Connective Tissue Disorder-Induced Diffuse Alveolar Hemorrhage: A Comprehensive Review with an Emphasis on Airway and Respiratory Management
Abstract
1. Introduction
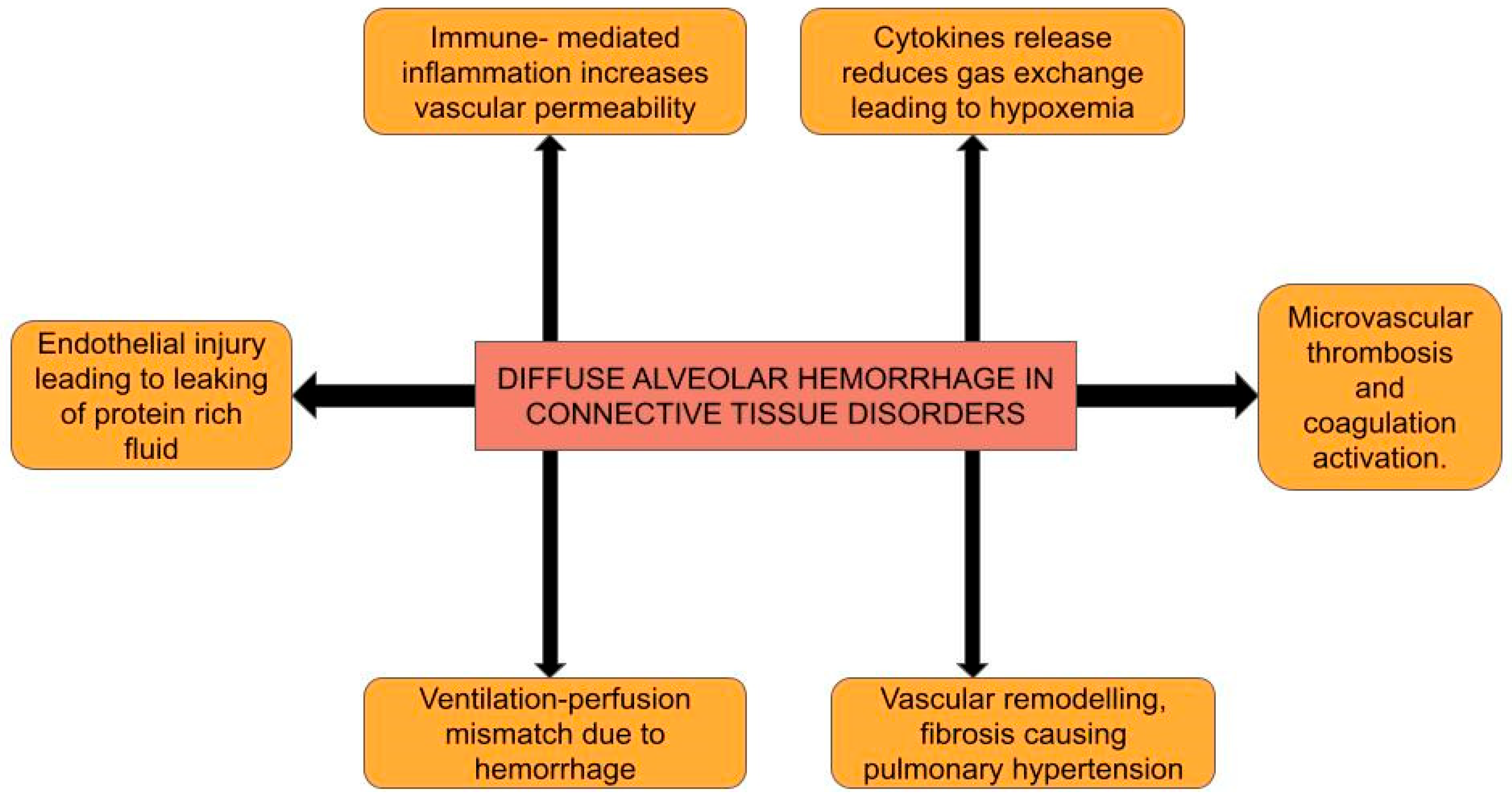
2. Pathophysiology
2.1. Immune Complex-Mediated Endothelial Injury
2.2. Cytokine Release, Signal Transduction, and Endothelial Barrier Disruption
2.3. Macrophage Activation and Polarization
2.4. Chronic Inflammation, Vascular Remodeling, and Fibrosis
3. Clinical Presentation
3.1. Classic Clinical Triad
- Hemoptysis occurs in 60–70% of cases; however, it may be absent in up to one-third of patients, particularly in those with severe anemia or non-massive hemorrhage [2]. Hemoptysis must be differentiated from hematemesis or pseudo-hemoptysis (denoting alveolar flooding that resembles blood, as noted in Serratia marcescens pneumonia, in which the reddish hue of the organism creates an impression of alveolar bleeding [16]).
- Anemia, either normochromic or due to iron deficiency, is characterized by a rapid decline in hemoglobin levels. It is frequently disproportionate to external blood loss [17].
3.2. Respiratory Symptoms
3.3. Systemic Characteristics of CTDs Associated with DAH
- Systemic Lupus Erythematosus (SLE)
- -
- DAH develops in up to 11% of patients with SLE [16].
- -
- Granulomatosis with Polyangiitis (GPA; originally Wegener’s)
- -
- Microscopic Polyangiitis (MPA)
- -
- Rheumatoid Arthritis (RA)
- -
- Anti-phospholipid Syndrome (APS)
- -
- -
- Scleroderma (Systemic Sclerosis)
- -
- While interstitial lung disease is more common, DAH can arise in the presence of renal crisis or pulmonary hypertension [28].
- Polymyositis and Dermatomyositis (PM-DM)
- -
- Pulmonary involvement precedes the muscular manifestations of PM-DM by many years or occurs simultaneously [29].
- Mixed connective tissue disorder
- -
- Approximately 73% of patients have pulmonary involvement, with few developing DAH [30].
4. Radiographic and Diagnostic Features of DAH in Connective Tissue Disorders
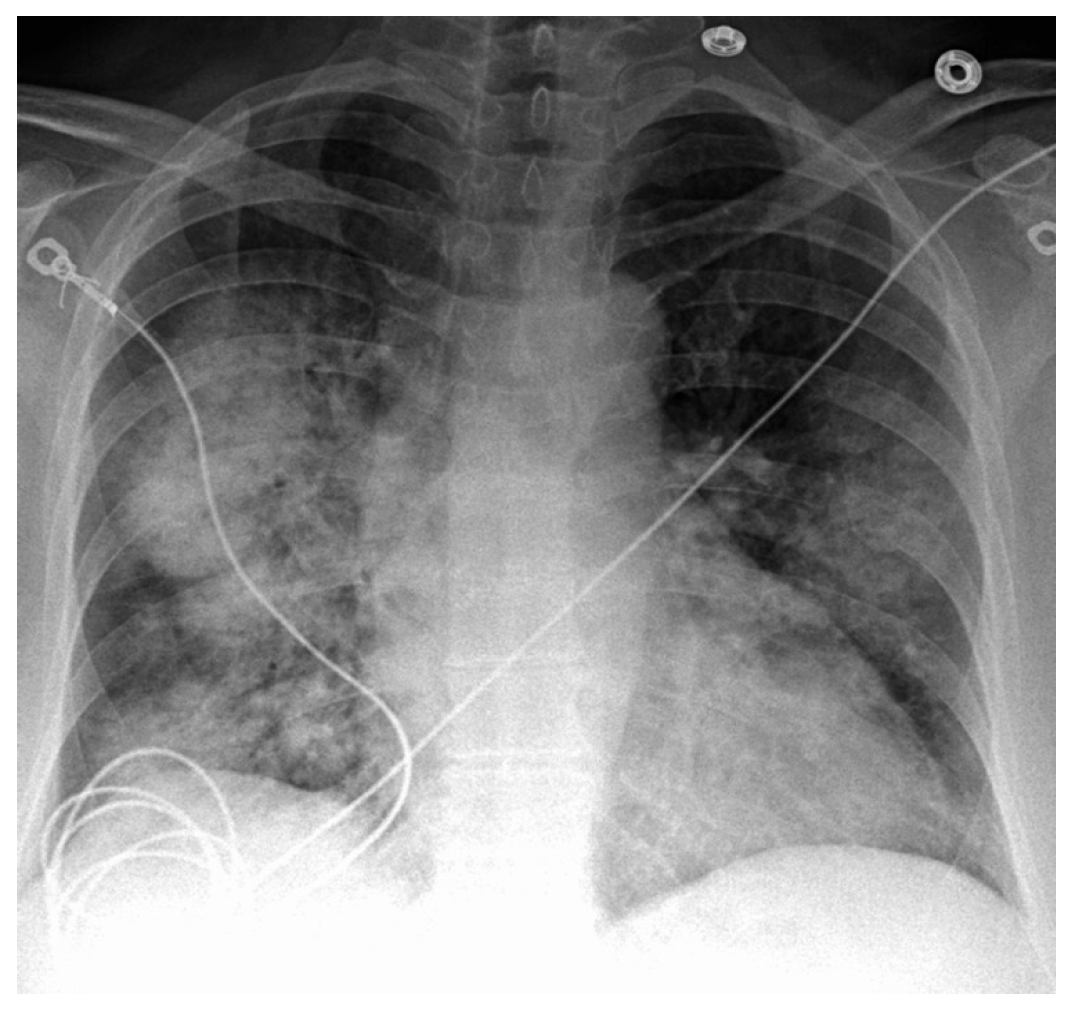
4.1. Chest X-Ray (CXR)
4.2. High-Resolution CT (HRCT) of the Chest
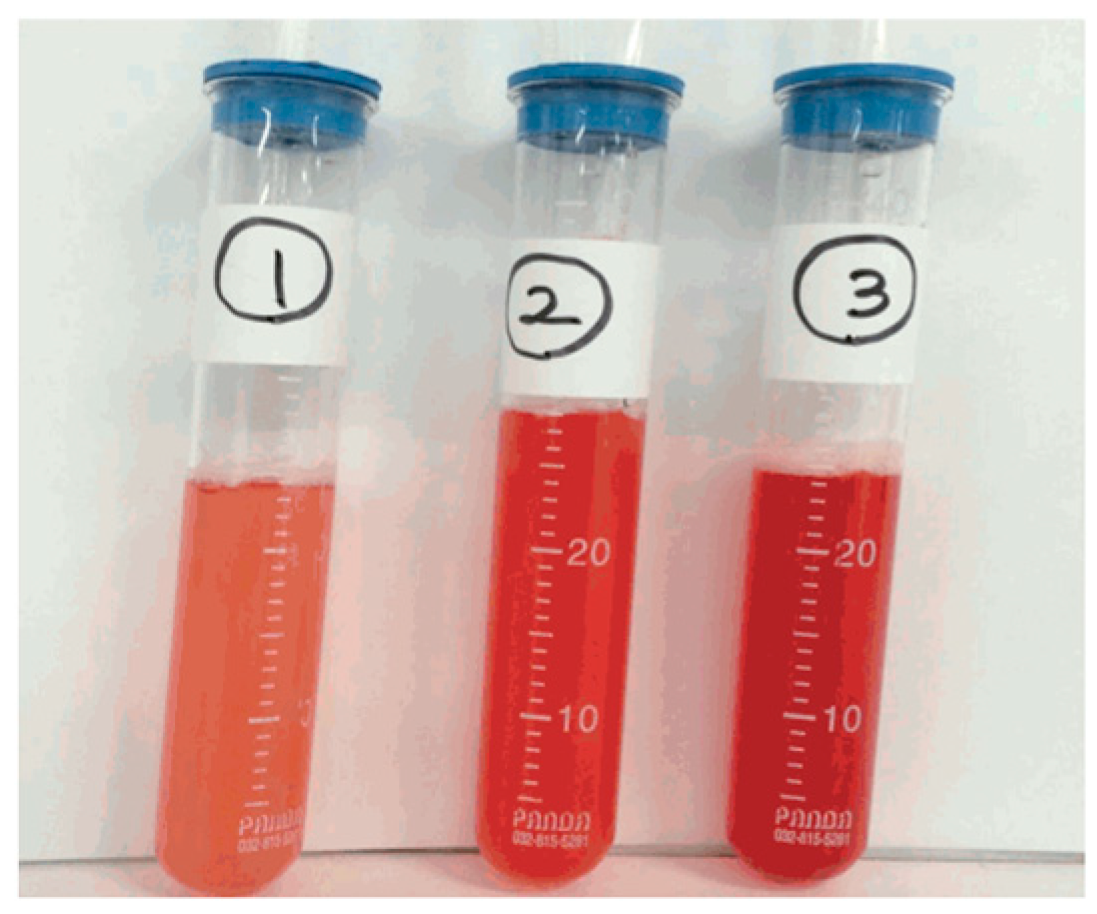
4.3. Bronchoalveolar Lavage (BAL)
4.4. Pulmonary Function Tests (PFTs)
4.5. Autoimmune Serological Analysis
4.6. Histopathological Examination
5. Management
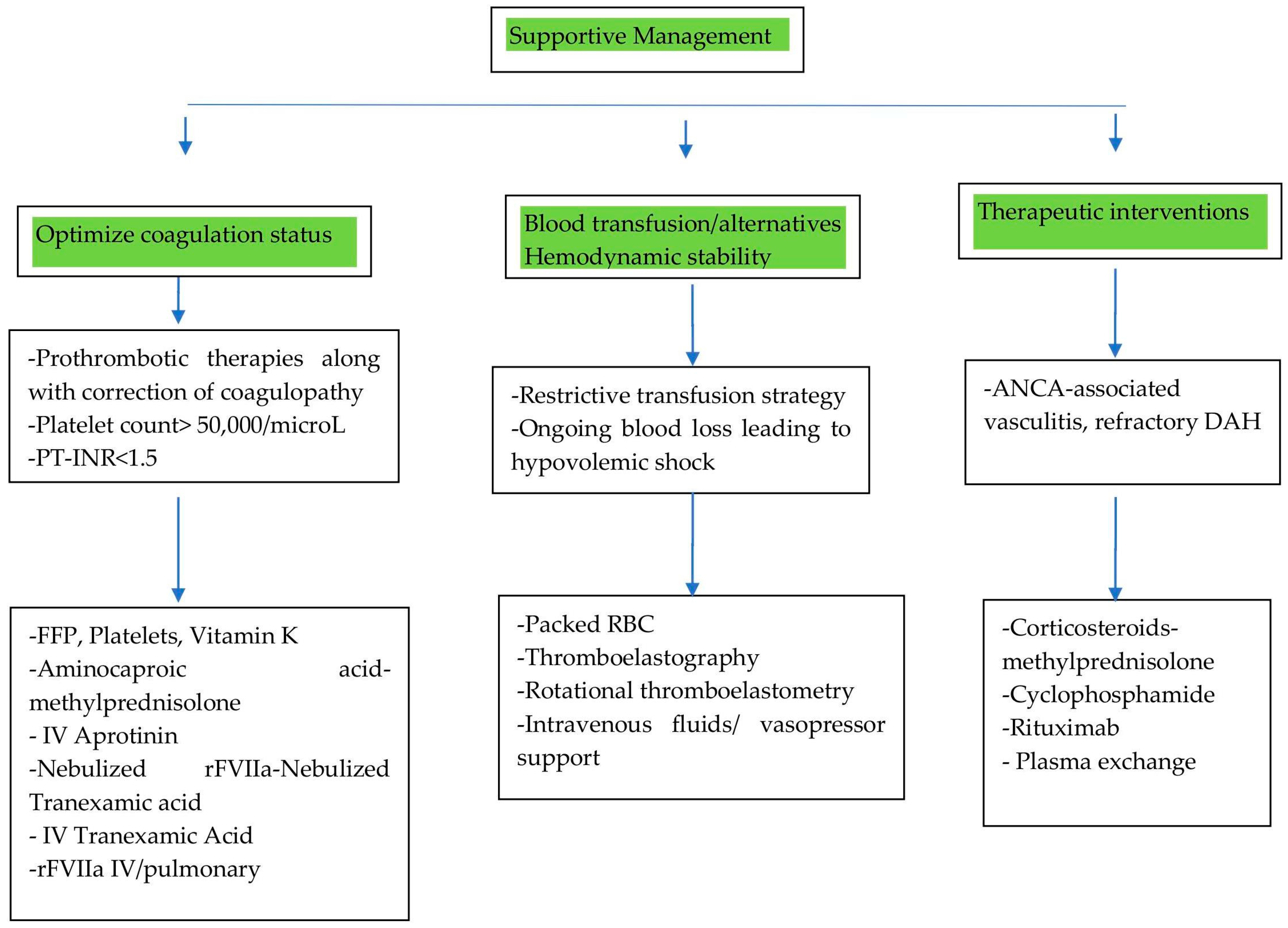
5.1. Supportive Care
5.1.1. Optimizing Coagulation Status
5.1.2. Managing Blood Transfusions: Strategies and Alternatives
5.1.3. Hemodynamic Stability
5.2. Therapeutic Interventions
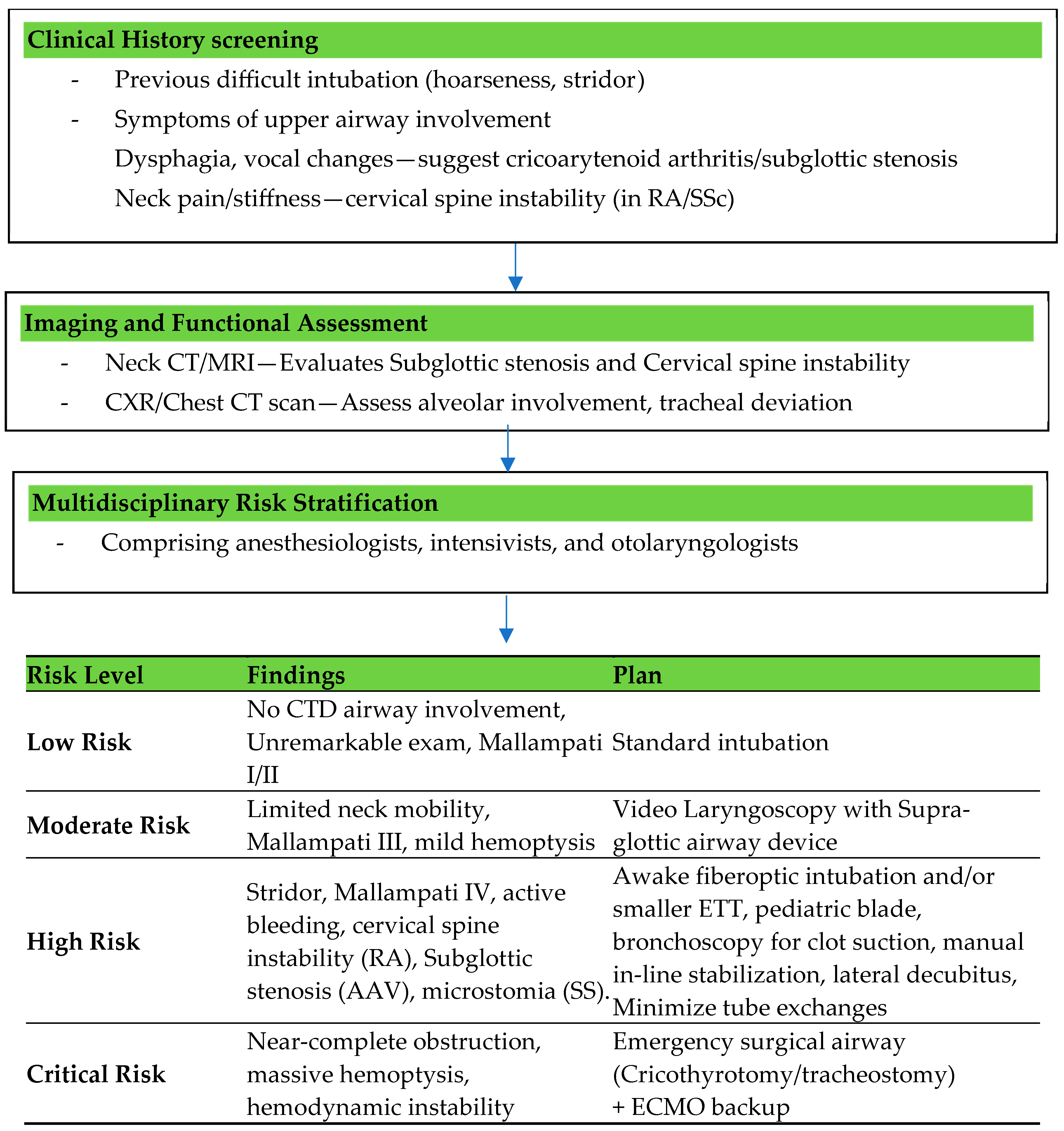
5.3. Airway Assessment
Challenges in Airway Management Specific to Connective Tissue Disorders
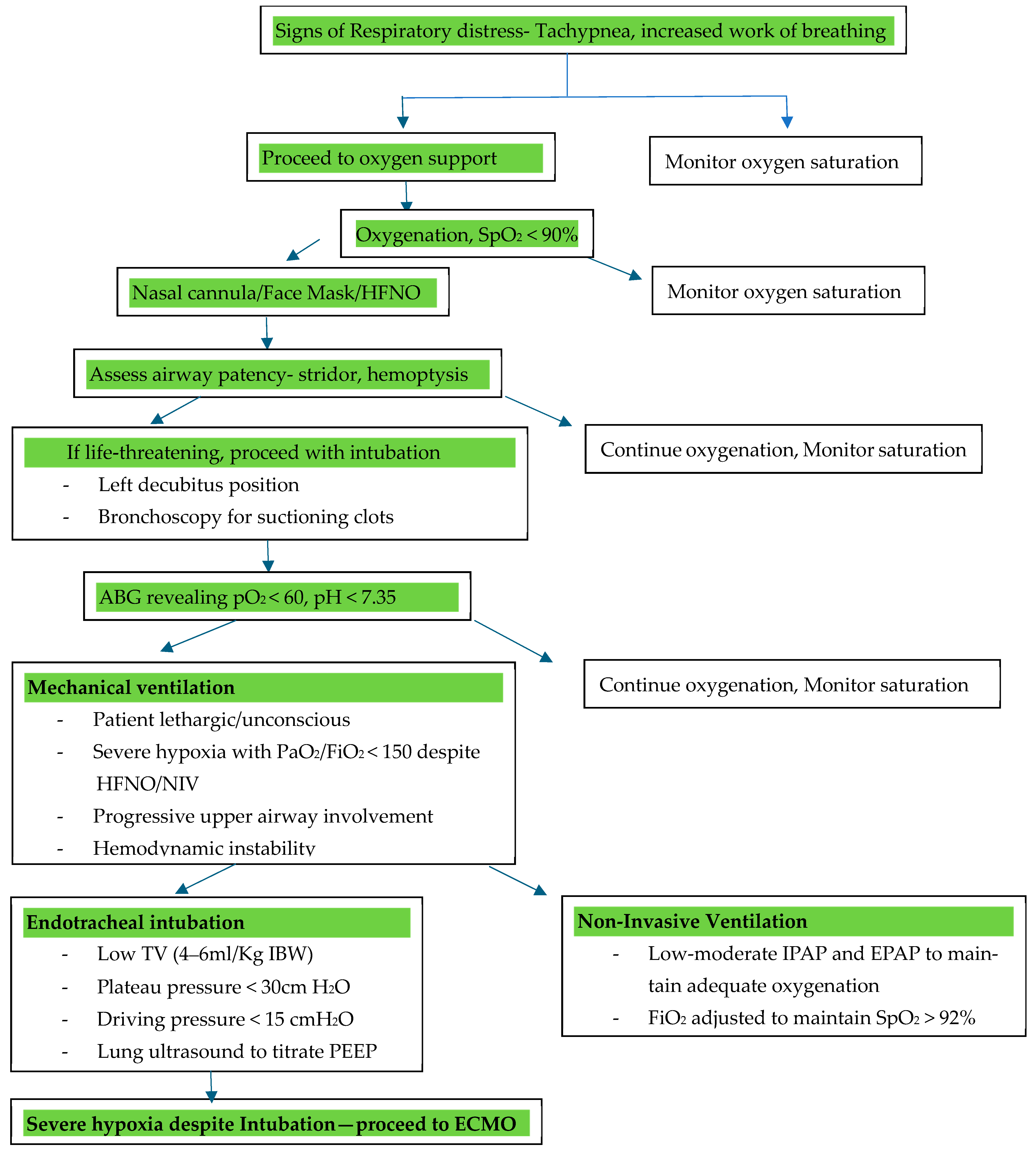
5.4. Oxygenation and Ventilation Strategies
5.4.1. Indications for Non-Invasive Ventilation
- Contraindications for Non-Invasive Ventilation
- Limitations to NIV
5.4.2. Use of High-Flow Nasal Oxygen (HFNO)
5.4.3. Indications for Intubation
- -
- Severe hypoxemia, defined as a PaO2/FiO2 (partial pressure of oxygen in arterial blood by the fraction of inspired oxygen) below 150 despite HFNO or NIV;
- -
- Respiratory failure, characterized by tachypnea greater than 35 breaths per minute, accessory muscle use, paradoxical breathing, or altered mental status due to hypoxia or hypercapnia;
- -
- Hemodynamic instability, including hypotension requiring vasopressor support or worsening shock states secondary to respiratory distress;
- -
- Inability to protect the airway due to altered consciousness, excessive airway bleeding, or progressive upper airway involvement from CTD-associated cricoarytenoid arthritis or subglottic stenosis;
- -
- Failure of non-invasive oxygenation strategies, including NIV intolerance or worsening respiratory parameters despite optimization, prompting consideration for early invasive airway management to prevent further decompensation.
- Invasive Ventilation
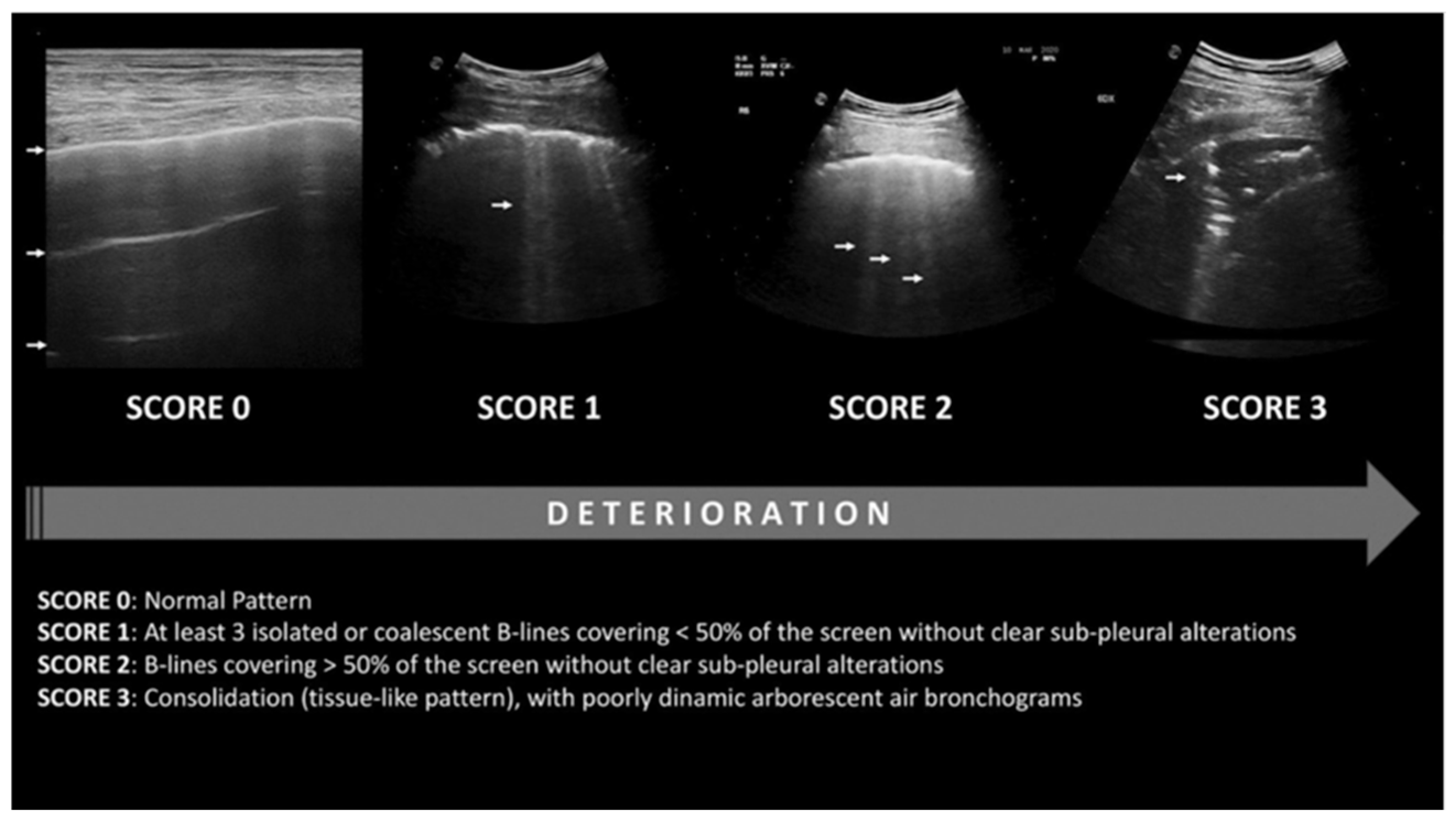
- Role of lung ultrasonography in diagnosing ARDS
- Role of lung ultrasonography in the management of ARDS
- Limitations of lung ultrasound in ARDS/CTD-induced DAH management
- -
- -
- The lung ultrasonography score has a strong correlation with PEEP-induced elevations in end-expiratory lung volume, which reflects enhanced gas entry into already inflated lung regions rather than re-inflation. It correlates with tissue density and lung aeration, but not with alterations in the score resulting from variations in PEEP [85]. This is probably due to the score remaining unaffected by alterations in consolidation size or a reduction in the number of B lines [86];
- -
- Information is contingent upon the operator and necessitates training for accurate interpretation.
5.4.4. Extracorporeal Membrane Oxygenation (ECMO)
- Types of ECMO:
- Veno-venous (VV) ECMO is used for respiratory failure with preserved cardiac function, such as in DAH, where massive pulmonary hemorrhage impairs gas exchange. It bypasses the lungs, supporting oxygenation and carbon dioxide removal. It can provide adequate gas exchange while allowing for lung-protective ventilation and mitigating ventilator-induced injury [83].
- Veno-arterial (VA) ECMO provides both respiratory and cardiac support, typically used in cases of combined heart and lung failure, such as right heart failure secondary to severe pulmonary hypertension in CTD patients [83].
- (1)
- Severe hypoxemia: PaO2/FiO2 < 80 mmHg despite optimal ventilation, including a trial of prone positioning;
- (2)
- Hypercapnia: pH < 7.25 with a partial pressure of carbon dioxide in arterial blood (PaCO2 ≥ 60 mmHg), despite conventional mechanical ventilation (with a respiratory rate of 35 bpm and a plateau pressure ≤ 30 cmH2O);
- (3)
- Ventilator settings with the use of ECMO
- Complications of ECMO in CTD-Induced DAH
- Hemorrhage: The most common and significant complication of ECMO in CTD-induced DAH is hemorrhage, primarily due to the systemic anticoagulation required to maintain circuit patency. Patients with CTDs are already at an increased risk of bleeding, particularly those with active disease and widespread vasculitis. The use of anticoagulants such as heparin increases the risk of bleeding within the lungs, worsening the alveolar hemorrhage and complicating management. Therefore, a delicate balance must be maintained between preventing clot formation in the ECMO circuit and avoiding further bleeding complications [86,87]. However, there have been some cases that are successfully managed without the use of anticoagulation, and other case reports utilizing systemic anticoagulation using a modified ACT target of 140 to 160 s [86,87].
- Thromboembolism: Although less frequent, thromboembolic events can occur if clot formation occurs within the ECMO circuit [85]. This is a particular concern in patients who have an increased tendency to form blood clots due to autoimmune factors or the use of certain immunosuppressive medications [85].
- Immunosuppressive Therapy and Drug Bioavailability: Patients with CTD-induced DAH typically require aggressive immunosuppressive therapy, such as corticosteroids, cyclophosphamide, or rituximab, to control disease activity and prevent further alveolar hemorrhage. However, the pharmacokinetics of these drugs in the context of ECMO remain poorly understood. ECMO itself can alter the bioavailability of medications, affecting drug absorption, distribution, metabolism, and elimination [86].
- Infection: Local infections at cannulation sites or systemic infections related to the ECMO circuit are possible. The immunosuppressive treatment used to control CTDs may increase the susceptibility to infections, complicating the management of patients on ECMO [85].
- Clinical outcomes of ECMO in CTD-induced DAH:
5.4.5. Sedation and Pain Management
6. Monitoring and Follow-Up
Strategies for Follow-Up
- Imaging and Pulmonary Function Monitoring:
- -
- Chest X-ray or HRCT at regular intervals to assess DAH resolution;
- -
- Pulmonary function tests (PFTs) assess residual restrictive lung disease or diffusion impairment following recovery [17].
- Immunosuppressive Therapy Optimization:
- -
- Depending on the CTD phenotype and severity, long-term immunosuppressive therapy, such as rituximab, cyclophosphamide, and mycophenolate mofetil, is used. A steroid-tapering strategy is used based on the clinical response [99].
- Preventive Strategies and Rehabilitation:
- -
- Administration of vaccinations (influenza, pneumococcal, and COVID-19) to prevent relapse-inducing infections [103];
- -
- Pulmonary rehabilitation is reserved for patients with persistent dyspnea or deconditioning [104];
- -
- Early rheumatology follow-up is recommended to prevent relapses and control the disease [104].
7. Prognosis
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alba, M.A.; Jennette, J.C.; Falk, R.J. Pathogenesis of ANCA-Associated Pulmonary Vasculitis. Semin. Respir. Crit. Care Med. 2018, 39, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S. Diffuse alveolar hemorrhage. Tuberc. Respir. Dis. 2013, 74, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, E.; Cuzzocrea, S. Role of TNF-alpha in lung tight junction alteration in mouse model of acute lung inflammation. Respir. Res. 2007, 8, 75. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta 2009, 1788, 864–871. [Google Scholar] [CrossRef]
- Clark, P.R.; Kim, R.K.; Pober, J.S.; Kluger, M.S. Tumor necrosis factor disrupts claudin-5 endothelial tight junction barriers in two distinct NF-kappaB-dependent phases. PLoS ONE 2015, 10, e0120075. [Google Scholar] [CrossRef]
- Zhuang, H.; Han, S.; Harris, N.S.; Reeves, W.H. MEK1/2- and ERK1/2-Mediated Lung Endothelial Injury and Altered Hemostasis Promote Diffuse Alveolar Hemorrhage in Murine Lupus. Arthritis Rheumatol. 2024, 76, 1538–1551. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef]
- Moschetti, L.; Piantoni, S.; Vizzardi, E.; Sciatti, E.; Riccardi, M.; Franceschini, F.; Cavazzana, I. Endothelial Dysfunction in Systemic Lupus Erythematosus and Systemic Sclerosis: A Common Trigger for Different Microvascular Diseases. Front. Med. 2022, 9, 849086. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, K.D.; Choubey, D. Cytokines in autoimmunity: Role in induction, regulation, and treatment. J. Interferon Cytokine Res. 2011, 31, 695–703. [Google Scholar] [CrossRef]
- Sweis, J.J.G.; Sweis, N.W.G.; Alnaimat, F.; Jansz, J.; Liao, T.E.; Alsakaty, A.; Azam, A.; Elmergawy, H.; Hanson, H.A.; Ascoli, C.; et al. Immune-mediated lung diseases: A narrative review. Front. Med. 2023, 10, 1160755. [Google Scholar] [CrossRef]
- Schultz, M.J.; Levi, M. Pulmonary coagulopathy: A potential therapeutic target in different forms of lung injury. Thorax 2007, 62, 563–564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barker, T.T.; Lee, P.Y.; Kelly-Scumpia, K.M.; Weinstein, J.S.; Nacionales, D.C.; Kumagai, Y.; Akira, S.; Croker, B.P.; Sobel, E.S.; Reeves, W.H.; et al. Pathogenic role of B cells in the development of diffuse alveolar hemorrhage induced by pristane. Lab Investig. 2011, 91, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Han, S.; Lee, P.Y.; Khaybullin, R.; Shumyak, S.; Lu, L.; Chatha, A.; Afaneh, A.; Zhang, Y.; Xie, C.; et al. Pathogenesis of Diffuse Alveolar Hemorrhage in Murine Lupus. Arthritis Rheumatol. 2017, 69, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Nelson-Maney, N.; Huang, Y.; Levescot, A.; Wang, Q.; Wei, K.; Cunin, P.; Li, Y.; Lederer, J.A.; Zhuang, H.; et al. High-dimensional analysis reveals a pathogenic role of inflammatory monocytes in experimental diffuse alveolar hemorrhage. JCI Insight. 2019, 4, e129703. [Google Scholar] [CrossRef]
- Laskin, D.L.; Malaviya, R.; Laskin, J.D. Role of Macrophages in Acute Lung Injury and Chronic Fibrosis Induced by Pulmonary Toxicants. Toxicol. Sci. 2019, 168, 287–301. [Google Scholar] [CrossRef]
- Ioachimescu, O.C.; Stoller, J.K. Diffuse alveolar hemorrhage: Diagnosing it and finding the cause. Cleve Clin. J. Med. 2008, 75, 258–280. [Google Scholar] [CrossRef]
- Wells, J.F.S. Alveolar Hemorrhage. In Orphan Lung Diseases; Springer: London, UK, 2014; pp. 155–175. [Google Scholar]
- Kazzaz, N.M.; Coit, P.; Lewis, E.E.; McCune, W.J.; Sawalha, A.H.; Knight, J.S. Systemic lupus erythematosus complicated by diffuse alveolar haemorrhage: Risk factors, therapy and survival. Lupus Sci. Med. 2015, 2, e000117. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Goyal, A.; Varacallo, M.A. Systemic Lupus Erythematosus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Da Silva, R.C.; Adhikari, P. Granulomatosis with Polyangiitis Presenting with Diffuse Alveolar Hemorrhage: A Systematic Review. Cureus 2022, 14, e29909. [Google Scholar] [CrossRef]
- Rout, P.; Garlapati, P.; Qurie, A. Granulomatosis with Polyangiitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557827/ (accessed on 20 March 2025).
- Tashiro, H.; Takahashi, K.; Sadamatsu, H.; Uchida, M.; Kimura, S.; Sueoka-Aragane, N. Chronic and Asymptomatic Diffuse Alveolar Haemorrhage with Microscopic Polyangiitis: A Case Report and Review of the Literature. Case Rep. Rheumatol. 2016, 2016, 1658126. [Google Scholar] [CrossRef]
- Hashmi, M.F.; Rout, P. Microscopic Polyangiitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531484/ (accessed on 20 March 2025).
- Osman, A.; Galiatsatos, P.; Bose, S.; Danoff, S. Rheumatoid arthritis causing diffuse alveolar haemorrhage: A novel therapeutic approach. BMJ Case Rep. 2017, 2017, bcr2017220509. [Google Scholar] [CrossRef]
- Chauhan, K.; Jandu, J.S.; Brent, L.H.; Al-Dhahir, M.A. Rheumatoid Arthritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Stoots, S.A.; Lief, L.; Erkan, D. Clinical Insights into Diffuse Alveolar Hemorrhage in Antiphospholipid Syndrome. Curr. Rheumatol. Rep. 2019, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.G.; Goyal, A.; Rout, P.; Singhal, M. Antiphospholipid Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430980/ (accessed on 20 March 2025).
- Saab, H.; Bajaj, T.; Bains, K.; Garcia-Pacheco, R. Recurrent Episodes of Diffuse Alveolar Hemorrhage in Systemic Sclerosis 30 Days Apart. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619846594. [Google Scholar] [CrossRef] [PubMed]
- Crestani, B. The respiratory system in connective tissue disorders. Allergy 2005, 60, 715–734. [Google Scholar] [CrossRef]
- Sapkota, B.; Khalili, Y.A. Mixed Connective Tissue Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542198/ (accessed on 20 March 2025).
- Brown, K.K. Pulmonary vasculitis. Proc. Am. Thorac. Soc. 2006, 3, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Jamsheer, F.; Alzayani, S.; Alsudairy, N. Diffuse Alveolar Hemorrhage as a Life-Threatening Complication of Systemic Lupus Erythematosus: A Case Report. Cureus 2024, 16, e73586. [Google Scholar] [CrossRef]
- Specks, U. Diffuse alveolar hemorrhage syndromes. Curr. Opin. Rheumatol. 2001, 13, 12–17. [Google Scholar] [CrossRef]
- Cortese, G.; Nicali, R.; Placido, R.; Gariazzo, G.; Anro, P. Radiological aspects of diffuse alveolar haemorrhage. Radiol. Med. 2008, 113, 16–28. [Google Scholar] [CrossRef]
- Shrestha, S.; Rai, P.; Kayastha, G. A Distinctive Encounter with Diffuse Alveolar Hemorrhage in Granulomatosis with Polyangiitis and Pneumonia. Clin. Case Rep. 2025, 13, e70026. [Google Scholar] [CrossRef]
- Stainer, A.; Rice, A.; Devaraj, A.; Barnett, J.L.; Donovan, J.; Kokosi, M.; Nicholson, A.G.; Cairns, T.; Wells, A.U.; Renzoni, E.A. Diffuse alveolar haemorrhage associated with subsequent development of ANCA positivity and emphysema in three young adults. BMC Pulm. Med. 2019, 19, 185. [Google Scholar] [CrossRef]
- De Lassence, A.; Fleury-Feith, J.; Escudier, E.; Beaune, J.; Bernaudin, J.F.; Cordonnier, C. Alveolar hemorrhage. Diagnostic criteria and results in 194 immunocompromised hosts. Am. J. Respir. Crit. Care Med. 1995, 151, 157–163. [Google Scholar] [CrossRef]
- Perez-Arellano, J.L.; Losa Garcia, J.E.; Garcia Macias, M.C.; Gomez Gomez, F.; Jimenez Lopez, A.; de Castro, S. Hemosiderin-laden macrophages in bronchoalveolar lavage fluid. Acta Cytol. 1992, 36, 26–30. [Google Scholar] [PubMed]
- Golde, D.W.; Drew, W.L.; Klein, H.Z.; Finley, T.N.; Cline, M.J. Occult pulmonary haemorrhage in leukaemia. Br. Med. J. 1975, 2, 166–168. [Google Scholar] [CrossRef]
- Moon, K.M.; Jung, S.Y.; Han, M.S.; Cho, Y.; Rah, Y.M.; Kim, J.W. Diffuse Alveolar Hemorrhage Confirmed by Bronchoalveolar Lavage in a Patient with Hemoptysis after Sildenafil Use for Erectile Dysfunction. Acute Crit. Care 2015, 30, 31–33. [Google Scholar] [CrossRef]
- Greening, A.P.; Hughes, J.M. Serial estimations of carbon monoxide diffusing capacity in intrapulmonary haemorrhage. Clin. Sci. 1981, 60, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Jog, N.R.; James, J.A. Biomarkers in connective tissue diseases. J. Allergy Clin. Immunol. 2017, 140, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Gertner, E. Diffuse alveolar hemorrhage in the antiphospholipid syndrome: Spectrum of disease and treatment. J. Rheumatol. 1999, 26, 805–807. [Google Scholar] [PubMed]
- Newsome, B.R.; Morales, J.E. Diffuse alveolar hemorrhage. South Med. J. 2011, 104, 269–274. [Google Scholar] [CrossRef]
- Travis, W.D.; Colby, T.V.; Lombard, C.; Carpenter, H.A. A clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation. Am. J. Surg. Pathol. 1990, 14, 1112–1125. [Google Scholar] [CrossRef]
- Susarla, S.C.; Fan, L.L. Diffuse alveolar hemorrhage syndromes in children. Curr. Opin. Pediatr. 2007, 19, 314–320. [Google Scholar] [CrossRef]
- Gomes, M.M.; Barros, C.; Luis, H.; Bilreiro, M.; Machado, B. Diffuse Alveolar Hemorrhage: An Unexpected Effect After Taking Acetylsalicylic Acid. Cureus 2022, 14, e21486. [Google Scholar] [CrossRef]
- Heslet, L.; Nielsen, J.D.; Levi, M.; Sengelov, H.; Johansson, P.I. Successful pulmonary administration of activated recombinant factor VII in diffuse alveolar hemorrhage. Crit. Care 2006, 10, R177. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.; Kuhn, J.; Gabriel, D.; Barrow, J.; Jennette, J.C.; Henke, D.C. Use of Activated Factor VII in Patients with Diffuse Alveolar Hemorrhage: A 10 Years Institutional Experience. Lung 2015, 193, 375–379. [Google Scholar] [CrossRef]
- Park, J.A. Treatment of Diffuse Alveolar Hemorrhage: Controlling Inflammation and Obtaining Rapid and Effective Hemostasis. Int. J. Mol. Sci. 2021, 22, 793. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; McArthur, J.; Morrison, R.R.; Ghafoor, S. Diffuse Alveolar Hemorrhage After Pediatric Hematopoietic Stem Cell Transplantation. Front. Oncol. 2020, 10, 1757. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Blumberg, N.; Culakova, E.; Refaai, M.A.; Lyman, G.H. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch. Intern. Med. 2008, 168, 2377–2381. [Google Scholar] [CrossRef]
- Stolla, M.; Refaai, M.A.; Heal, J.M.; Spinelli, S.L.; Garraud, O.; Phipps, R.P.; Blumberg, N. Platelet transfusion—The new immunology of an old therapy. Front. Immunol. 2015, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Wikkelso, A.; Wetterslev, J.; Moller, A.M.; Afshari, A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst. Rev. 2016, 2016, CD007871. [Google Scholar] [CrossRef]
- de Prost, N.; Parrot, A.; Picard, C.; Ancel, P.Y.; Mayaud, C.; Fartoukh, M.; Cadranel, J. Diffuse alveolar haemorrhage: Factors associated with in-hospital and long-term mortality. Eur. Respir. J. 2010, 35, 1303–1311. [Google Scholar] [CrossRef]
- Walsh, M.; Merkel, P.A.; Peh, C.A.; Szpirt, W.M.; Puechal, X.; Fujimoto, S.; Hawley, C.M.; Khalidi, N.; Floßmann, O.; Wald, R.; et al. Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis. N. Engl. J. Med. 2020, 382, 622–631. [Google Scholar] [CrossRef]
- Apfelbaum, J.L.; Hagberg, C.A.; Connis, R.T.; Abdelmalak, B.B.; Agarkar, M.; Dutton, R.P.; Fiadjoe, J.E.; Greif, R.; Klock, P.A.; Mercier, D.; et al. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology 2022, 136, 31–81. [Google Scholar] [CrossRef]
- Law, J.A.; Broemling, N.; Cooper, R.M.; Drolet, P.; Duggan, L.V.; Griesdale, D.E.; Hung, O.R.; Jones, P.M.; Kovacs, G.; Massey, S.; et al. The difficult airway with recommendations for management—Part 2—The anticipated difficult airway. Can. J. Anaesth. 2013, 60, 1119–1138. [Google Scholar] [CrossRef] [PubMed]
- Al-Adhoubi, N.K.; Bystrom, J. Systemic lupus erythematosus and diffuse alveolar hemorrhage, etiology and novel treatment strategies. Lupus 2020, 29, 355–363. [Google Scholar] [CrossRef]
- Collard, H.R.; Schwarz, M.I. Diffuse alveolar hemorrhage. Clin. Chest Med. 2004, 25, 583–592. [Google Scholar] [CrossRef]
- Renda, T.; Corrado, A.; Iskandar, G.; Pelaia, G.; Abdalla, K.; Navalesi, P. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br. J. Anaesth. 2018, 120, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.R.; Warner, M.L.; Tuder, R.; Schwarz, M.I. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine 1997, 76, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Lauque, D.; Cadranel, J.; Lazor, R.; Pourrat, J.; Ronco, P.; Guillevin, L.; Cordier, J.F. Microscopic polyangiitis with alveolar hemorrhage. A study of 29 cases and review of the literature. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P). Medicine 2000, 79, 222–233. [Google Scholar] [CrossRef]
- Lara, A.R.; Schwarz, M.I. Diffuse alveolar hemorrhage. Chest 2010, 137, 1164–1171. [Google Scholar] [CrossRef]
- Tucci, M.R.; Costa, E.L.; Nakamura, M.A.; Morais, C.C. Noninvasive ventilation for acute respiratory distress syndrome: The importance of ventilator settings. J. Thorac. Dis. 2016, 8, E982–E986. [Google Scholar] [CrossRef]
- Cabrini, L.; Landoni, G.; Oriani, A.; Plumari, V.P.; Nobile, L.; Greco, M.; Pasin, L.; Beretta, L.; Zangrillo, A. Noninvasive ventilation and survival in acute care settings: A comprehensive systematic review and metaanalysis of randomized controlled trials. Crit. Care Med. 2015, 43, 880–888. [Google Scholar] [CrossRef]
- Criner, G.J.; Gayen, S.; Zantah, M.; Dominguez Castillo, E.; Naranjo, M.; Lashari, B.; Pourshahid, S.; Gangemi, A. Clinical review of non-invasive ventilation. Eur. Respir. J. 2024, 64, 2400396. [Google Scholar] [CrossRef]
- Grieco, D.L.; Maggiore, S.M.; Roca, O.; Spinelli, E.; Patel, B.K.; Thille, A.W.; Barbas, C.S.V.; de Acilu, M.G.; Cutuli, S.L.; Bongiovanni, F.; et al. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med. 2021, 47, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Frat, J.P.; Brugiere, B.; Ragot, S.; Chatellier, D.; Veinstein, A.; Goudet, V.; Coudroy, R.; Petitpas, F.; Robert, R.; Thille, A.W.; et al. Sequential application of oxygen therapy via high-flow nasal cannula and noninvasive ventilation in acute respiratory failure: An observational pilot study. Respir. Care 2015, 60, 170–178. [Google Scholar] [CrossRef]
- Laghi, F.; Shaikh, H.; Caccani, N. Basing intubation of acutely hypoxemic patients on physiologic principles. Ann. Intensive Care 2024, 14, 86. [Google Scholar] [CrossRef]
- Hakim, R.; Watanabe-Tejada, L.; Sukhal, S.; Tulaimat, A. Acute respiratory failure in randomized trials of noninvasive respiratory support: A systematic review of definitions, patient characteristics, and criteria for intubation. J. Crit. Care 2020, 57, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994, 149 Pt 1, 818–824. [Google Scholar] [CrossRef]
- Bernard, G.R. Acute respiratory distress syndrome: A historical perspective. Am. J. Respir. Crit. Care Med. 2005, 172, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar]
- Kusunoki, M.; Umegaki, T.; Shoji, T.; Nishimoto, K.; Anada, N.; Ando, A.; Uba, T.; Oku, K.; Hakata, S.; Hagihira, S.; et al. Severe Progressive Diffuse Alveolar Hemorrhage in a Patient with Systemic Lupus Erythematosus. Case Rep. Crit. Care 2018, 2018, 9790459. [Google Scholar] [CrossRef]
- Amato, M.B.; Barbas, C.S.; Medeiros, D.M.; Schettino Gde, P.; Lorenzi Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Morais, C.; Fernandes, E.d.O.; Takagaki, T.Y. Beneficial effects of the “open lung approach” with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am. J. Respir. Crit. Care Med. 1995, 152 Pt 1, 1835–1846. [Google Scholar] [CrossRef]
- Wu, S.J.; Hsu, Y.C.; Wang, K.L.; Fu, P.K. Prone Positioning May Improve the Treatment of Diffuse Alveolar Hemorrhage and Severe Acute Respiratory Distress Syndrome (ARDS) Secondary to ANCA Associated Vasculitis: A Case Report. Life 2022, 12, 235. [Google Scholar] [CrossRef]
- Boumans, M.M.A.; Aerts, W.; Pisani, L.; Bos, L.D.J.; Smit, M.R.; Tuinman, P.R. Diagnostic accuracy of lung ultrasound in diagnosis of ARDS and identification of focal or non-focal ARDS subphenotypes: A systematic review and meta-analysis. Crit. Care 2024, 28, 224. [Google Scholar] [CrossRef]
- Dell’Aquila, P.; Raimondo, P.; Racanelli, V.; De Luca, P.; De Matteis, S.; Pistone, A.; Melodia, R.; Crudele, L.; Lomazzo, D.; Solimando, A.G.; et al. Integrated lung ultrasound score for early clinical decision-making in patients with COVID-19: Results and implications. Ultrasound. J. 2022, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Brower, R.G.; Lanken, P.N.; MacIntyre, N.; Matthay, M.A.; Morris, A.; Ancukiewicz, M. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2004, 351, 327–336. [Google Scholar] [PubMed]
- Salem, M.S.; Eltatawy, H.S.; Abdelhafez, A.A.; Alsherif, S.E.-d.I. Lung ultrasound-versus FiO2-guided PEEP in ARDS patients. Egypt. J. Anaesth. 2020, 36, 31–37. [Google Scholar] [CrossRef]
- Gurubhagavatula, I.; Palevsky, H.I. Pulmonary hypertension in systemic autoimmune disease. Rheum. Dis. Clin. N. Am. 1997, 23, 365–394. [Google Scholar] [CrossRef]
- Allen, S.; Holena, D.; McCunn, M.; Kohl, B.; Sarani, B. A review of the fundamental principles and evidence base in the use of extracorporeal membrane oxygenation (ECMO) in critically ill adult patients. J. Intensive Care Med. 2011, 26, 13–26. [Google Scholar] [CrossRef]
- Brodie, D.; Bacchetta, M. Extracorporeal membrane oxygenation for ARDS in adults. N. Engl. J. Med. 2011, 365, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Tonna, J.E.; Abrams, D.; Brodie, D.; Greenwood, J.C.; Rubio Mateo-Sidron, J.A.; Usman, A.; Fan, E. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021, 67, 601–610. [Google Scholar] [CrossRef]
- Pacheco Claudio, C.; Charbonney, E.; Durand, M.; Kolan, C.; Laskine, M. Extracorporeal membrane oxygenation in diffuse alveolar hemorrhage secondary to systemic lupus erythematosus. J. Clin. Med. Res. 2014, 6, 145–148. [Google Scholar]
- Ahmed, S.H.; Aziz, T.; Cochran, J.; Highland, K. Use of extracorporeal membrane oxygenation in a patient with diffuse alveolar hemorrhage. Chest 2004, 126, 305–309. [Google Scholar] [CrossRef]
- Goel, M.K.; Chauhan, M.; Kumar, A.; Wadwa, P.; Maitra, G.; Talegaonkkar, M. A Case of Refractory Hypoxemic Respiratory Failure due to Antineutrophil Cytoplasmic Antibodies-associated Diffuse Alveolar Hemorrhage Rescued by Extracorporeal Membrane Oxygenation. Indian J. Crit. Care Med. 2020, 24, 879–881. [Google Scholar] [PubMed]
- Pais, F.; Fayed, M.; Evans, T. The Successful Use of Extracorporeal Membrane Oxygenation in Systemic Lupus Erythematosus-Induced Diffuse Alveolar Haemorrhage. Eur. J. Case Rep. Intern. Med. 2017, 4, 000515. [Google Scholar] [CrossRef]
- Lewis, K.; Balas, M.C.; Stollings, J.L.; McNett, M.; Girard, T.D.; Chanques, G.; Kho, M.E.; Pandharipande, P.P.; Weinhouse, G.L.; Brummel, N.E.; et al. A Focused Update to the Clinical Practice Guidelines for the Prevention and Management of Pain, Anxiety, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2025, 53, e711–e727. [Google Scholar] [CrossRef] [PubMed]
- Moller, M.H.; Alhazzani, W.; Lewis, K.; Belley-Cote, E.; Granholm, A.; Centofanti, J.; McIntyre, W.B.; Spence, J.; Al Duhailib, Z.; Needham, D.M.; et al. Use of dexmedetomidine for sedation in mechanically ventilated adult ICU patients: A rapid practice guideline. Intensive Care Med. 2022, 48, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.W.; Skrobik, Y.; Gelinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- White, P.F.; Kehlet, H.; Neal, J.M.; Schricker, T.; Carr, D.B.; Carli, F. The role of the anesthesiologist in fast-track surgery: From multimodal analgesia to perioperative medical care. Anesth. Analg. 2007, 104, 1380–1396. [Google Scholar] [CrossRef]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gelinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef]
- Chanques, G.; Constantin, J.M.; Devlin, J.W.; Ely, E.W.; Fraser, G.L.; Gelinas, C.; Girard, T.D.; Guérin, C.; Jabaudon, M.; Jaber, S.; et al. Analgesia and sedation in patients with ARDS. Intensive Care Med. 2020, 46, 2342–2356. [Google Scholar] [CrossRef]
- Battaglini, D.; Fazzini, B.; Silva, P.L.; Cruz, F.F.; Ball, L.; Robba, C.; Rocco, P.R.M.; Pelosi, P. Challenges in ARDS Definition, Management, and Identification of Effective Personalized Therapies. J. Clin. Med. 2023, 12, 1381. [Google Scholar] [CrossRef]
- Grotberg, J.C.; Reynolds, D.; Kraft, B.D. Extracorporeal Membrane Oxygenation for Respiratory Failure: A Narrative Review. J. Clin. Med. 2024, 13, 3795. [Google Scholar] [CrossRef]
- Demiselle, J.; Auchabie, J.; Beloncle, F.; Gatault, P.; Grange, S.; Du Cheyron, D.; Dellamonica, J.; Boyer, S.; Beauport, D.T.; Piquilloud, L.; et al. Patients with ANCA-associated vasculitis admitted to the intensive care unit with acute vasculitis manifestations: A retrospective and comparative multicentric study. Ann. Intensive Care 2017, 7, 39. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Lan, L.; Ren, P.; Ni, A.; Ma, Y.; Wang, Y.; Zhu, Y.; Xu, Y.; Chen, J.; et al. Antineutrophil Cytoplasmic Antibody-Associated Vasculitis with Acute Kidney Injury: Short-Term Recovery Predicts Long-Term Outcome. Front. Immunol. 2021, 12, 641655. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, B.; Sanchez-Alamo, B.; Schirmer, J.H.; Berti, A.; Blockmans, D.; Cid, M.C.; Holle, J.U.; Hollinger, N.; Karadag, O.; Kronbichler, A.; et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann. Rheum. Dis. 2024, 83, 30–47. [Google Scholar] [CrossRef]
- Khangoora, V.; Bernstein, E.J.; King, C.S.; Shlobin, O.A. Connective tissue disease-associated pulmonary hypertension: A comprehensive review. Pulm. Circ. 2023, 13, e12276. [Google Scholar] [CrossRef] [PubMed]
- Biciusca, V.; Rosu, A.; Stan, S.I.; Cioboata, R.; Biciusca, T.; Balteanu, M.A.; Florescu, C.; Camen, G.C.; Cimpeanu, O.; Bumbea, A.M.; et al. A Practical Multidisciplinary Approach to Identifying Interstitial Lung Disease in Systemic Autoimmune Rheumatic Diseases: A Clinician’s Narrative Review. Diagnostics 2024, 14, 2674. [Google Scholar] [CrossRef]
- Olivieri, B.; Betterle, C.; Zanoni, G. Vaccinations and Autoimmune Diseases. Vaccines 2021, 9, 815. [Google Scholar] [CrossRef]
- Seleoglu, I.; Demirel, A. Pulmonary rehabilitation in connective tissue disease-associated interstitial lung disease: A systematic review. Sarcoidosis Vasc. Diffuse Lung Dis. 2024, 41, e2024061. [Google Scholar] [PubMed]
- Picard, C.; Parrot, A.; Mayaud, C.; Cadranel, J. Diffuse alveolar hemorrhage in the immunocompetent host: Diagnostic and therapeutic management. Presse Med. 2009, 38, 1343–1352. [Google Scholar] [CrossRef]
- Cartin-Ceba, R.; Diaz-Caballero, L.; Al-Qadi, M.O.; Tryfon, S.; Fervenza, F.C.; Ytterberg, S.R.; Specks, U. Diffuse Alveolar Hemorrhage Secondary to Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: Predictors of Respiratory Failure and Clinical Outcomes. Arthritis Rheumatol. 2016, 68, 1467–1476. [Google Scholar] [CrossRef]
- Mirouse, A.; Parrot, A.; Audigier, V.; Demoule, A.; Mayaux, J.; Geri, G.; Mariotte, E.; Bréchot, N.; de Prost, N.; Vautier, M.; et al. Severe diffuse alveolar hemorrhage related to autoimmune disease: A multicenter study. Crit. Care 2020, 24, 231. [Google Scholar] [CrossRef]

| Connective Tissue Disorder | Specific Serological Labs | Non-Specific Labs |
|---|---|---|
| SLE | ANA, anti-dsDNA, anti-SM, anti | Increased ESR |
| -histone antibody | ||
| Anti-phospholipid antibody syndrome | APL, lupus anticoagulant, | Eosinophilia |
| anti-CL, anti-β2GP1 antibody | ||
| Rheumatoid arthritis | RF, anti-CCP | Hematuria |
| Polymyositis and Dermatomyositis | Anti-Jo-1 | |
| Systemic sclerosis | Anti-topoisomerase I (Scl-70), | Anemia |
| Anti-RNA polymerase III antibodies | ||
| Mixed connective tissue disorder | Anti-RNP | |
| Granulomatosis with polyangiitis (GPA) | c-ANCA | Prolonged PT/aPTT/PT INR |
| Microscopic polyangiitis (MPA) | p-ANCA | Thrombocytopenia |
| Lung Ultrasound Findings | CTD-Induced DAH Adaptation | Clinical Decision-Making |
|---|---|---|
| Focal/non-focal sub-phenotypes | Non-focal: uniform PEEP | |
| Non-focal in most CTDs, | Focal: lower PEEP with | |
| Focal in GPA | lateral decubitus on | |
| bleeding side. | ||
| B-lines | Fibrin reduces compliance, | |
| leading to less PEEP | ||
| Responsiveness—start lower PEEP | ||
| Patchy/asymmetric due to blood | and adjust accordingly | |
| -SS-induced fibrosis can mask B- | ||
| lines, adjust PEEP to avoid | ||
| pneumothorax [28]. | ||
| -SLE/APS leads to increased | ||
| vascular fragility, titrate | ||
| PEEP cautiously [82]. | ||
| Consolidations | Clotted blood | Avoid overdistension |
| (barotrauma), lesser | ||
| PEEP max, use ECMO early if | ||
| worsening (described below). | ||
| Pleural line | CTDs can cause pleural | |
| Thickened/irregular | inflammation correlating | |
| with CTD activity—titrate | ||
| immunosuppression. Effusions | ||
| can reduce compliance, titrate | ||
| PEEP and monitor for | ||
| hemodynamic compromise. | ||
| Zones involved | Anterior/lateral | Blood pools in gravity |
| -dependent areas early | ||
| Improvement in B-lines | Improving DAH | -Cautiously reduce PEEP |
| New B-lines/consolidation | Worsening DAH | -Assess for re-bleeding, titrate |
| Supportive therapies, avoid increasing | ||
| PEEP |
| Category | Key Component | Clinical Action |
|---|---|---|
| Respiratory monitoring | -ABGs -Serial HRCT -POCUS (lung aeration, volume status) -SpO2/EtCO2 (on ventilated patients) [96,97] | -Early intubation if PaO2/FiO2 < 200 -Lung-protective ventilation |
| Hemodynamic monitoring | -Arterial line for continuous blood pressure monitoring. -Fluid resuscitation guided by CVP measurement, POCUS [98] | -Vasopressor support for hemodynamic instability/shock -Avoid volume overload as can worsen pulmonary edema |
| Renal monitoring | -Daily creatinine/electrolytes -Urine output -Lupus nephritis/ANCA-GN screening [99] | -Nephrology consultation for AKI -CRRT if refractory acidosis /volume overload |
| Hematologic/Coagulation | -Hb, platelets, Fibrinogen, D-Dimer -TMA screening (SLE/APS) [100] | -PRBC transfusion if Hb < 7 g/dL -PLEX for ANCA vasculitis/catastrophic APS |
| Ventilation Strategy | -Low TV -Moderate PEEP -Permissive hypercapnia [73,74] | -Avoid barotrauma -ECMO if refractory hypoxia |
| Multidisciplinary Team approach | Address respiratory failure, CTD-induced immune dysfunction, and anatomical risks and provide longitudinal care. -Pulmonology: bronchoscopy, imaging -Rheumatology: immuno- suppression -Medical intensive care: ECMO/hemodynamics -Anesthesiology: address difficult airway -ENT surgeons: manage structural complications -Hematology: coagulopathy -Nephrology: AKI/CRRT -Respiratory therapy: ventilator weaning | -Daily interdisciplinary rounds [101,102] -Tailor therapy to CTD subtype (rituximab for AAV, anticoagulation for APS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudgal, M.; Balaji, S.; Gajendiran, A.P.; Subramanya, A.; Murugan, S.K.; Gondhi, V.; Bhatnagar, A.R.; Gunasekaran, K. Connective Tissue Disorder-Induced Diffuse Alveolar Hemorrhage: A Comprehensive Review with an Emphasis on Airway and Respiratory Management. Life 2025, 15, 793. https://doi.org/10.3390/life15050793
Mudgal M, Balaji S, Gajendiran AP, Subramanya A, Murugan SK, Gondhi V, Bhatnagar AR, Gunasekaran K. Connective Tissue Disorder-Induced Diffuse Alveolar Hemorrhage: A Comprehensive Review with an Emphasis on Airway and Respiratory Management. Life. 2025; 15(5):793. https://doi.org/10.3390/life15050793
Chicago/Turabian StyleMudgal, Mayuri, Swetha Balaji, Ajeetha Priya Gajendiran, Ananthraj Subramanya, Shanjai Krishnan Murugan, Venkatesh Gondhi, Aseem Rai Bhatnagar, and Kulothungan Gunasekaran. 2025. "Connective Tissue Disorder-Induced Diffuse Alveolar Hemorrhage: A Comprehensive Review with an Emphasis on Airway and Respiratory Management" Life 15, no. 5: 793. https://doi.org/10.3390/life15050793
APA StyleMudgal, M., Balaji, S., Gajendiran, A. P., Subramanya, A., Murugan, S. K., Gondhi, V., Bhatnagar, A. R., & Gunasekaran, K. (2025). Connective Tissue Disorder-Induced Diffuse Alveolar Hemorrhage: A Comprehensive Review with an Emphasis on Airway and Respiratory Management. Life, 15(5), 793. https://doi.org/10.3390/life15050793






