Microfluidic Sorting Can Be Applied for Assisted Reproduction Sperm Selection in Different Cases of Semen Abnormalities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Human Semen Samples
2.3. Microfluidic Sperm Sorting
2.4. Pellet Swim-Up Technique
2.5. Direct Swim-Up Technique
2.6. Assessment of Sperm Intracellular ROS
2.7. Assessment of Sperm DNA Fragmentation
2.8. Flow Cytometry
2.9. Assessment of Sperm Kinematic Parameters and Hyperactivated Motility
2.10. Assessment of Sperm Chromatin Compaction by Chromomycin A3
2.11. Statistical Analysis:
3. Results
3.1. Comparison of MSS and SU Selection Methods in Hyperviscous and Normally Viscous Semen Samples
3.2. Comparison of MSS and SU Selection Methods in Oligozoospermic and Asthenozoospermic Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto, S.; Carrageta, D.F.; Alves, M.G.; Rocha, A.; Agarwal, A.; Barros, A.; Oliveira, P.F. Sperm selection strategies and their impact on assisted reproductive technology outcomes. Andrologia 2021, 53, e13725. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.T.Y.; Lee, C.L.; Tian, X.; Lam, K.K.W.; Li, R.H.W.; Ng, E.H.Y.; Yeung, W.S.B.; Chiu, P.C.N. Simulating nature in sperm selection for assisted reproduction. Nat. Rev. Urol. 2022, 19, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Doostabadi, M.R.; Mangoli, E.; Marvast, L.D.; Dehghanpour, F.; Maleki, B.; Torkashvand, H.; Talebi, A.R. Microfluidic devices employing chemo- and thermotaxis for sperm selection can improve sperm parameters and function in patients with high DNA fragmentation. Andrologia 2022, 54, e14623. [Google Scholar] [CrossRef]
- Akerlöf, E.; Fredricson, B.; Gustafsson, O.; Lundin, A.; Lunell, N.O.; Nylund, L.; Rosenborg, L.; Pousette, A. Comparison between a swim-up and a Percoll gradient technique for the separation of human spermatozoa. Int. J. Androl. 1987, 10, 663–669. [Google Scholar] [CrossRef]
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Agarwal, A.; Ikemoto, I.; Loughlin, K.R. Effect of sperm washing on levels of reactive oxygen species in semen. Arch. Androl. 1994, 33, 157–162. [Google Scholar] [CrossRef]
- Rappa, K.L.; Rodriguez, H.F.; Hakkarainen, G.C.; Anchan, R.M.; Mutter, G.L.; Asghar, W. Sperm processing for advanced reproductive technologies: Where are we today? Biotechnol. Adv. 2016, 34, 578–587. [Google Scholar] [CrossRef]
- Zini, A.; Finelli, A.; Phang, D.; Jarvi, K. Influence of semen processing technique on human sperm DNA integrity. Urology 2000, 56, 1081–1084. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Cambi, M.; Boni, L.; Iorio, A.L.; Passaro, C.; Luppino, B.; Nadalini, M.; Marchiani, S.; Tamburrino, L.; et al. Variation of DNA Fragmentation Levels During Density Gradient Sperm Selection for Assisted Reproduction Techniques: A Possible New Male Predictive Parameter of Pregnancy? Medicine 2016, 95, e3624. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm selection with density gradient centrifugation and swim up: Effect on DNA fragmentation in viable spermatozoa. Sci. Rep. 2019, 9, 7492. [Google Scholar] [CrossRef]
- Kim, S.W.; Jee, B.C.; Kim, S.K.; Kim, S.H. Sperm DNA fragmentation and sex chromosome aneuploidy after swim-up versus density gradient centrifugation. Clin. Exp. Reprod. Med. 2017, 44, 201–206. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J. Androl. 1988, 9, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Katigbak, R.D.; Turchini, G.M.; de Graaf, S.P.; Kong, L.; Dumée, L.F. Review on Sperm Sorting Technologies and Sperm Properties toward New Separation Methods via the Interface of Biochemistry and Material Science. Adv. Biosyst. 2019, 3, e1900079. [Google Scholar] [CrossRef] [PubMed]
- Ahmadkhani, N.; Hosseini, M.; Saadatmand, M.; Abbaspourrad, A. The influence of the female reproductive tract and sperm features on the design of microfluidic sperm-sorting devices. J. Assist. Reprod. Genet. 2022, 39, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Schuster, T.G.; Cho, B.; Keller, L.M.; Takayama, S.; Smith, G.D. Isolation of motile spermatozoa from semen samples using microfluidics. Reprod. Biomed. Online 2003, 7, 75–81. [Google Scholar] [CrossRef]
- Cho, B.S.; Schuster, T.G.; Zhu, X.; Chang, D.; Smith, G.D.; Takayama, S. Passively driven integrated microfluidic system for separation of motile sperm. Anal. Chem. 2003, 75, 1671–1675. [Google Scholar] [CrossRef]
- Shirota, K.; Yotsumoto, F.; Itoh, H.; Obama, H.; Hidaka, N.; Nakajima, K.; Miyamoto, S. Separation efficiency of a microfluidic sperm sorter to minimize sperm DNA damage. Fertil. Steril. 2016, 105, 315–321.e1. [Google Scholar] [CrossRef]
- Sarbandi, I.R.; Lesani, A.; Moghimi Zand, M.; Nosrati, R. Rheotaxis-based sperm separation using a biomimicry microfluidic device. Sci. Rep. 2021, 11, 18327. [Google Scholar] [CrossRef]
- Sharma, S.; Kabir, M.A.; Asghar, W. Selection of healthy sperm based on positive rheotaxis using a microfluidic device. Analyst 2022, 147, 1589–1597. [Google Scholar] [CrossRef]
- Zaferani, M.; Cheong, S.H.; Abbaspourrad, A. Rheotaxis-based separation of sperm with progressive motility using a microfluidic corral system. Proc. Natl. Acad. Sci. USA 2018, 115, 8272–8277. [Google Scholar] [CrossRef]
- Huang, C.H.; Chen, C.H.; Huang, T.K.; Lu, F.; Jen Huang, J.Y.; Li, B.R. Design of a gradient-rheotaxis microfluidic chip for sorting of high-quality Sperm with progressive motility. iScience 2023, 26, 107356. [Google Scholar] [CrossRef]
- Koyama, S.; Amarie, D.; Soini, H.A.; Novotny, M.V.; Jacobson, S.C. Chemotaxis assays of mouse sperm on microfluidic devices. Anal. Chem. 2006, 78, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ma, R.; Han, C.; Su, K.; Zhang, Q.; Qiu, T.; Wang, L.; Huang, G.; Qiao, J.; Wang, J.; et al. Integration of sperm motility and chemotaxis screening with a microchannel-based device. Clin. Chem. 2010, 56, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, R.R.; Yin, T.; Zou, W.; Tang, Y.; Ding, J.; Yang, J. Generation of Gradients on a Microfluidic Device: Toward a High-Throughput Investigation of Spermatozoa Chemotaxis. PLoS ONE 2015, 10, e0142555. [Google Scholar] [CrossRef]
- Li, K.; Li, R.; Ni, Y.; Sun, P.; Liu, Y.; Zhang, D.; Huang, H. Novel distance-progesterone-combined selection approach improves human sperm quality. J. Transl. Med. 2018, 16, 203. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Qiu, T.; Xie, L.; Chen, W.; Liu, R.; Lu, Y.; Mitchelson, K.; Wang, J.; Qiao, J.; et al. The construction of an interfacial valve-based microfluidic chip for thermotaxis evaluation of human sperm. Biomicrofluidics 2014, 8, 024102. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; Laguna-Barraza, R.; de Castro, A.C.; Sánchez-Calabuig, M.J.; Cano-Oliva, E.; de Castro-Pita, F.J.; Montoro-Buils, L.; Pericuesta, E.; Fernández-González, R.; Gutiérrez-Adán, A. Sperm selection by thermotaxis improves ICSI outcome in mice. Sci. Rep. 2018, 8, 2902. [Google Scholar] [CrossRef]
- Ko, Y.J.; Maeng, J.H.; Hwang, S.Y.; Ahn, Y. Design, Fabrication, and Testing of a Microfluidic Device for Thermotaxis and Chemotaxis Assays of Sperm. SLAS Technol. 2018, 23, 507–515. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, B.; Fu, Q.; Wu, J.; Liu, R. A fully integrated biomimetic microfluidic device for evaluation of sperm response to thermotaxis and chemotaxis. Lab Chip 2021, 21, 310–318. [Google Scholar] [CrossRef]
- Gode, F.; Gürbüz, A.S.; Tamer, B.; Pala, I.; Isik, A.Z. The Effects of Microfluidic Sperm Sorting, Density Gradient and Swim-up Methods on Semen Oxidation Reduction Potential. Urol. J. 2020, 17, 397–401. [Google Scholar]
- Pujol, A.; García-Peiró, A.; Ribas-Maynou, J.; Lafuente, R.; Mataró, D.; Vassena, R. A microfluidic sperm-sorting device reduces the proportion of sperm with double-stranded DNA fragmentation. Zygote 2022, 30, 200–205. [Google Scholar] [CrossRef]
- Hsu, C.T.; Lee, C.I.; Lin, F.S.; Wang, F.Z.; Chang, H.C.; Wang, T.E.; Huang, C.C.; Tsao, H.M.; Lee, M.S.; Agarwal, A. Live motile sperm sorting device for enhanced sperm-fertilization competency: Comparative analysis with density-gradient centrifugation and microfluidic sperm sorting. J. Assist. Reprod. Genet. 2023, 40, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, N.; Eyni, H.; Sabz, F.T.K.; Narimani, N.; Zandieh, Z.; Amjadi, F. Microfluidic in compared with Zeta potential, MACS and swim up methods, resulted in improved chromatin integrity and high quality sperms. JBRA Assist. Reprod. 2024, 29, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Sheibak, N.; Amjadi, F.; Shamloo, A.; Zarei, F.; Zandieh, Z. Microfluidic sperm sorting selects a subpopulation of high-quality sperm with a higher potential for fertilization. Hum. Reprod. 2024, 39, 902–911. [Google Scholar] [CrossRef]
- Ferreira Aderaldo, J.; da Silva Maranhão, K.; Ferreira Lanza, D.C. Does microfluidic sperm selection improve clinical pregnancy and miscarriage outcomes in assisted reproductive treatments? A systematic review and meta-analysis. PLoS ONE 2023, 18, e0292891. [Google Scholar] [CrossRef]
- Parrella, A.; Keating, D.; Cheung, S.; Xie, P.; Stewart, J.D.; Rosenwaks, Z.; Palermo, G.D. A treatment approach for couples with disrupted sperm DNA integrity and recurrent ART failure. J. Assist. Reprod. Genet. 2019, 36, 2057–2066. [Google Scholar] [CrossRef]
- Kocur, O.M.; Xie, P.; Souness, S.; Cheung, S.; Rosenwaks, Z.; Palermo, G.D. Assessing male gamete genome integrity to ameliorate poor assisted reproductive technology clinical outcome. F S Sci. 2023, 4, 2–10. [Google Scholar] [CrossRef]
- Kocur, O.M.; Xie, P.; Cheung, S.; Souness, S.; McKnight, M.; Rosenwaks, Z.; Palermo, G.D. Can a sperm selection technique improve embryo ploidy? Andrology 2023, 11, 1605–1612. [Google Scholar] [CrossRef]
- Banti, M.; Van Zyl, E.; Kafetzis, D. Sperm Preparation with Microfluidic Sperm Sorting Chip May Improve Intracytoplasmic Sperm Injection Outcomes Compared to Density Gradient Centrifugation. Reprod. Sci. 2024, 31, 1695–1704. [Google Scholar] [CrossRef]
- Traini, G.; Tamburrino, L.; Vignozzi, L.; Baldi, E.; Marchiani, S. Is oxidative stress evaluated in viable human spermatozoa a marker of good semen quality? Front. Endocrinol. 2022, 13, 1012416. [Google Scholar] [CrossRef]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Tocci, V.; Failli, P.; Forti, G.; Baldi, E. Nuclear staining identifies two populations of human sperm with different DNA fragmentation extent and relationship with semen parameters. Hum. Reprod. 2008, 23, 1035–1043. [Google Scholar] [CrossRef]
- Mortimer, S.T.; Swan, M.A.; Mortimer, D. Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Hum. Reprod. 1998, 13, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, T.; Asadi, N.; Jadid, L.; Kazerooni, M.; Ghanadi, A.; Ghaffarpasand, F.; Kazerooni, Y.; Zolghadr, J. Evaluation of sperm’s chromatin quality with acridine orange test, chromomycin A3 and aniline blue staining in couples with unexplained recurrent abortion. J. Assist. Reprod. Genet. 2009, 26, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, S.; Tamburrino, L.; Olivito, B.; Betti, L.; Azzari, C.; Forti, G.; Baldi, E.; Muratori, M. Characterization and sorting of flow cytometric populations in human semen. Andrology 2014, 2, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Cambi, M.; Lotti, F.; Natali, I.; Filimberti, E.; Noci, I.; Forti, G.; Maggi, M.; et al. DNA fragmentation in brighter sperm predicts male fertility independently from age and semen parameters. Fertil. Steril. 2015, 104, 582–590.e4. [Google Scholar] [CrossRef]
- Ogle, R.A.; Netherton, J.; Schneider, E.; Velkov, T.; Zhang, H.; Cole, N.; Hetherington, L.; Villaverde, A.I.S.B.; Baker, M.A. Nuclear heterogeneity is prevalent in high-quality fractionated human sperm cells typically used for assisted conception. Hum. Reprod. 2021, 36, 2073–2082. [Google Scholar] [CrossRef]
- René, C.; Landry, I.; de Montigny, F. Couples’ experiences of pregnancy resulting from assisted reproductive technologies: A qualitative meta-synthesis. Int. J. Nurs. Stud. Adv. 2021, 4, 100059. [Google Scholar] [CrossRef]
- Anbari, F.; Khalili, M.A.; Sultan Ahamed, A.M.; Mangoli, E.; Nabi, A.; Dehghanpour, F.; Sabour, M. Microfluidic sperm selection yields higher sperm quality compared to conventional method in ICSI program: A pilot study. Syst. Biol. Reprod. Med. 2021, 67, 137–143. [Google Scholar] [CrossRef]
- Feyzioglu, B.S.; Avul, Z. Effects of sperm separation methods before intrauterine insemination on pregnancy outcomes and live birth rates: Differences between the swim-up and microfluidic chip techniques. Medicine 2023, 102, e36042. [Google Scholar] [CrossRef]
- Zaha, I.; Naghi, P.; Stefan, L.; Bunescu, C.; Radu, M.; Muresan, M.E.; Sandor, M.; Sachelarie, L.; Huniadi, A. Comparative Study of Sperm Selection Techniques for Pregnancy Rates in an Unselected IVF-ICSI Population. J. Pers. Med. 2023, 13, 619. [Google Scholar] [CrossRef]
- Quinn, M.M.; Jalalian, L.; Ribeiro, S.; Ona, K.; Demirci, U.; Cedars, M.I.; Rosen, M.P. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared with density gradient centrifugation with swim-up in split semen samples. Hum. Reprod. 2018, 33, 1388–1393. [Google Scholar] [CrossRef]
- Aydın, Ş.; Bulgan Kılıçdağ, E.; Çağlar Aytaç, P.; Çok, T.; Şimşek, E.; Haydardedeoğlu, B. Prospective randomized controlled study of a microfluidic chip technology for sperm selection in male infertility patients. Andrologia 2022, 54, e14415. [Google Scholar] [CrossRef] [PubMed]
- Delaroche, L.; Caillou, H.; Lamazou, F.; Genauzeau, E.; Meicler, P.; Oger, P.; Dupont, C.; Humaidan, P. Live birth after intrauterine insemination: Is there an upper cut-off for the number of motile spermatozoa inseminated? Reprod. Biomed. Online 2020, S1472–S6483, 30522–30528. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil. Steril. 2014, 102, 998–1005.e8. [Google Scholar] [CrossRef] [PubMed]
- Cissen, M.; Wely, M.V.; Scholten, I.; Mansell, S.; Bruin, J.P.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. Measuring Sperm DNA Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef]
- Robinson, L.; Gallos, I.D.; Conner, S.J.; Rajkhowa, M.; Miller, D.; Lewis, S.; Kirkman-Brown, J.; Coomarasamy, A. The effect of sperm DNA fragmentation on miscarriage rates: A systematic review and meta-analysis. Hum. Reprod. 2012, 27, 2908–2917. [Google Scholar] [CrossRef]
- Tamburrino, L.; Traini, G.; Ragosta, M.E.; Dabizzi, S.; Vezzani, S.; Scarpa, F.; Vignozzi, L.; Baldi, E.; Marchiani, S. Semen cryopreservation and storage in liquid nitrogen: Impact on chromatin compaction. Andrology, 2024; early view. [Google Scholar] [CrossRef]
- Cho, C.L.; Agarwal, A. Role of sperm DNA fragmentation in male factor infertility: A systematic review. Arab. J. Urol. 2017, 16, 21–34. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Singh, R.; Palani, A. Sperm DNA Fragmentation in Reproductive Medicine: A Review. J. Hum. Reprod. Sci. 2022, 15, 206–218. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum. Reprod. Update 2003, 9, 331–345. [Google Scholar] [CrossRef]
- Esteves, S.C.; Agarwal, A.; Majzoub, A. An evidence-based perspective on the role of sperm chromatin integrity and sperm DNA fragmentation testing in male infertility. Transl. Androl. Urol. 2017, 6, S665–S672. [Google Scholar] [CrossRef]
- Le, M.T.; Dang, H.N.T.; Nguyen, T.V.; Nguyen, T.T.T.; Nguyen, Q.H.V.; Cao, N.T. Effects of sperm preparation techniques on sperm survivability and DNA fragmentation. J. Int. Med. Res. 2022, 50, 3000605221097492. [Google Scholar] [CrossRef] [PubMed]
- Amano, K.; Oigawa, S.; Ichizawa, K.; Tokuda, Y.; Unagami, M.; Sekiguchi, M.; Furui, M.; Nakaoka, K.; Ito, A.; Hayashi, R.; et al. Swim-up method is superior to density gradient centrifugation for preserving sperm DNA integrity during sperm processing. Reprod. Med. Biol. 2024, 23, e12562. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Wang, L.; Gao, X.; Lu, X.; Wu, Z.; Wang, Y.; Liu, K.; Tao, J.; Wu, Y. Assessment of density gradient centrifugation (DGC) and sperm chromatin dispersion (SCD) measurements in couples with male factor infertility undergoing ICSI. J. Assist. Reprod. Genet. 2014, 31, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Finnie, J.M.; Muscio, L.; Whiting, S.; Connaughton, H.S.; Kuczera, L.; Rothkirch, T.B.; De Iuliis, G.N. Potential importance of transition metals in the induction of DNA damage by sperm preparation media. Hum. Reprod. 2014, 29, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Mirsanei, J.S.; Sheibak, N.; Zandieh, Z.; Mehdizadeh, M.; Aflatoonian, R.; Tabatabaei, M.; Mousavi, A.S.; Amjadi, F. Microfluidic chips as a method for sperm selection improve fertilization rate in couples with fertilization failure. Arch. Gynecol. Obstet. 2022, 306, 901–910. [Google Scholar] [CrossRef]
- Odhiambo, J.F.; DeJarnette, J.M.; Geary, T.W.; Kennedy, C.E.; Suarez, S.S.; Sutovsky, M.; Sutovsky, P. Increased conception rates in beef cattle inseminated with nanopurified bull semen. Biol. Reprod. 2014, 91, 97. [Google Scholar] [CrossRef]
- Omar, M.A.; Raouf, K.S.; ElGuindy, T.; Kamel, N.; Arafa, S.S.; Gebril, A. Magnetic-activated sperm enrichment (MASE) versus density gradient centrifugation (DGC) impact on ICSI outcome. IJGO 2020, 2, 10–15. [Google Scholar]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Nallella, K.P.; Thomas, A.J., Jr.; Alvarez, J.G.; Sikka, S.C. Reactive oxygen species as an independent marker of male factor infertility. Fertil. Steril. 2006, 86, 878–885. [Google Scholar] [CrossRef]
- de Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive oxygen species and sperm physiology. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Chen, S.J.; Allam, J.P.; Duan, Y.G.; Haidl, G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch. Gynecol. Obstet. 2013, 288, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, X.; Li, H. Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 2025, 16, 1520835. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; Burjaq, H.; Gotlieb, L.; Casper, R.F. Seminal hyperviscosity is associated with poor outcome of in vitro fertilization and embryo transfer: A prospective study. Fertil. Steril. 2008, 90, 1739–1743. [Google Scholar] [CrossRef]
- Bedwal, R.G.; Nair, N.; Pareek, C.; More, A.; Kalbande, A. Enhancing the Fertility Potential: A Case Report on the Management of Oligoasthenozoospermia Using a Microfluidic Device. Cureus 2024, 16, e61737. [Google Scholar] [CrossRef] [PubMed]
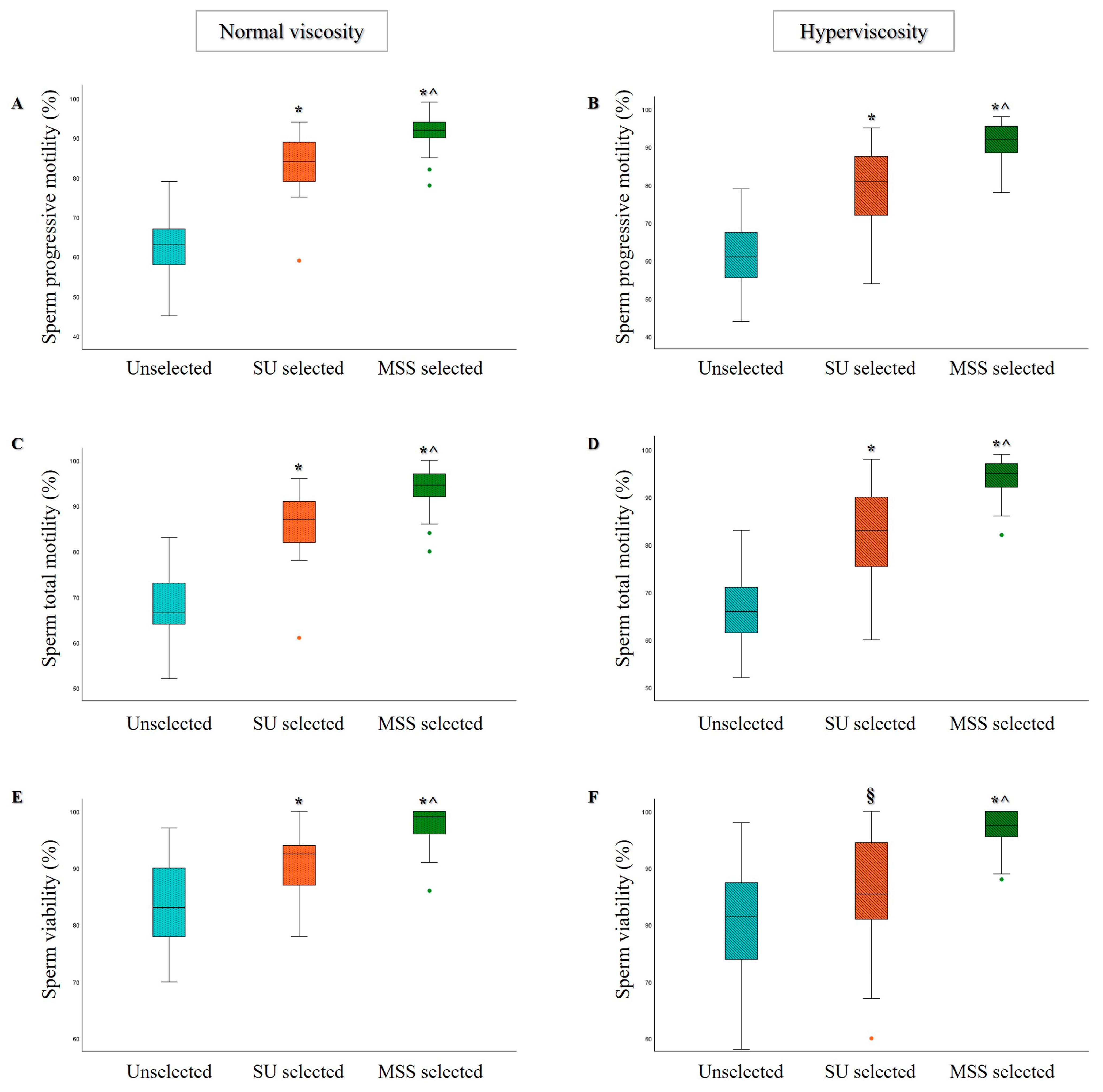
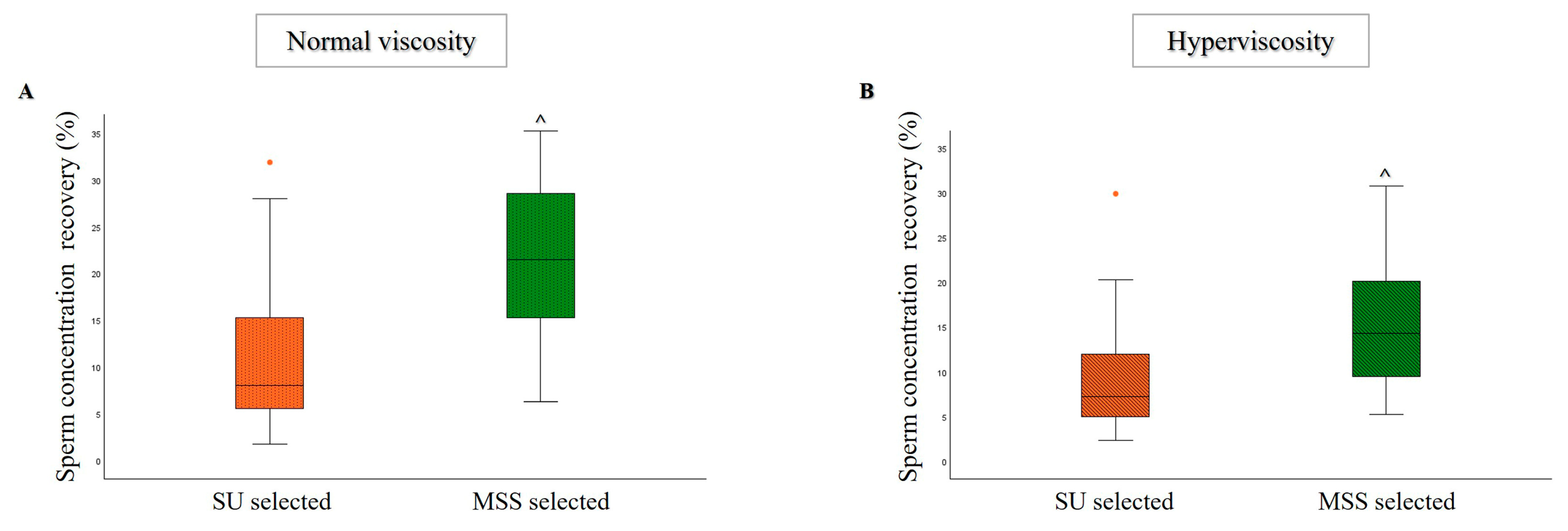
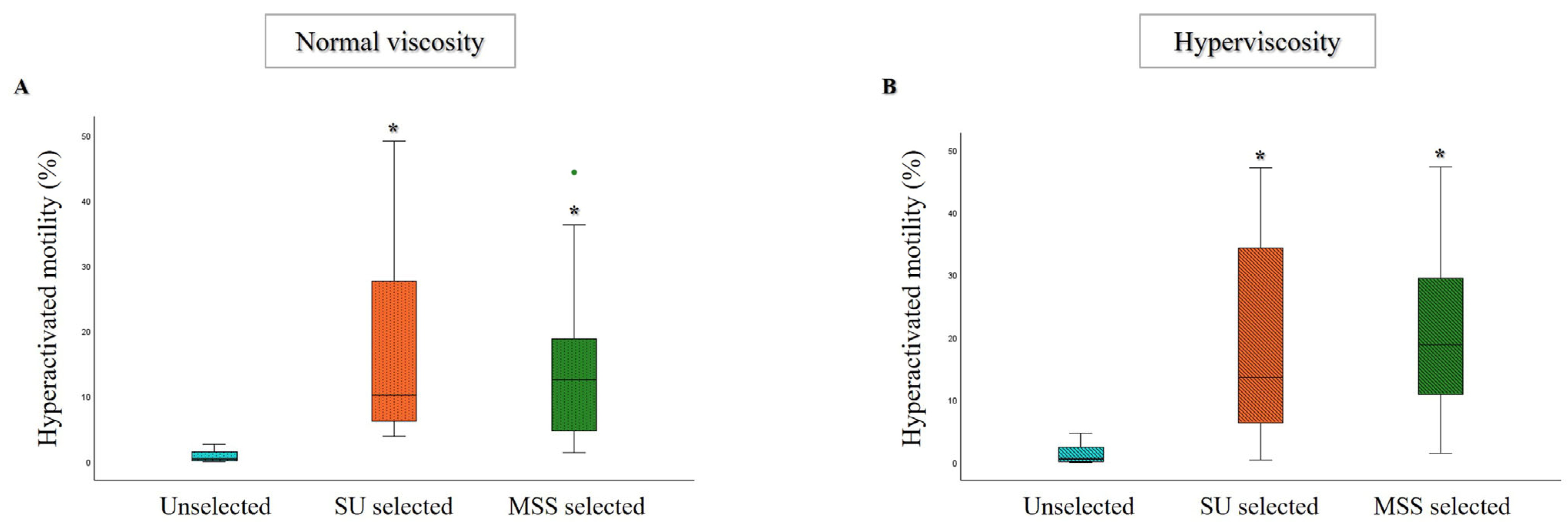

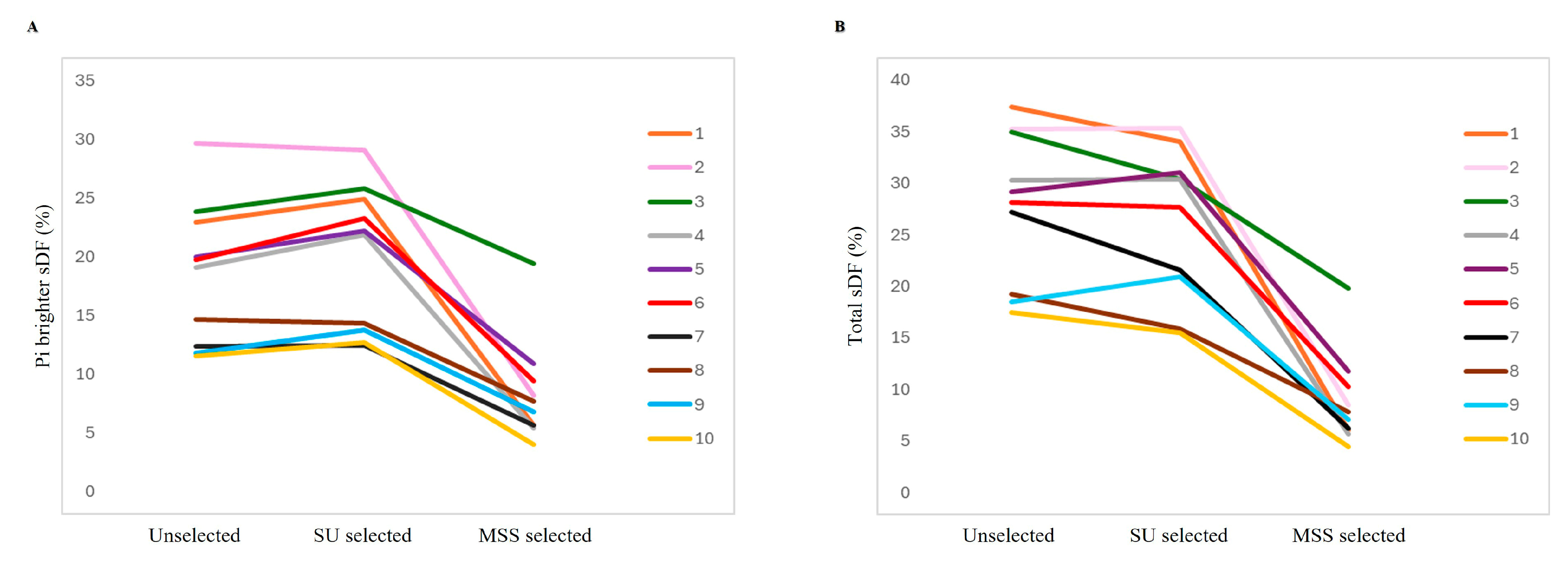
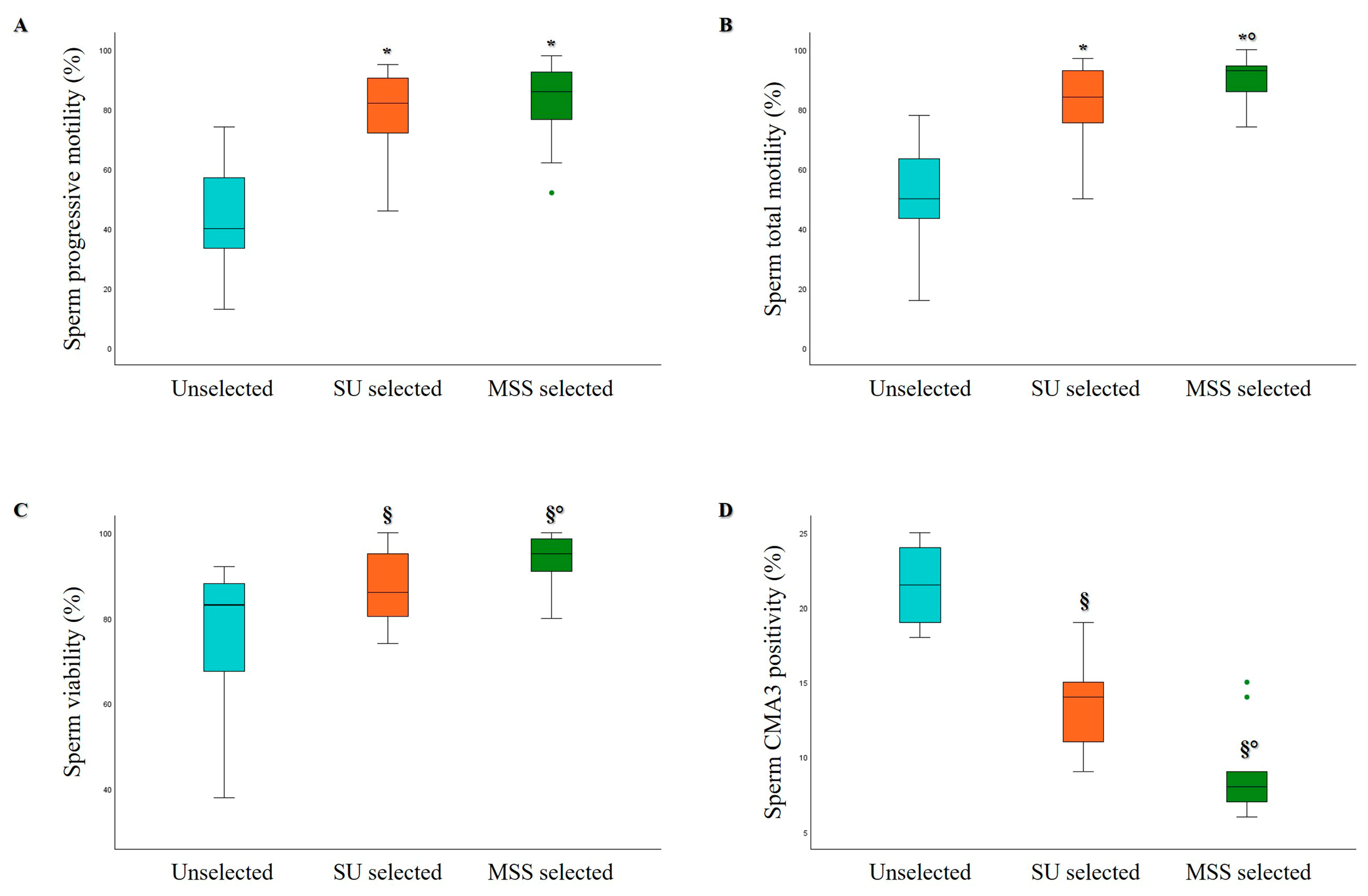
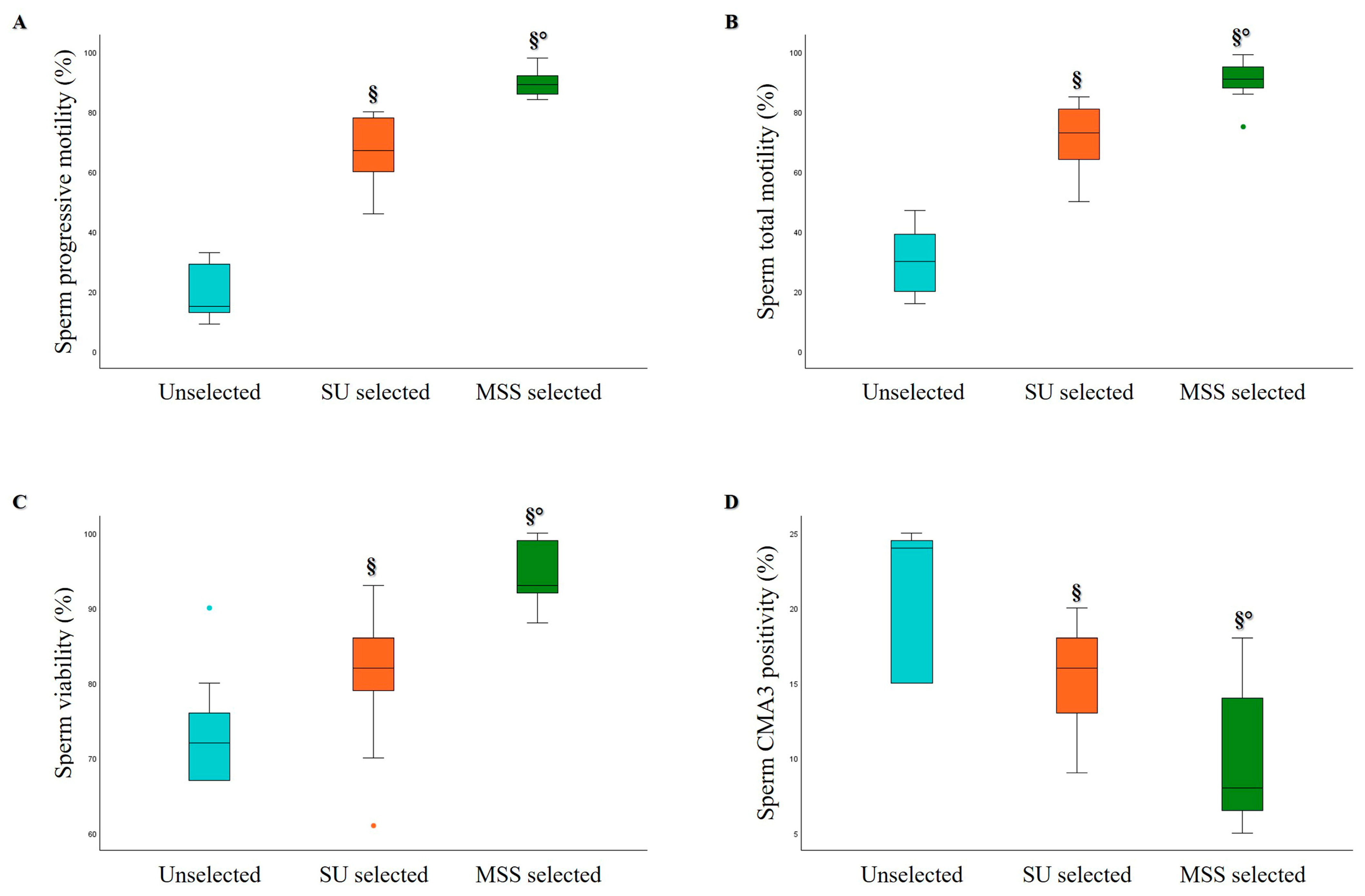

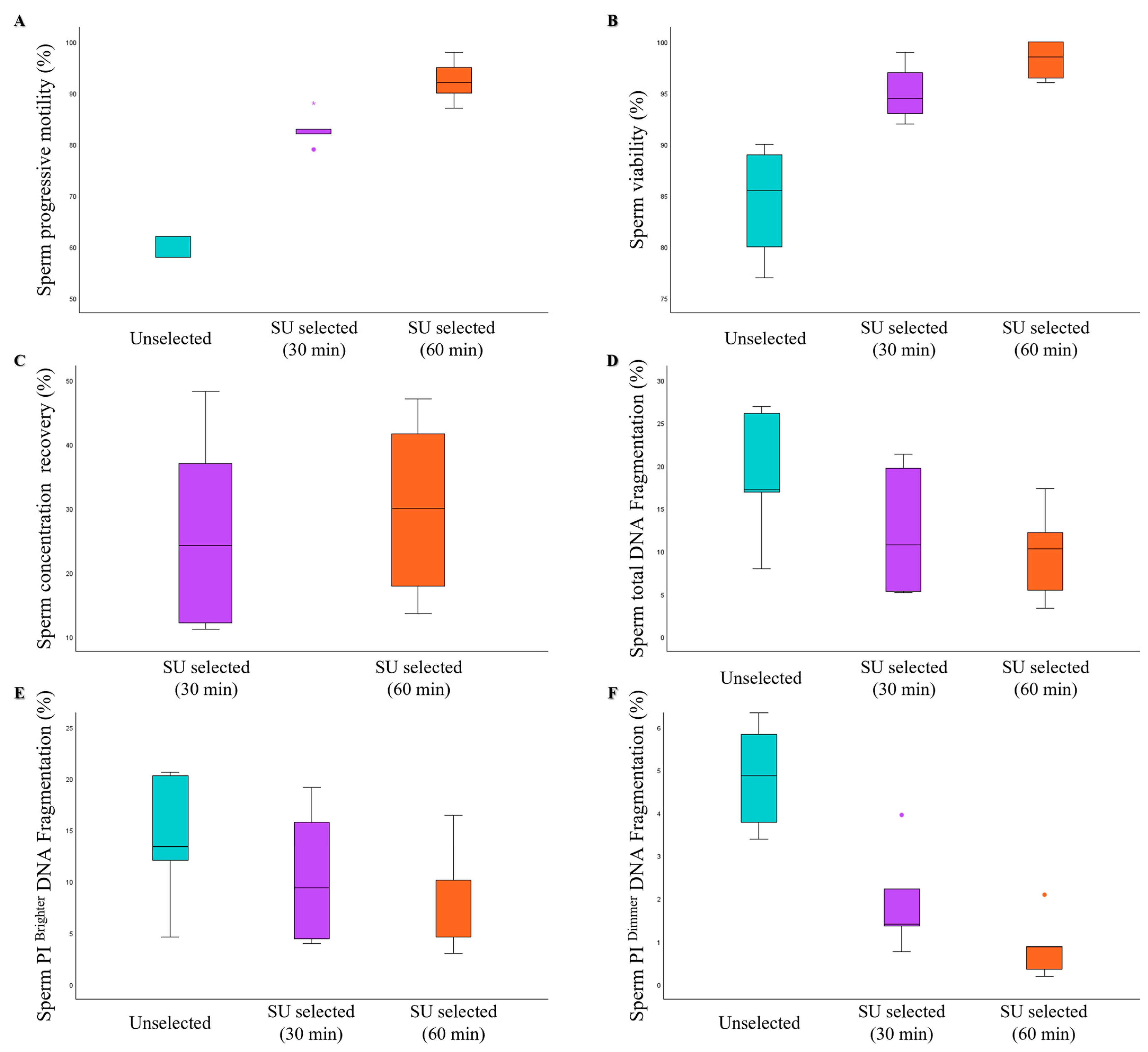
| Age (Years) | Abstinence (Days) | Volume (mL) | pH | Progressive Motility (%) | Total Motility (%) | Concentration (×106/mL) | Normal Morphology (%) | |
|---|---|---|---|---|---|---|---|---|
| All samples (n = 57) | 34 [28–37] | 4 [3–6] | 5.1 [4.1–5.6] | 7.8 [7.6–7.8] | 62.0 [56.0–67.5] | 66.0 [63.0–71.0] | 77.0 [52.0–119.5] | 5.0 [4.0–7.0] |
| Normal viscosity (n = 26) | 35.0 [30.5–37.0] | 5.0 [3.7–6.0] | 5.2 [4.5–5.7] | 7.8 [7.6–7.8] | 63.0 [57.5–67.5] | 66.5 [64.0–73.3] | 87.8 [59.8–172.5] | 5.0 [4.0–8.0] |
| Hyperviscosity (n = 31) | 33.0 [24.0–36.0] | 4.0 [3.0–6.0] | 4.9 [4.0–5.6] | 7.8 [7.6–8.0] | 61.0 [55.0–68.0] | 66.0 [60.0–71.0] | 71.0 [48.0–116.0] | 5.0 [4.0–6.0] |
| p | ns | ns | ns | ns | ns | ns | ns | ns |
| VAP (µm/s) | VSL (µm/s) | VCL (µm/s) | ALH (µm) | BCF (Hz) | LIN (%) | STR (%) | ||
|---|---|---|---|---|---|---|---|---|
| Normal viscosity | Unselected | 37.2 [33.9–40.6] | 28.2 [24.0–31.1] | 57.9 [35.1–66.4] | 4.2 [3.7–4.6] | 21.5 [20.3–24.6] | 45.0 [41.4–48.3] | 72.1 [68.0–75.5] |
| SU selected | 62.6 [55.7–72.2] * | 49.1 [41.3–57.3] * | 100.9 [92.4–126.1] * | 5.8 [5.1–6.9] * | 23.0 [21.0–24.8] | 49.3 [38.7–53.8] | 77.6 [70.8–80.1] § | |
| MSS selected | 67.8 [55.5–73.0] *° | 49.0 [44.9–62.2] *° | 108.9 [92.1–128.4] * | 6.2 [5.4–7.6]* | 21.1 [19.2–22.6] ^ | 50.8 [44.1–55.1] § | 77.4 [72.9–81.0] * | |
| Hyperviscosity | Unselected | 40.8 [31.0–44.0] | 31.0 [22.0–35.1] | 61.4 [51.8–70.5] | 3.9 [3.5–4.6] | 24.2 [21.5–26.4] | 44.6 [39.2–50.1] | 70.7 [66.9–77.7] |
| SU selected | 62.2 [48.2–73.4] * | 45.3 [37.3–55.5] * | 108.2 [82.2–141.7] * | 5.8 [4.2–8.0] * | 24.8 [22.2–28.6] | 44.1 [40.5–51.8] | 74.4 [68.9–80.3] § | |
| MSS selected | 68.2 [62.4–76.9] *° | 56.1 [47.3–62.9] *^ | 113.2 [105.1–136.2] *° | 6.8 [5.6–7.7] *° | 22.3 [20.1–23.5] §° | 46.9 [42.9–53.6] §° | 77.5 [73.3–82.7] *° | |
| Progressive Motility (%) | Total Motility (%) | Viability (%) | CellROX® Orange Positivity (%) | sDF (%) | |||
|---|---|---|---|---|---|---|---|
| Pi Brighter | Pi Dimmer | Total | |||||
| Unselected | 63.5 [55.5–69.5] | 67.5 [60.8–74.5] | 83.0 [73.8–91.3] | 9.8 [8.4–20.5] | 24.2 [19.6–28.7] | 11.2 [7.7–12.8] | 34.4 [30.0–38.7] |
| Direct SU selected | 85.5 [82.8–90.3] * | 90.0 [86.0–94.0] * | 96.5 [92.8–98.0] § | 11.4 [8.4–25.3] | 12.2 [8.3–15.2] * | 1.1 [0.4–3.5] * | 12.8 [9.1–17.3] * |
| SU selected | 85.5 [76.8–91.0] * | 89.0 [78.0–94.3] *# | 94.0 [87.3–96.5] *# | 17.8 [4.8–30.7] | 20.9 [13.2–24.6] §# | 5.4 [1.2–8.5] §# | 29.0 [18.2–33.3] §# |
| MSS selected | 93.0 [90.0–95.0] *°@ | 96.0 [93.8–97.3] *^@ | 100.0 [99.8–100.0] *^@ | 22.7 [9.1–29.6] | 9.9 [5.6–12.8] *° | 0.3 [0.2–0.6] *#° | 10.3 [6.0–14.1] *#^ |
| Age (Years) | Abstinence (Days) | Volume (mL) | pH | Progressive Motility (%) | Total Motility (%) | Concentration (×106/mL) | Normal Morphology (%) | |
|---|---|---|---|---|---|---|---|---|
| Oligozoospermic samples (n = 15) | 33.0 [26.0–37.0] | 3.0 [3.0–5.0] | 4.5 [3.8–5.8] | 7.8 [7.6–7.8] | 40.0 [33.0–57.0] | 50.0 [43.0–64.0] | 6.2 [4.1–8.8] | 2.0 [1.0–4.0] |
| Asthenozoospermic samples (n = 9) | 40.0 [28.5–44.0] | 4.0 [3.0–5.0] | 5.0 [4.3–6.1] | 7.8 [7.6–7.8] | 15.0 [11.5–30.5] | 30.0 [18.0–41.5] | 15.5 [8.1–19.0] | 1.0 [0.0–2.0] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traini, G.; Ragosta, M.E.; Tamburrino, L.; Papini, A.; Cipriani, S.; Vignozzi, L.; Baldi, E.; Marchiani, S. Microfluidic Sorting Can Be Applied for Assisted Reproduction Sperm Selection in Different Cases of Semen Abnormalities. Life 2025, 15, 790. https://doi.org/10.3390/life15050790
Traini G, Ragosta ME, Tamburrino L, Papini A, Cipriani S, Vignozzi L, Baldi E, Marchiani S. Microfluidic Sorting Can Be Applied for Assisted Reproduction Sperm Selection in Different Cases of Semen Abnormalities. Life. 2025; 15(5):790. https://doi.org/10.3390/life15050790
Chicago/Turabian StyleTraini, Giulia, Maria Emanuela Ragosta, Lara Tamburrino, Alice Papini, Sarah Cipriani, Linda Vignozzi, Elisabetta Baldi, and Sara Marchiani. 2025. "Microfluidic Sorting Can Be Applied for Assisted Reproduction Sperm Selection in Different Cases of Semen Abnormalities" Life 15, no. 5: 790. https://doi.org/10.3390/life15050790
APA StyleTraini, G., Ragosta, M. E., Tamburrino, L., Papini, A., Cipriani, S., Vignozzi, L., Baldi, E., & Marchiani, S. (2025). Microfluidic Sorting Can Be Applied for Assisted Reproduction Sperm Selection in Different Cases of Semen Abnormalities. Life, 15(5), 790. https://doi.org/10.3390/life15050790










