Physiological Response of Citrus reticulata Blanco var. Gonggan Seedlings to High-Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Determination of Physiological Parameters in Gonggan Seedlings
2.2.1. Experimental Design
2.2.2. Detection of Membrane Permeability in Gonggan Leaves
2.2.3. Determination of Chlorophyll Content
2.2.4. Determination of MDA Content
2.2.5. Determination of POD Activity
2.2.6. Determination of Photosynthetic Metrics
2.3. Statistical Analysis
3. Results
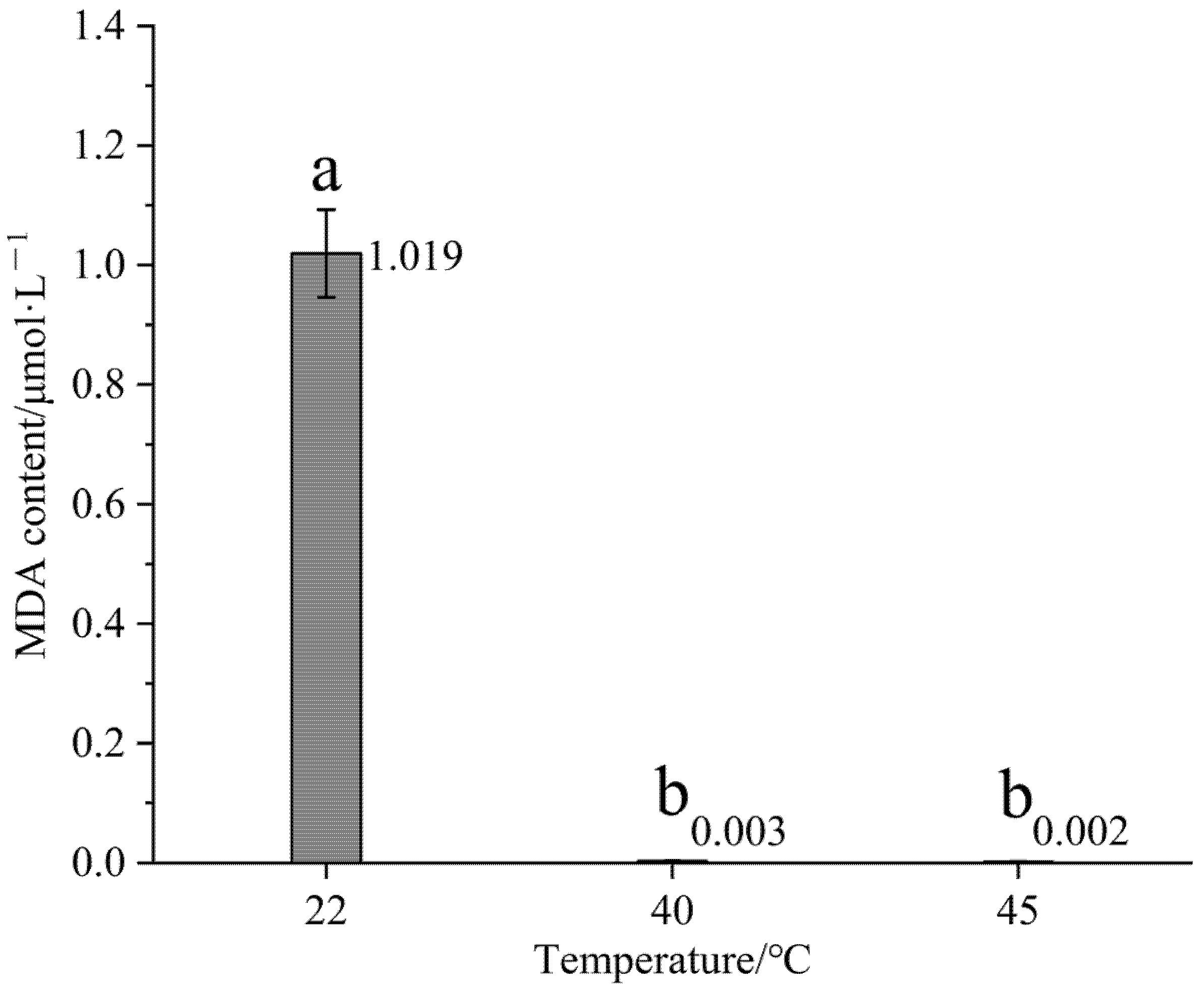
3.1. Effect of High-Temperature Stress on MDA Content
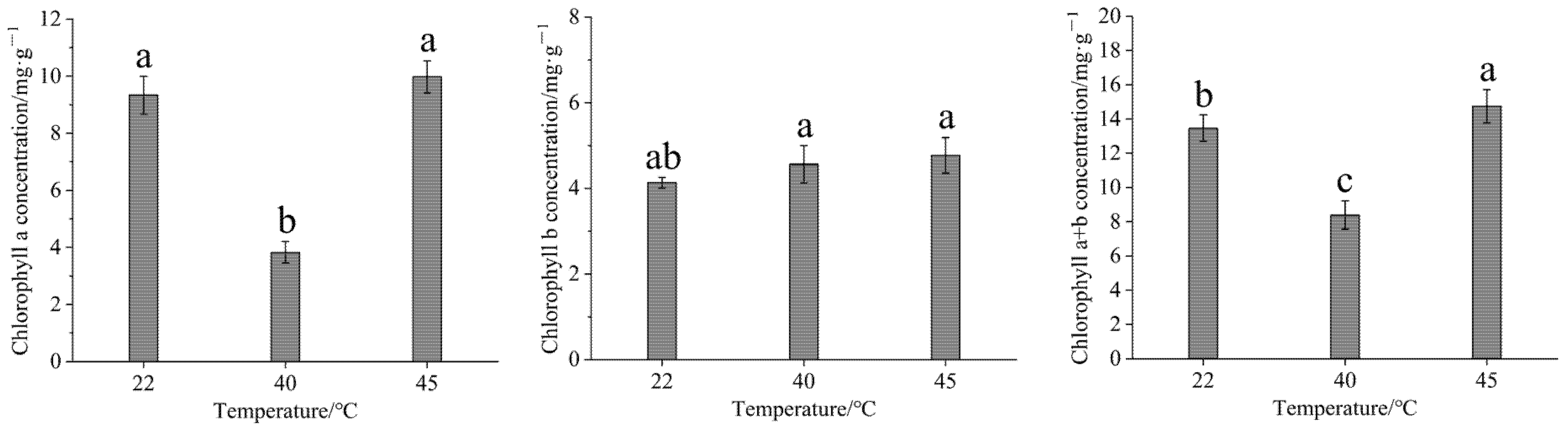
3.2. Effect of High-Temperature Stress on Chlorophyll Content
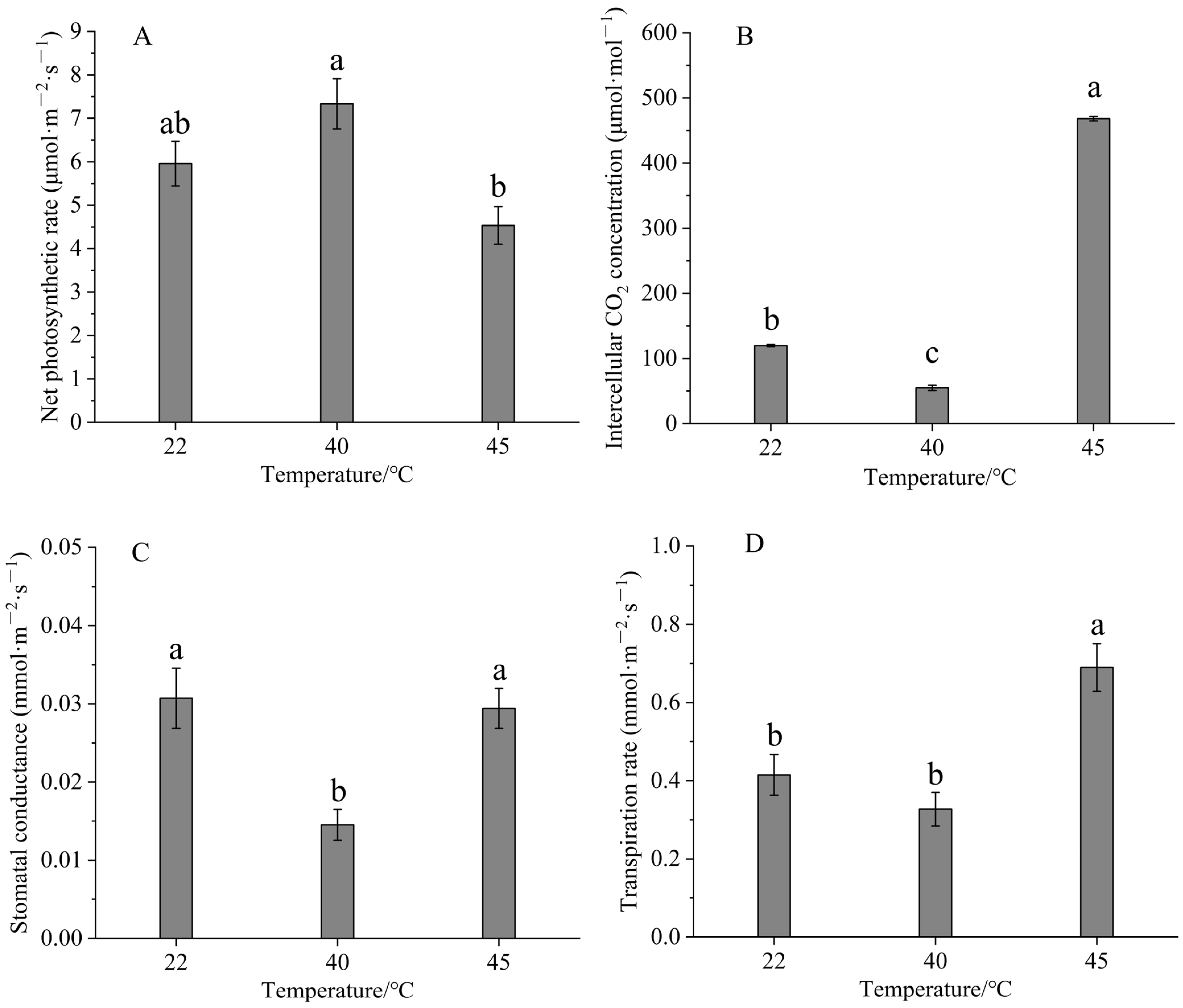
3.3. Effects of High-Temperature Stress on Photosynthetic Metrics
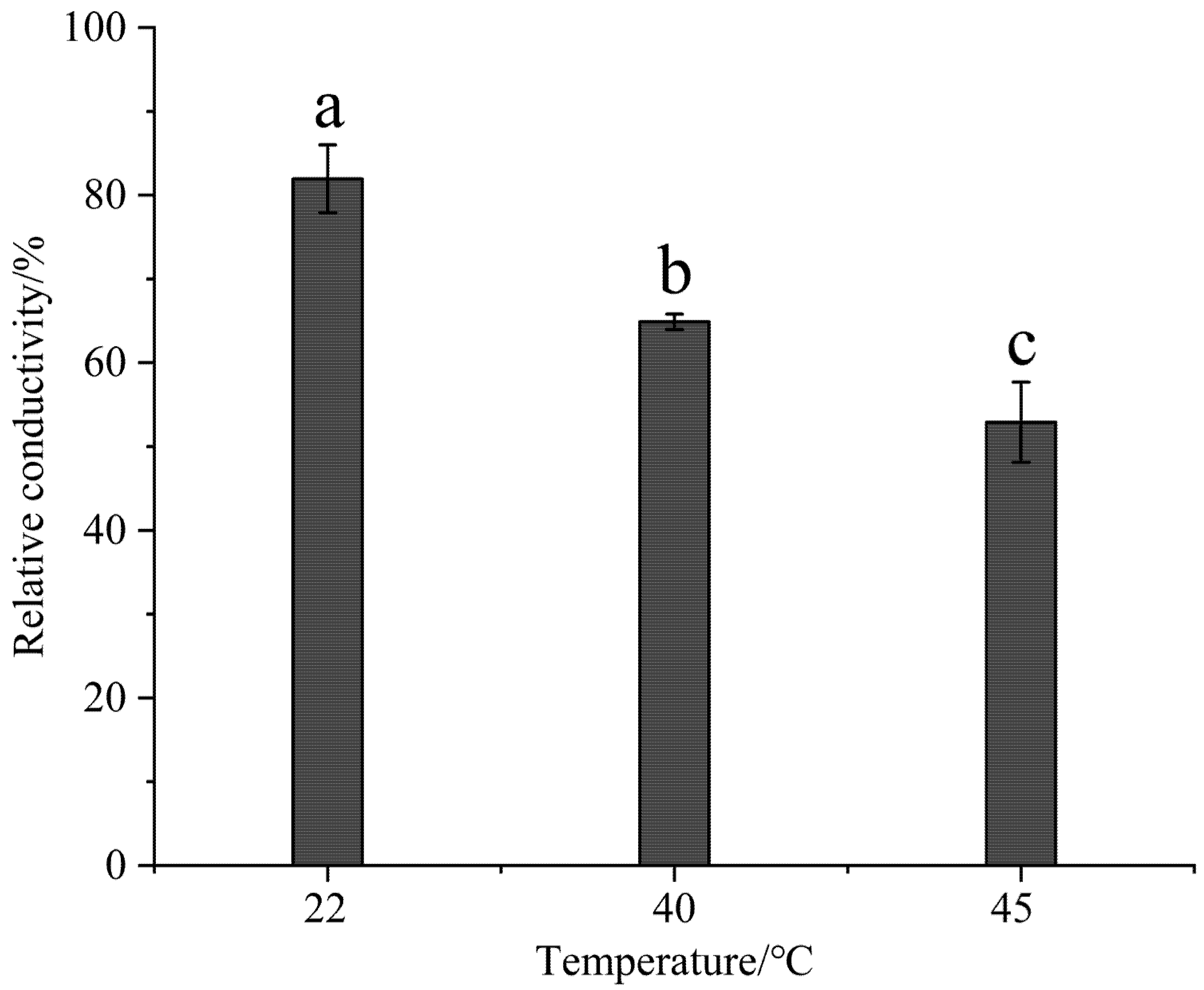
3.4. Effect of High-Temperature Stress on the Cytoplasmic Membrane Permeability
3.5. Effect of High-Temperature Stress on POD Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, C.; Gu, M.; Huang, Y.; You, C.; Bao, S.; Xie, S.; Gong, J.; Deng, G.; Wu, P.; Wu, W.; et al. Integrated genomic and transcriptomic analysis reveals the mechanisms underlying leaf variegation in ‘Gonggan’ mandarin. BMC Plant Biol. 2025, 25, 472. [Google Scholar] [CrossRef]
- Liu, D.J.; Shen, Q.M.; Lin, K.W.; Wang, F.; Bu, Z.B.; Peng, J.; Brennan, C.; Benjakul, S.; Xiao, G.S.; Ma, L.K. The aroma profiles of dried gonggans: Characterization of volatile compounds in oven-dried and freeze-dried gonggan. Food Res. Int. 2024, 191, 114716. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.M.; Chao, M. Studies on Extraction of Flavonoids from Tribute orange Peels and its Antibacterial Activities. Food Ind. 2013, 34, 21–23. [Google Scholar]
- Liu, J.; Zhang, R.; Xu, X.; Fowler, J.C.; Miller, T.E.X.; Dong, T. Effect of summer warming on growth, photosynthesis and water status in female and male Populus cathayana: Implications for sex-specific drought and heat tolerances. Tree Physiol. 2020, 40, 1178–1191. [Google Scholar] [CrossRef]
- Akter, N.; Islam, M.R. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 37. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef]
- Zhang, X.X.; Cai, J.; Wollenweber, B.; Liu, F.L.; Dai, T.B.; Cao, W.X.; Jiang, D. Multiple heat and drought events affect grain yield and accumulations of high molecular weight glutenin subunits and glutenin macropolymers in wheat. J. Cereal Sci. 2013, 57, 134–140. [Google Scholar] [CrossRef]
- Peng, S.B.; Huang, J.L.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.H.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Xu, J.M.; Henry, A.; Sreenivasulu, N. Rice yield formation under high day and night temperatures—A prerequisite to ensure future food security. Plant Cell Environ. 2020, 43, 1595–1608. [Google Scholar] [CrossRef]
- Wang, W.L.; Cai, C.; He, J.; Gu, J.F.; Zhu, G.L.; Zhang, W.Y.; Zhu, J.G.; Liu, G. Yield, dry matter distribution and photosynthetic characteristics of rice under elevated CO2 and increased temperature conditions. Field Crops Res. 2020, 248, 107605. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Gaiser, T.; Naab, J.; Ewert, F. HS in cereals: Mechanisms and modelling. Eur. J. Agron. 2015, 64, 98–113. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Maestri, E.; Klueva, N.; Perrotta, C.; Gulli, M.; Nguyen, H.T.; Marmiroli, N. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol. Biol. 2002, 48, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Ruiz, J.M.; Garcia, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Yang, H.; Tang, Y.; Liu, X.; Liu, B.; Hu, X.; Hu, Z. Quantifying High-Temperature-Induced Injury in Nanfeng Tangerine Plants: Insights from Photosynthetic and Biochemical Mechanisms. Agronomy 2024, 14, 648. [Google Scholar] [CrossRef]
- Talanova, V.V.; Akimova, T.V.; Titov, A.F. Effect of whole plant and local heating on the ABA content in cucumber seedling leaves and roots and on their heat tolerance. Russ. J. Plant Physiol. 2003, 50, 90–94. [Google Scholar] [CrossRef]
- Larkindale, J.; Huang, B.R. Thermotolerance and antioxidant systems in Agrostis stolonifera: Involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide and ethylene. J. Plant Physiol. 2004, 161, 405–413. [Google Scholar] [CrossRef]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhang, Y.C.; Niu, S.C.; Hao, L.H.; Yu, W.B.; Chen, D.F.; Xiang, D.Y. Response of Dahlia Photosynthesis and Transpiration to High-Temperature Stress. Horticulturae 2023, 9, 1047. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Liu, H.; Meng, H.; Cheng, Z. Physiological Responses of Cucumber Seedlings to Combined High-Temperature and High-Humidity Stress at Different Leaf Stages. Horticulturae 2024, 10, 1369. [Google Scholar] [CrossRef]
- Jiang, N.; Yang, Z.; Zhang, H.; Xu, J.; Li, C. Effect of Low Temperature on Photosynthetic Physiological Activity of Different Photoperiod Types of Strawberry Seedlings and Stress Diagnosis. Agronomy 2023, 13, 1321. [Google Scholar] [CrossRef]
- Hong, E.; Xia, X.; Ji, W.; Li, T.; Xu, X.; Chen, J.; Chen, X.; Zhu, X. Effects of High Temperature Stress on the Physiological and Biochemical Characteristics of Paeonia ostii. Int. J. Mol. Sci. 2023, 24, 11180. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.Y.; Zeng, P.S.; Cui, J.B.; Zhang, Y.T.; Yang, J.J.; Zhu, L.J.; Hu, H.L.; Xu, J. Physiological and gene expression changes of Cryptomeria fortunei Hooibrenk families under heat stress. Front. Plant Sci. 2023, 14, 1083847. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.C.; Kitto, D.; Kamcev, J. Accurately measuring the ionic conductivity of membranes via the direct contact method. J. Membr. Sci. 2023, 669, 121304. [Google Scholar] [CrossRef]
- Brignot, H.; Rayot, C.; Buiret, G.; Thomas-Danguin, T.; Feron, G. Determination of the optimal method for measuring malondialdehyde in human saliva. MethodsX 2025, 14, 103070. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.S.; Gao, X.F. A New Method for Accurate Determination of Peroxidase Activity Based on Fluorescence Decrease of Guaiacol. Chin. J. Anal. Chem. 2015, 43, 1040–1046. [Google Scholar]
- Sato, K. Abiotic Stress Biology in Horticultural Plants; Springer: Tokyo, Japan, 2015; pp. 77–86. [Google Scholar] [CrossRef]
- Morales, M.; Munne-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Luan, Q.F.; Jiang, J.M.; Li, Y.J. Prediction and Utilization of Malondialdehyde in Exotic Pine Under Drought Stress Using Near-Infrared Spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef]
- Xing, Y.H.; Lu, H.Y.; Zhu, X.F.; Deng, Y.F.; Xie, Y.J.; Luo, Q.H.; Yu, J.S. How Rice Responds to Temperature Changes and Defeats Heat Stress. Rice 2024, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Li, G.; Huang, J.; Zheng, Y. Physiological Response of Bitter Gourd Seedlings to High Temperature Stress and Preliminary Evaluation of Heat Resistance. Guangdong Agric. Sci. 2023, 50, 155–164. (In Chinese) [Google Scholar]
- Jing, H.D.; Zhao, L.; Shi, X.H.; Du, L.J. Heat tolerance of nine Helleborus orientlis cultivars. J. Northwest A F Univ. (Nat. Sci. Ed.) 2023, 51, 112–122. (In Chinese) [Google Scholar]
- Haddaji, D.; Bousselmi, L.; Saadani, O.; Nouairi, I.; Ghrabi-Gammar, Z. Enzymatic degradation of azo dyes using three macrophyte species: Arundo donax, Typha angustifolia and Phragmites australis. Desalination Water Treat. 2015, 53, 1129–1138. [Google Scholar] [CrossRef]
- Chang, C.F.; Guo, L.J.; Yu, H.; Hang, Y.M.; Zhu, J.L.; Wu, R.H. Leaf Morphology and Physiological Response of Four Hebes Varieties Under High Temperature Stress. North. Hortic. 2024, 11, 56–63. (In Chinese) [Google Scholar]
- Ye, B.Y.; Li, X.Q.; Chi, Y.; Jin, P.; Jin, S.H. Effects of High Temperature Stress on Photosynthesis and Antioxidant System of Carya cathayensis. Mol. Plant Breed. 2024, 22, 4018–4024. (In Chinese) [Google Scholar]
- Yang, L.; Yang, Z.Q.; Lu, S.Y.; Zhang, Y.D.; Zheng, H. Effect Mechanism of High Temperature and High Humidity Stress on Yield Formation of cucumber. Chin. J. Agrometeorol. 2022, 43, 392–407. (In Chinese) [Google Scholar]
- Xue, J.P.; Wang, X.; Zhang, A.M.; Chang, L. Changes of photosynthesis parameters and chlorophyll fluorescence around Sprout tumble of Pinellia ternata under high temperature stress. China J. Chin. Mater. Medica 2010, 35, 2232–2235. (In Chinese) [Google Scholar]
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Kausar, A.; Siddique, K.H.M.; Farooq, M. Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Jiang, Y.H.; Qiu, R.J.; Gong, X.W.; Agathokleous, E.; Hu, W.; Clothier, B. Heat stress decreased transpiration but increased evapotranspiration in gerbera. Front. Plant Sci. 2023, 14, 1119076. [Google Scholar] [CrossRef]
- Wu, X.M.; Yang, M.; Zhou, X.P.; Kuang, R.B.; Liu, C.H.; He, H.; Xu, Z.; Wei, Y.R. Effects of Different Concentrations of Melatonin on the Physiological Characteristics of Strawberry Seedlings under High temperature Stress. Biotechnol. Bull. 2025, 41, 181–189. (In Chinese) [Google Scholar]


| Characters | No | Min | Max | Ave | STDEV |
|---|---|---|---|---|---|
| Width | 36 | 9.00 | 17.00 | 13.08 | 2.38 |
| Length | 36 | 39.00 | 105.00 | 68.75 | 14.20 |
| Number of leaves | 36 | 6.00 | 14.00 | 8.67 | 1.57 |
| Plant height | 36 | 5.00 | 8.80 | 6.14 | 0.95 |
| Leaf area | 36 | 1.13 | 4.55 | 2.59 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Liao, J.; Ye, C.; Chen, S.; Wang, Y.; Zhang, X.; Huang, J.; Chen, C. Physiological Response of Citrus reticulata Blanco var. Gonggan Seedlings to High-Temperature Stress. Life 2025, 15, 806. https://doi.org/10.3390/life15050806
Wu S, Liao J, Ye C, Chen S, Wang Y, Zhang X, Huang J, Chen C. Physiological Response of Citrus reticulata Blanco var. Gonggan Seedlings to High-Temperature Stress. Life. 2025; 15(5):806. https://doi.org/10.3390/life15050806
Chicago/Turabian StyleWu, Shaoping, Jinyan Liao, Chunxing Ye, Shanyi Chen, Yingshan Wang, Xiaochun Zhang, Junwen Huang, and Cong Chen. 2025. "Physiological Response of Citrus reticulata Blanco var. Gonggan Seedlings to High-Temperature Stress" Life 15, no. 5: 806. https://doi.org/10.3390/life15050806
APA StyleWu, S., Liao, J., Ye, C., Chen, S., Wang, Y., Zhang, X., Huang, J., & Chen, C. (2025). Physiological Response of Citrus reticulata Blanco var. Gonggan Seedlings to High-Temperature Stress. Life, 15(5), 806. https://doi.org/10.3390/life15050806







