1. Introduction
Heat stress (HS) is a critical environmental stressor that impacts human and animal well-being globally [
1], with particularly pronounced effects in tropical and subtropical climatic zones [
2]. This complex physiological challenge arises from the synergistic interaction of multiple environmental parameters, predominantly encompassing elevated ambient temperature, increased relative humidity, solar radiation intensity, and airflow velocity. Among these, high temperature and high humidity are the main factors influencing the heat stress response. Environmental conditions characterized by high temperatures and humidity can lead to reduced reproductive performance, diminished animal welfare, decreased fertility, increased susceptibility to diseases, and, in extreme cases, elevated mortality rates [
3]. Heat stress can also trigger physiological responses in animals, such as reduced feed intake, water loss, body weight reduction, elevated rectal temperature, and increased respiratory and heart rates [
4]. However, the molecular mechanisms underlying the perception and regulation of temperature fluctuations are still unclear.
The heart is a highly heat-stress-sensitive organ. Under heat stress, excessive reactive oxygen species (ROS) are generated in the hearts of animals. The overproduction of ROS continuously attacks macromolecules, such as nucleic acids, proteins, and lipids, within cardiomyocytes, disrupting their structure and function while also inhibiting the biological activity of antioxidant enzymes. This leads to oxidative stress damage in cardiomyocytes and, in severe cases, may even result in cardiomyocyte apoptosis and necrosis [
5]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a pivotal transcriptional regulator that governs cellular redox balance and constitutes a fundamental defense mechanism against oxidative damage. Under normal physiological conditions, Nrf2 forms a complex with Kelch-like ECH-associated protein 1 (Keap1) in the cytosol, undergoing subsequent degradation through the ubiquitin-proteasome system mediated by Keap1, thereby maintaining low levels of expression. However, under oxidative stress conditions, elevated levels of ROS oxidize key cysteine residues in Keap1, leading to the inactivation of the Keap1-Cul3 complex and thereby inhibiting the ubiquitination and degradation of Nrf2 [
6]. Subsequently, Nrf2 undergoes nuclear translocation and associates with antioxidant response elements (AREs), thereby activating the expression of downstream genes encoding crucial antioxidant enzymes, such as glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) [
7]. Hayes et al. demonstrated that activation of the Keap1-Nrf2 signaling pathway upregulates the expression of GSH (Glutathione), SOD, and CAT [
8].
Heat stress significantly affects the small intestine (duodenum, jejunum, and ileum) and gut microbiota in rats. Studies have shown that heat stress triggers significant architectural remodeling of the intestinal morphology, manifesting as marked villus atrophy and concurrent crypt hyperplasia in the duodenal mucosa. These structural alterations are accompanied by the induction of oxidative stress and inflammatory responses, ultimately compromising the intestinal digestive capacity and nutrient absorption efficiency [
9]. In the jejunum, heat stress suppresses the activity of digestive enzymes (such as lactase and sucrase), reduces the efficiency of nutrient digestion, and induces epithelial cell apoptosis, further compromising its absorptive capacity. Furthermore, heat stress significantly inhibits the secretory function of goblet cells, leading to thinning of the intestinal mucus layer and weakening of its physical barrier against pathogenic bacteria [
10]. Goblet cells are a critical component of the intestinal epithelium and are responsible for secreting mucus to form the mucus layer, which protects the intestinal epithelium from pathogens and toxins [
11]. Heat stress reduces both the number and function of goblet cells, resulting in decreased mucus layer thickness. This increases the likelihood of direct contact between pathogens and epithelial cells, further exacerbating intestinal inflammation and damage [
10]. In the ileum, heat stress manifests as a reduction in beneficial bacteria (such as
Lactobacillus and
Bifidobacterium) and an increase in opportunistic pathogens (such as
Salmonella), leading to microbial dysbiosis and localized inflammatory responses [
12]. Additionally, heat stress significantly reduces gut microbiota diversity and inhibits the production of short-chain fatty acids (SCFAs), thereby weakening the protective effects of these bacteria on intestinal epithelial cells [
13,
14,
15]. Therefore, mitigating the negative impacts of heat stress on the small intestine and gut microbiota may be a crucial strategy for improving overall health.
Modern medical research has shown that the heart supplies oxygen and nutrients to the small intestine through blood circulation, while the nutrients absorbed by the small intestine enter the liver via the portal vein system and are subsequently distributed throughout the body. Cardiac dysfunction may reduce intestinal blood flow, leading to intestinal ischemia and impaired barrier function, which can result in bacterial and endotoxin translocation, exacerbating systemic inflammation and increasing the cardiac burden [
16]. Tang et al. found that myocardial infarction may trigger a systemic inflammatory response, impair intestinal barrier function, and increase intestinal permeability. The entry of intestinal endotoxins into the bloodstream may further aggravate cardiac inflammation and injury [
17]. The gut microbiota, as endogenous microorganisms of the small intestine, is closely associated with small intestinal dysfunction. Gut microbiota dysbiosis can impair the small intestine’s ability to properly “transform and transport” nutrients, thereby affecting blood circulation and contributing to the development of cardiovascular diseases [
18].
Currently, the common approaches to managing heat stress include rapid cooling, fluid replacement, and pharmacological interventions [
19]. Studies have shown that effective antioxidants can mitigate heat-stress-induced damage. However, current strategies for mitigating heat stress are constrained, and effective therapeutic interventions are notably deficient. Therefore, identifying effective natural remedies to prevent and alleviate heat stress is crucial. Sheng Mai San (SMS), a classic formulation for replenishing qi and nourishing yin, is clinically used to treat critical conditions such as heatstroke, heart failure, liver failure, and shock, as well as chronic diseases like chronic bronchitis, tuberculosis, and diabetes, demonstrating favorable therapeutic outcomes. However, the mechanisms underlying the effects of Sheng Mai San on heat stress-induced cardiac and intestinal injuries and gut microbiota dysbiosis remain unclear. Therefore, this study established a heat stress model to investigate the regulatory mechanisms of Sheng Mai San on the heart-small intestine-gut microbiota axis under heat stress.
2. Materials and Methods
2.1. Reagents
The BCA protein concentration assay kit (Catalog No.: PC0020) and tissue cytoplasm/nucleus separation kit (Catalog No.: EX2660) were purchased from Solarbio (Beijing, China). CAT (Catalog No.: A007-2-1), SOD (Catalog No.: A001-3-2), GSH (Catalog No.: A006-2-1), T-AOC (Total Antioxidant Capacity) (Catalog No.: A015-2-1), and MDA (Malondialdehyde) (Catalog No.: A003-1-2) assay kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Antibodies against Nrf2 (Catalog No.: 16396-1-AP) and Keap1 (Catalog No.: 10503-2-AP) were acquired from Proteintech (Wuhan, China). Enzyme-linked immunosorbent assay (ELISA) kits for inter-leukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China).
2.2. Preparation of Sheng Mai San
Sheng Mai San was prepared following standardized traditional Chinese medicine decoction protocols as previously described [
20,
21]. The herbal components of Sheng Mai San (
Panax ginseng C.A. Mey.,
Ophiopogon japonicus (
L.f.)
Ker-Gawl., and
Schisandra chinensis (
Turcz.)
Baill.) in a 3:3:2 ratio were pulverized and sieved through a 20-mesh screen, followed by soaking in 10 volumes of distilled water for 30 min. The mixture was decocted by rapid boiling and subsequent simmering for 60 min, filtered, and then refluxed with 8 volumes of distilled water for 40 min. After combining the filtrates, the solution was centrifuged at 3000 rpm for 15 min, concentrated under reduced pressure at 60 °C to 1.0 g crude herb/mL, and lyophilized to obtain the final freeze-dried powder.
2.3. Establishment of a Rat Model of Heat Stress
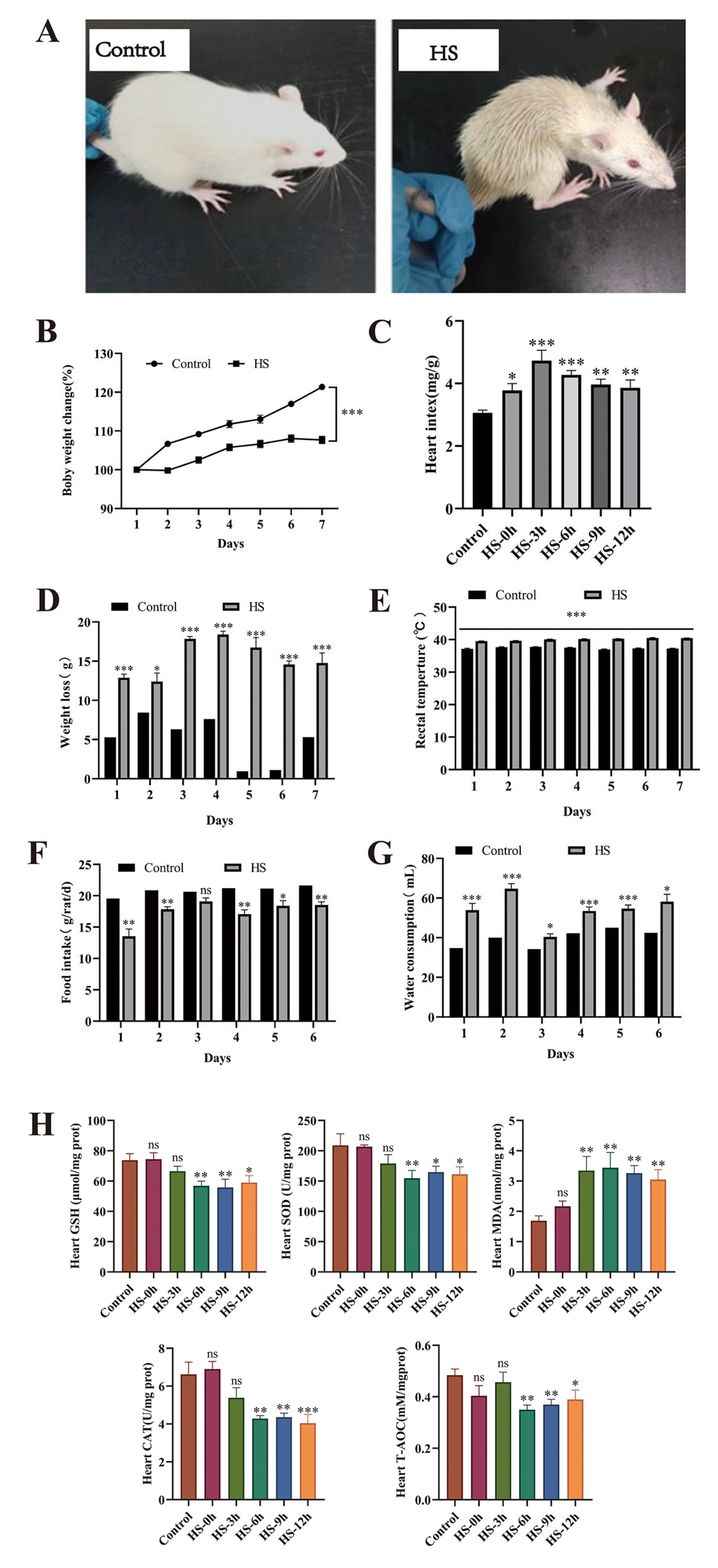
Forty-eight male SPF-grade healthy Sprague-Dawley (SD) rats (body weight 200 ± 20 g) were obtained from the Lanzhou Veterinary Research Institute of the Chinese Academy of Agricultural Sciences [Laboratory Animal License No.: SCXK (Gan) 2020-0002] and acclimatized for 7 days in a standard facility (temperature 23 ± 1 °C, relative humidity 45 ± 5%, 12 h/12 h light/dark cycle) with free access to food and water. Prior to the experiments, baseline body weight was recorded, and core temperature was measured using a rectal thermometer (inserted 2 cm deep). The heat stress (HS) model was established according to a previously published protocol [
22]. Rats were exposed to 38 ± 1 °C and 75 ± 5% relative humidity in an artificial climate chamber for 2 h daily (10:00–12:00) for 7 consecutive days, with food and water deprivation during HS sessions. Body weight and temperature were measured immediately after each HS session before returning the animals to the standard conditions. Following the final HS exposure, samples were collected at 0, 3, 6, 9, and 12 h time points under anesthesia induced by intraperitoneal injection of 2% sodium pentobarbital.
2.4. Drug Intervention Treatment with Sheng Mai San
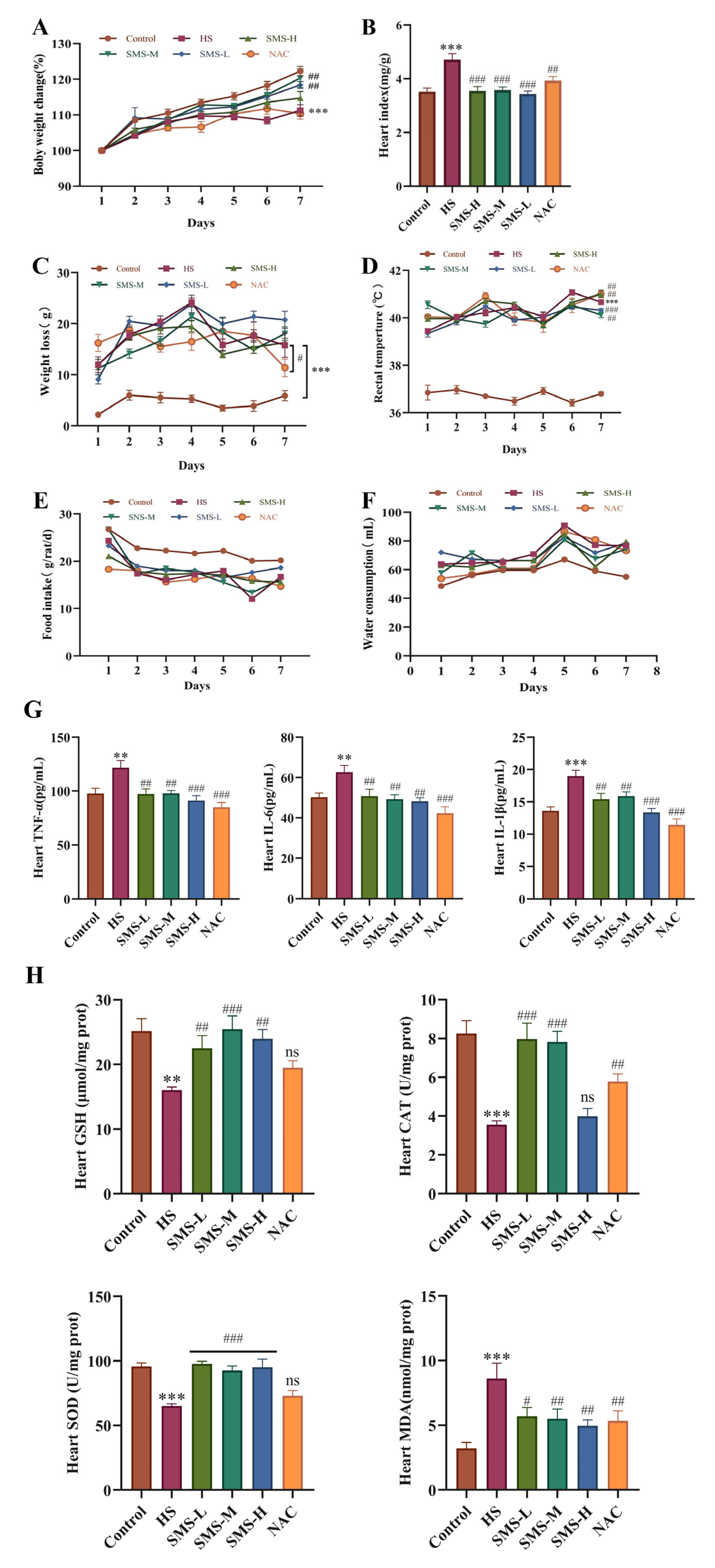
Sixty SD rats were divided into six groups and administered treatments by gavage 2 h before exposure to daily heat stress. The control and HS groups were administered a normal saline solution daily. The dosage of Sheng Mai San for rats was calculated based on human clinical equivalent dose conversion using the formula Dr = (Dh/W) × F, where Dr represents the rat dose (g/kg), Dh is the human clinical dose (g), W is the standard human body weight (60 kg), and F is the species conversion factor (6.3) for rat-to-human dose translation according to body surface area normalization principles. The Sheng Mai San intervention groups were administered SMS-H (5.04 g/kg), SMS-M (2.52 g/kg), and SMS-L (1.26 g/kg). Additionally, a positive control group received N-acetylcysteine (NAC) (150 mg/kg) [
23]. The experimental rats were administered the treatment once a day for 7 days. The control group was maintained at an ambient temperature of 23 ± 1 °C and relative humidity of 45 ± 5%. The heat stress model and treatment groups were subjected to heat stress conditions for 7 consecutive days, as per the established model, with sampling conducted 6 h after the final heat stress session.
2.5. Behavioral and Physiological Observations in Rats
Behavioral changes in rats were observed before and after exposure to heat stress. Body weight and body temperature were recorded for each group before and after heat stress, along with daily food intake and the amount of water consumed.
2.6. Measurement of Heart Index
After collecting blood from the abdominal aorta, the heart was quickly excised, the surface moisture was absorbed, and the heart weight was recorded. The cardiac index was calculated as the ratio of the whole heart weight (HW) to the body weight (BW).
2.7. Determination of CAT, SOD, GSH, T-AOC, and MDA Levels in the Heart
Heart tissue was collected, rinsed with PBS, immediately snap-frozen in liquid nitrogen, and preserved at −80 °C for further biochemical analysis. A 10% heart tissue homogenate was prepared and centrifuged at 3000× g for 15 min at 4 °C. The protein concentration of the samples was determined using a BCA assay kit. The cardiac tissue concentrations of CAT, SOD, GSH, T-AOC, and MDA were quantified according to the manufacturer’s protocols for the respective commercial assay kits.
2.8. Determination of IL-1β, IL-6, and TNF-α Levels in the Heart
The concentrations of IL-1β, IL-6, and TNF-α in the cardiac tissue were determined in strict accordance with the manufacturer’s protocols provided with the respective assay kits.
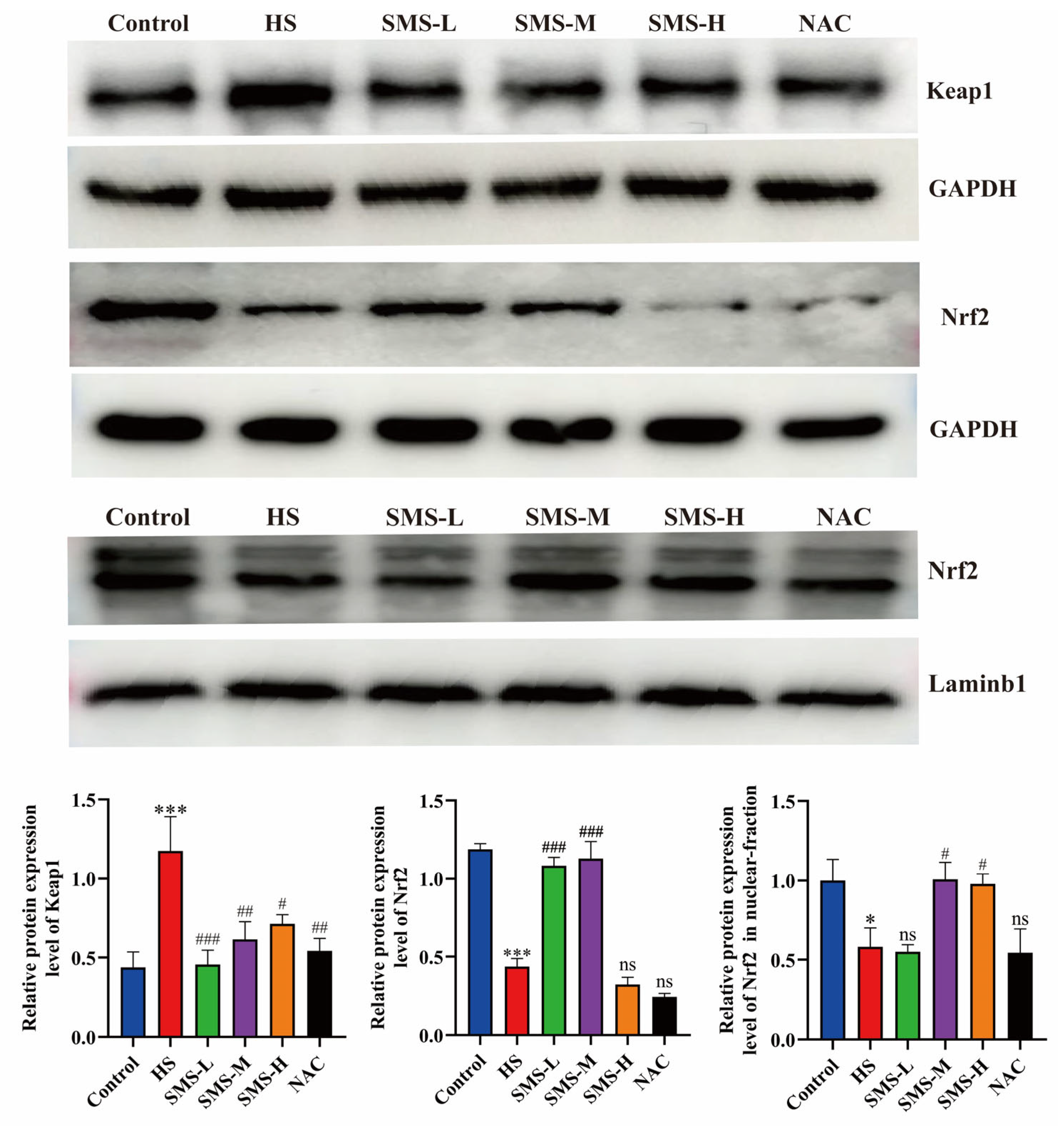
2.9. Western Blotting Analysis
2.9.1. Determination of Total Protein Content in the Heart
Total protein was extracted from rat heart tissue using RIPA lysis buffer, and the protein concentrations were determined using a BCA assay kit. The loading amounts for each sample were subsequently calculated based on the quantified protein concentrations. SDS-PAGE gels were prepared, and electrophoresis was performed at 80 V for 40 min, followed by 100 V for 80 min. Proteins were transferred to PVDF membranes at 200 mA for 90 min. The membranes were subjected to a 2 h blocking procedure at ambient temperature, subsequently incubated with specific primary antibodies targeting Keap1 (1:3000 dilution), Nrf2 (1:3000 dilution), and GAPDH (1:2000 dilution) at 4 °C for overnight incubation. The membranes were subjected to extensive washing with TBST for ten cycles, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies at ambient temperature for 1 h, and subsequently washed thoroughly with TBST for an additional ten cycles. Protein bands were visualized using an ECL detection reagent, and images were captured. The relative expression levels of the target proteins were quantified by densitometric analysis using ImageJ software (v1.8.0; National Institutes of Health, Bethesda, MD, USA).
2.9.2. Determination of Nuclear Protein Content in the Heart
Approximately 50 mg of heart tissue was weighed and homogenized in 500 μL of reagent A containing protease inhibitors at −10 °C for 20 min. The homogenate was then oscillated at 4 °C for 30 min and centrifuged at 1200×
g and 4 °C for 5 min. The resulting supernatant, representing the cytoplasmic fraction, was carefully aspirated and transferred to a pre-cooled sterile centrifuge tube for subsequent analysis. The remaining pellet was reconstituted in ice-cold PBS and centrifuged at 2000×
g for 5 min at 4 °C, followed by complete removal of the supernatant. The remaining pellet, representing the nuclear fraction, was resuspended in 200 μL of preservation buffer B, and the protein concentration was determined using a BCA assay kit. The nuclear translocation of Nrf2 in the heart was measured as described in
Section 2.9.1.
2.10. Histopathological Observation
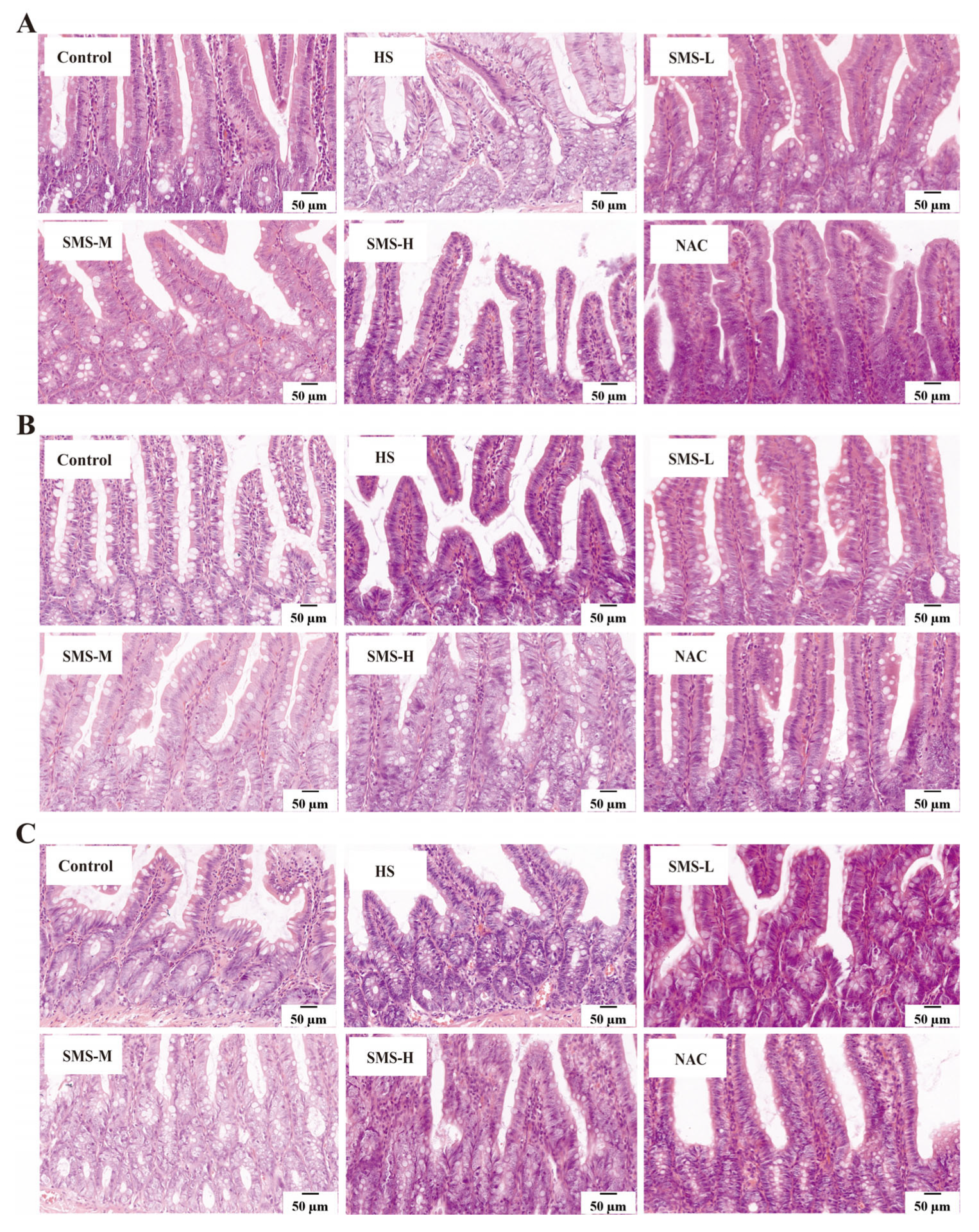
2.10.1. HE (Hematoxylin and Eosin) Staining
The duodenum, jejunum, and ileum tissues were immersed in 10% neutral formalin and rinsed under running water for 24 h. Subsequent dehydration, embedding, and sectioning were performed using an automatic tissue processor, yielding sections with a thickness of 4 μm. The sections were then baked at 60 °C for 4 h. Dewaxing of the sections was carried out using xylene I, xylene II, benzene-alcohol, and a graded series of ethanol (100% ethanol I, 100% ethanol II, 90% ethanol I, 90% ethanol II, and 80% ethanol). The tissue sections were stained with hematoxylin for 5 min and subsequently washed with continuous running deionized water for 15 min to ensure complete removal of excess stain. Differentiation was performed using a differentiating solution, and the sections were rinsed again with running water for 15 min. Eosin staining was performed for 15 min, after which the sections were gradually dehydrated in ascending concentrations of alcohol and cleared. Finally, the sections were mounted. A digital slice scanner (DX1; Shandong, China) was used to collect pictures and observe the damage to the duodenum, jejunum, and ileum tissues.
2.10.2. AB-PAS Staining (Alcian Blue-Periodic Acid-Schiff Staining)
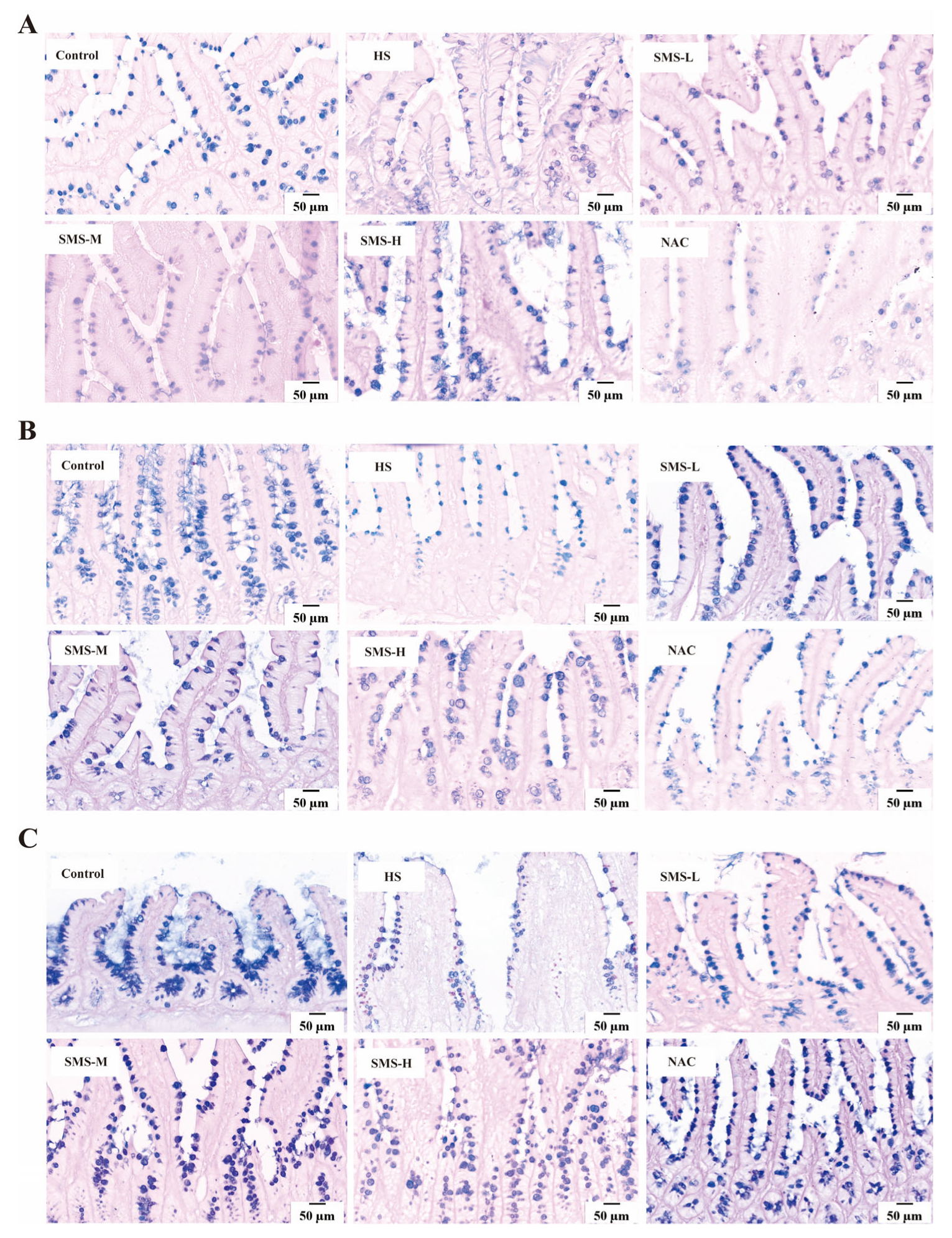
The tissue sections underwent standard dewaxing procedures, followed by a 15-min rinse under continuous running deionized water. Subsequently, the sections were stained with Alcian Blue for 15 min, gently washed with distilled water, treated with 1% periodic acid solution for 5 min, and an additional 10-min distilled water rinse. The sections were then incubated with Schiff’s reagent for 15 min and thoroughly rinsed under running deionized water for 10 min. Finally, the sections were processed through a graded ethanol series for dehydration, cleared in xylene, and permanently mounted using a synthetic resin. Images were collected using a digital slice scanner (DX1; Shandong, China), and the number of goblet cells and mucin secretion in the duodenum, jejunum, and ileum were observed.
2.11. High-Throughput Sequencing Analysis of the Gut Microbiota
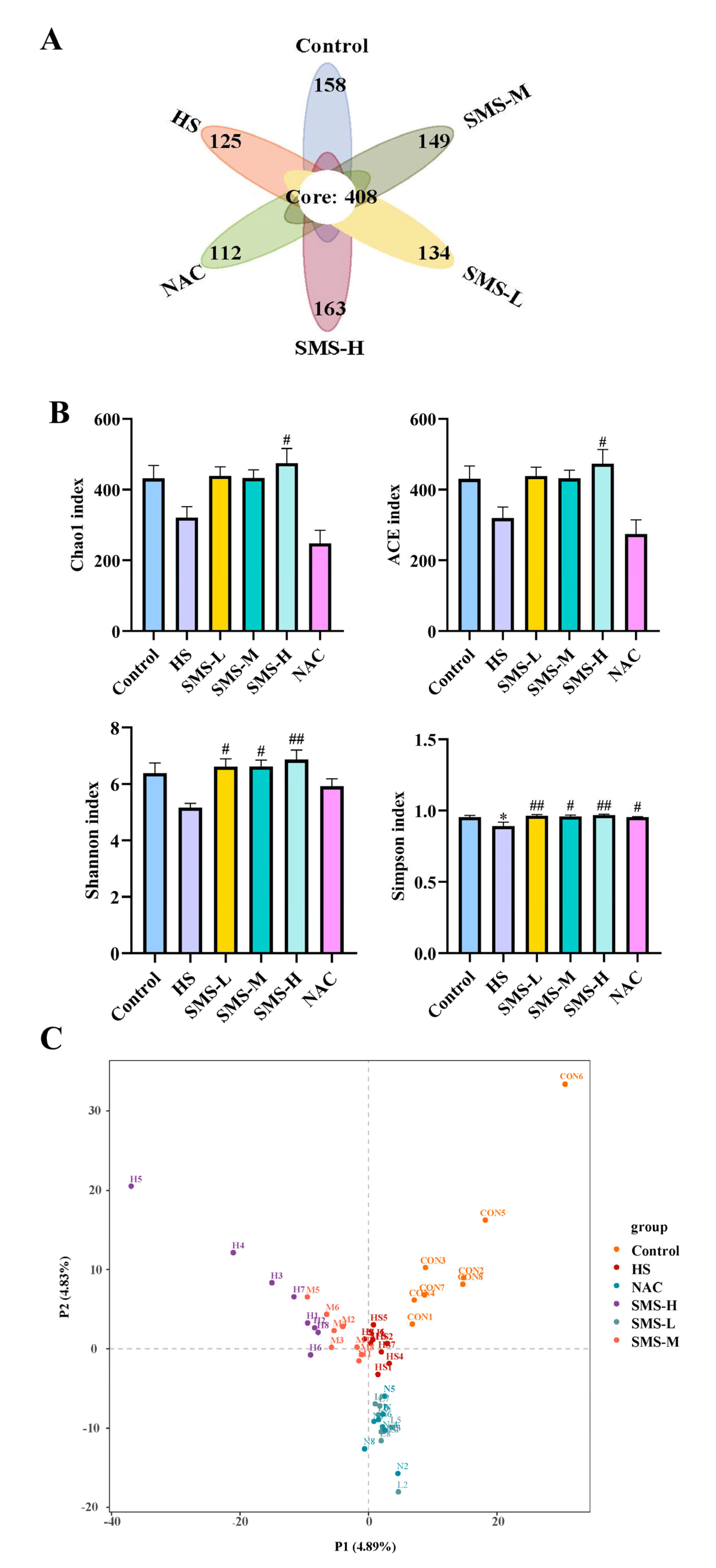
Upon completion of the experimental protocol, fecal specimens were collected from the rat subjects. Genomic DNA was extracted using a kit with bead-beating lysis (3 × 60 s) and RNase A treatment, followed by quality verification. The V3–V4 region of bacterial 16S rRNA genes was amplified using primers 341F/805R under standardized PCR conditions, followed by AMPure XP bead purification and Illumina Nextera XT indexing. The libraries were sequenced using an Illumina NovaSeq 6000 platform. Raw FASTQ data were processed in QIIME2 through (1) quality filtering and denoising (DADA2 for ASV generation), (2) taxonomic classification against the SILVA 138 database, and (3) diversity analyses (alpha: Shannon/Chao1; beta: weighted UniFrac PCoA). Differential microbial analysis was performed using one-way ANOVA.
2.12. Statistical Analysis
The experimental results are expressed as the mean ± standard error (SEM). Statistical analyses were performed using SPSS software (version 26.0; IBM Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) was used for multiple comparisons, while independent two-sample t-tests were applied for comparisons between two groups. Graphs were generated using GraphPad Prism 9 software. A p-value of <0.05 was considered statistically significant.
4. Discussion
Heat stress can disrupt thermoregulatory mechanisms, leading to a series of physiological and behavioral changes [
24]. Our study found that heat stress resulted in reduced body weight, food intake, and water consumption in rats, which may be associated with imbalances in energy metabolism and increased protein breakdown [
25]. Under high-temperature conditions, rats reduce heat-producing activities to lower their body temperature, and since feeding and digestion processes are accompanied by increased heat production, rats instinctively decrease their food intake. Reduced food intake leads to insufficient energy intake, while the metabolic rate increases to dissipate heat, resulting in higher energy expenditure. Additionally, high temperatures can diminish thirst sensations in rats, suppressing their drinking behavior. Furthermore, heat stress, a common environmental stressor, has been shown to significantly affect multiple organ systems, with the heart being particularly vulnerable due to its central role in maintaining circulatory and metabolic homeostasis. Studies have indicated that heat stress-induced cardiac injury primarily occurs within 6–12 h of heat stress exposure, a critical time window characterized by significant changes in cardiac function and biochemical markers. Specifically, this period is marked by a notable increase in the cardiac index, decline in key antioxidants (such as GSH, SOD, CAT, and T-AOC), and elevated levels of the lipid peroxidation product MDA. These changes collectively suggest that oxidative stress and impaired antioxidant defense mechanisms play pivotal roles in heat-stress-induced cardiac injury. It was found that during the early stages (within 6 h) of heat stress, the cardiac index increased significantly. This elevation may reflect a compensatory mechanism to meet the heightened metabolic demands and increased circulatory load caused by heat stress (Gathiram et al., 1987) [
26]. However, this compensatory mechanism is limited, and prolonged elevation of the cardiac index can lead to myocardial injury and deterioration of cardiac function [
26]. Antioxidant enzymes, which are capable of scavenging or neutralizing free radicals and reactive oxygen species, play a major role in maintaining intracellular redox balance and protecting against oxidative damage. Among these, SOD, CAT, and GPx are critical components of the antioxidant enzyme system [
27]. The experimental results revealed that heat stress exposure induced a significant suppression of SOD and GPx enzymatic activities in porcine myocardial tissue, concurrent with a marked elevation in MDA levels, indicative of enhanced lipid peroxidation (McCord et al., 1969) [
28]. Although heat stress rapidly induces excessive generation of ROS, the peak effects of oxidative stress are often delayed. The investigation revealed that 6 h post-heat stress exposure, significant elevations in LDH levels were observed in both serum and myocardial tissues of murine models, concomitant with a pronounced reduction in SOD activity in myocardial tissues. Parallel in vitro experiments substantiated these findings, demonstrating that heat-stressed cells exhibited marked alterations in cellular viability, antioxidant capacity (as reflected by SOD and GSH activities), ROS accumulation, and LDH release (Wang et al., 2024) [
29], consistent with our research findings. These changes collectively demonstrate that oxidative stress and impaired antioxidant defense mechanisms play critical roles in heat stress-induced cardiac injury.
According to traditional Chinese (veterinary) medicine, the heart governs blood circulation and supports small intestine function through heart yang (warming) and heart blood (nourishing). The small intestine digests food, absorbs nutrients, and transports them to the heart for blood production. These organs are interconnected as exterior-interior pairs, meaning that dysfunction in one can affect the other. For example, heart fire can cause small intestine heat symptoms, while small intestine heat may lead to heart-related issues [
30]. Sheng Mai San, a classic formulation for replenishing qi and nourishing yin, is clinically used to treat cardiovascular diseases, diabetes, liver injury, heatstroke, and shock [
31,
32,
33,
34,
35,
36]. However, research on Sheng Mai San for mitigating heat stress injury is limited. In our study, we intervened in heat-stressed rats with Sheng Mai San and found that it reduced the cardiac index, thereby alleviating the cardiac load. It significantly increased the levels of GSH, SOD, and CAT, while decreasing MDA content in the heart. Additionally, Sheng Mai San reduced the levels of IL-1β, IL-6, and TNF-α in the heat-stressed hearts. These results indicate that Sheng Mai San effectively mitigates heat-stress-induced cardiac injury by enhancing antioxidant defense mechanisms and suppressing inflammatory responses. The Keap1-Nrf2 pathway is a critical regulatory mechanism that enables cells to combat oxidative stress. Under normal conditions, Keap1 binds to Nrf2, promoting its ubiquitination and degradation, thereby maintaining low levels of Nrf2. The heart is a high-energy-demand organ that is highly sensitive to oxidative stress. Heat stress leads to the accumulation of ROS in cardiomyocytes, triggering apoptosis, inflammation, and fibrosis. According to our research, heat stress inhibits the expression of the Keap1-Nrf2 pathway in the heart. Additionally, we found that Sheng Mai San intervention activated the Keap1-Nrf2 pathway, promoting the nuclear translocation of Nrf2, thereby enhancing antioxidant capacity and mitigating cardiac injuries.
Heat stress not only directly causes cardiac injury but may also further induce small intestinal damage through the “heart-gut axis” mechanism. Cardiac injury induced by heat stress leads to decreased cardiac function, which subsequently affects systemic blood circulation, resulting in insufficient intestinal blood perfusion and triggering intestinal ischemia and hypoxia [
37]. Furthermore, inflammatory cytokines and oxidative stress mediators released during cardiac injury can systemically disseminate through the circulatory system, thereby exacerbating intestinal mucosal damage and potentiating pathological alterations in the small intestine [
38]. According to our research, the duodenum, jejunum, and ileum of rats can sustain pathological damage from heat stress. This damage is mostly characterized by edema, villus shedding, unclear epithelial cell shape, and a decrease in goblet cell count. However, intervention with Sheng Mai San significantly mitigated heat-stress-induced pathological damage in the rat small intestine. Using HE staining, Zhang et al. observed that heat stress caused severe damage to the small intestinal epithelium. Compared to the control tissues, heat-stressed tissues exhibited detachment of epithelial cells from the basement membrane at the villus tips. At higher magnification, vacuolization of epithelial cells was also observed, with severe cases showing exposed lamina propria [
39]. This disruption of barrier function provides an opportunity for the colonization and proliferation of pathogenic bacteria while potentially inhibiting the growth of beneficial bacteria, leading to intestinal dysbiosis [
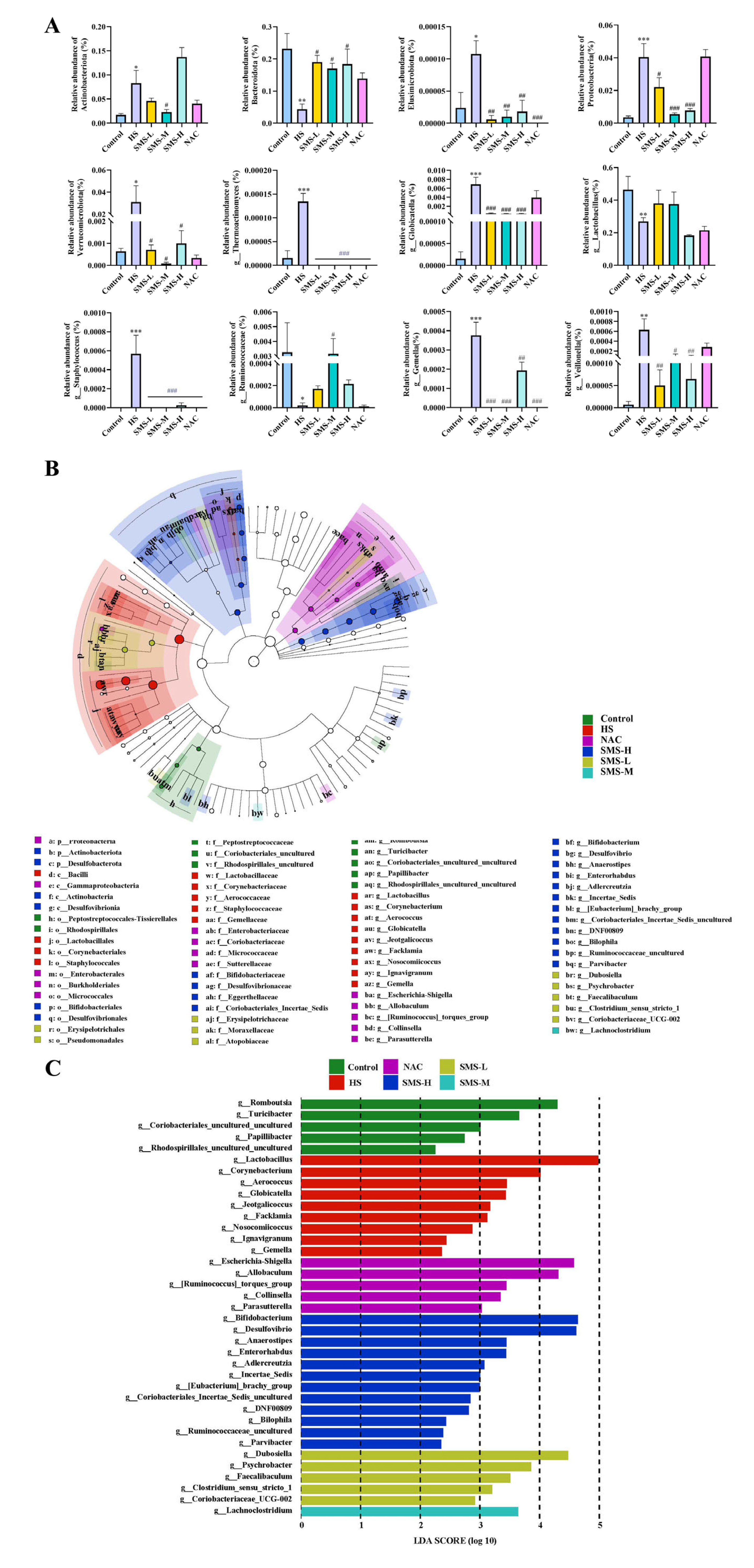
40]. Our results demonstrated that heat-stressed rats exhibited significant reductions in microbial alpha-diversity indices, including Chao1, ACE, Shannon, and Simpson indices, suggesting that heat stress may reduce the diversity and richness of the gut microbiota in rats. Through differential microbiota analysis, it was revealed that heat stress resulted in increased expression of harmful bacteria, such as
g_Globicatella,
g_Thermoactinomyces,
g_Staphylococcus,
g_Gemella, and
g_Veillonella, and decreased expression of
g_Lactobacillus and
g_Ruminococcaceae. However, these bacterial populations were partially restored after intervention with Sheng Mai San. The overproliferation of
g_Globicatella,
g_Gemella,
g_Staphylococcus, and
g_Veillonella may exacerbate systemic inflammation by producing pro-inflammatory metabolites, thereby promoting the development of cardiac diseases, such as atherosclerosis and endocarditis. These findings reveal that cardiac and intestinal diseases mutually influence each other, and that there is a close association between gut microbiota and cardiac health. This underscores the importance of maintaining gut microecological balance for the prevention and treatment of cardiovascular diseases and provides new research directions for future interventions in cardiovascular diseases through the modulation of gut microbiota. Furthermore, in patients with inflammatory bowel disease (IBD),
g_Staphylococcus and
g_Veillonella are significantly elevated, and they can disrupt intestinal epithelial cells and the mucus layer, increasing intestinal permeability and leading to bacterial and endotoxin translocation, which further exacerbates systemic inflammation [
41,
42].
g_Lactobacillus, a crucial probiotic in the gut, plays a vital role in maintaining intestinal barrier function, exerting antioxidant effects, regulating immune responses, and improving microbial balance [
43]. We observed a significant reduction in
g_Lactobacillus abundance in heat-stressed rats. Heat stress also led to a notable decrease in
g_Ruminococcaceae, a key fiber-degrading bacterium in the gut that ferments dietary fiber to produce SCFAs, such as butyrate, acetate, and propionate. These microbial metabolites are vital for maintaining intestinal homeostasis and the integrity of the mucosal barrier. Future research should further explore the specific mechanisms of
g_Thermoactinomyces under heat stress conditions and its impact on the host. These findings demonstrate that Sheng Mai San alleviates heat stress-induced cardiac injury, small intestinal pathological changes, and gut microbiota dysbiosis through multi-target and multi-pathway mechanisms, providing a scientific basis for the application of traditional Chinese medicine in modern medicine.