.25 ± 6.27 | 0.62 | 18.93 ± 9.98 | 23.13 ± 4.44 | 0.22 |
| Nighttime HRV | ||||||
| SDNN(ms) | 97.50 ± 39.75 | 99.67 ± 27.59 | 0.10 | 99.83 ± 54.15 | 127.17 ± 46.78 | 0.06 |
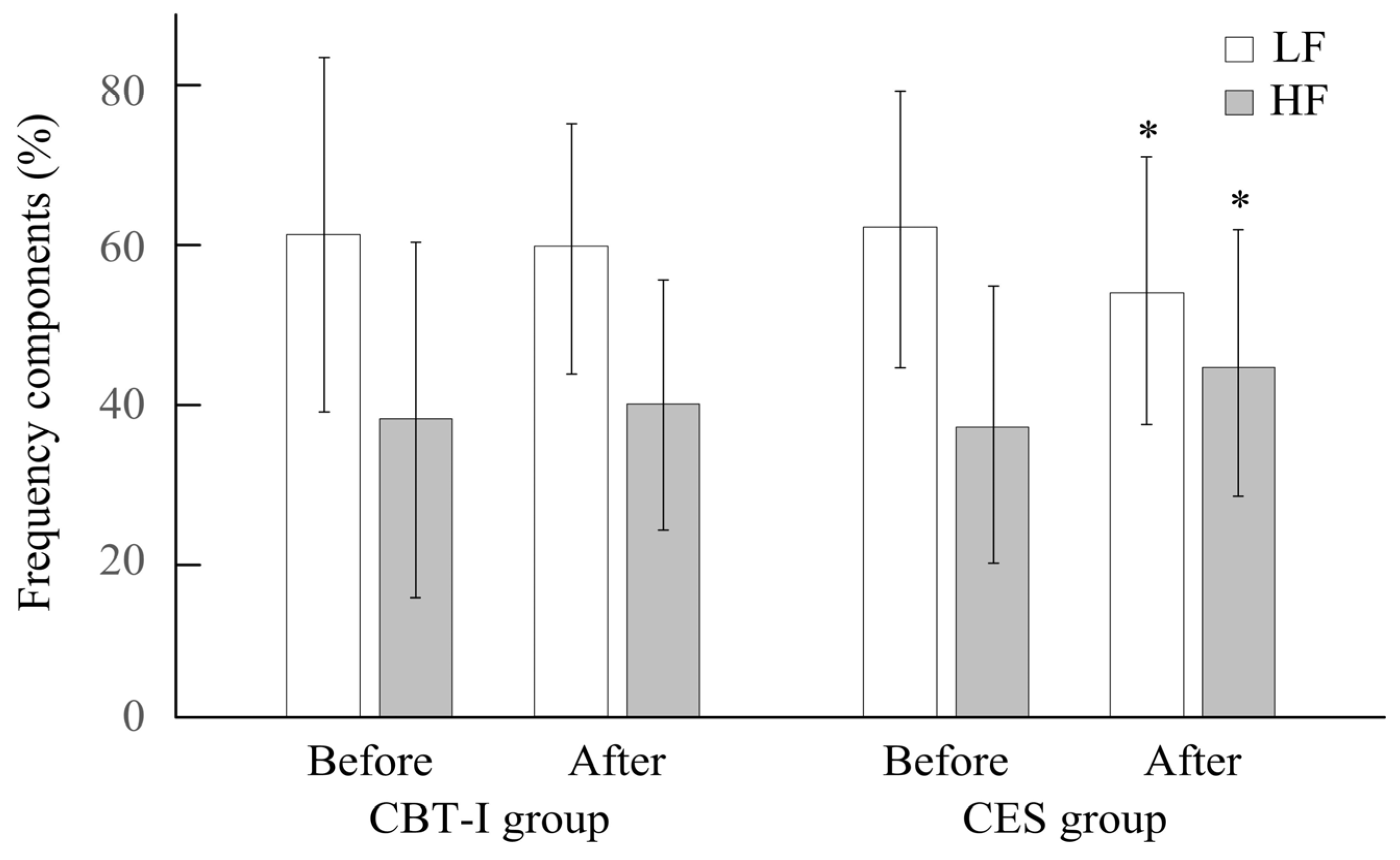
| LF/HF ratio | 2.67 ± 2.36 | 1.90 ± 1.28 | 0.40 | 2.24 ± 1.42 | 1.45 ± 0.83 | 0.04 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsou, Y.-A.; Hung, B.-L.; Chang, W.-D. Comparing the Effects of Cranial Electrotherapy Stimulation and Cognitive Behavioral Therapy for Insomnia on Daily Mood and Physiological Sleep Parameters in Athletes with Poor Pre-Competition Sleep Quality. Life 2025, 15, 905. https://doi.org/10.3390/life15060905
Tsou Y-A, Hung B-L, Chang W-D. Comparing the Effects of Cranial Electrotherapy Stimulation and Cognitive Behavioral Therapy for Insomnia on Daily Mood and Physiological Sleep Parameters in Athletes with Poor Pre-Competition Sleep Quality. Life. 2025; 15(6):905. https://doi.org/10.3390/life15060905
Chicago/Turabian StyleTsou, Yung-An, Bao-Lien Hung, and Wen-Dien Chang. 2025. "Comparing the Effects of Cranial Electrotherapy Stimulation and Cognitive Behavioral Therapy for Insomnia on Daily Mood and Physiological Sleep Parameters in Athletes with Poor Pre-Competition Sleep Quality" Life 15, no. 6: 905. https://doi.org/10.3390/life15060905
APA StyleTsou, Y.-A., Hung, B.-L., & Chang, W.-D. (2025). Comparing the Effects of Cranial Electrotherapy Stimulation and Cognitive Behavioral Therapy for Insomnia on Daily Mood and Physiological Sleep Parameters in Athletes with Poor Pre-Competition Sleep Quality. Life, 15(6), 905. https://doi.org/10.3390/life15060905








