Real-World Study of Tildrakizumab Survival in Psoriasis: Impact of Arthritis, Hypertension, and Prior Biologic Use
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Drug Survival
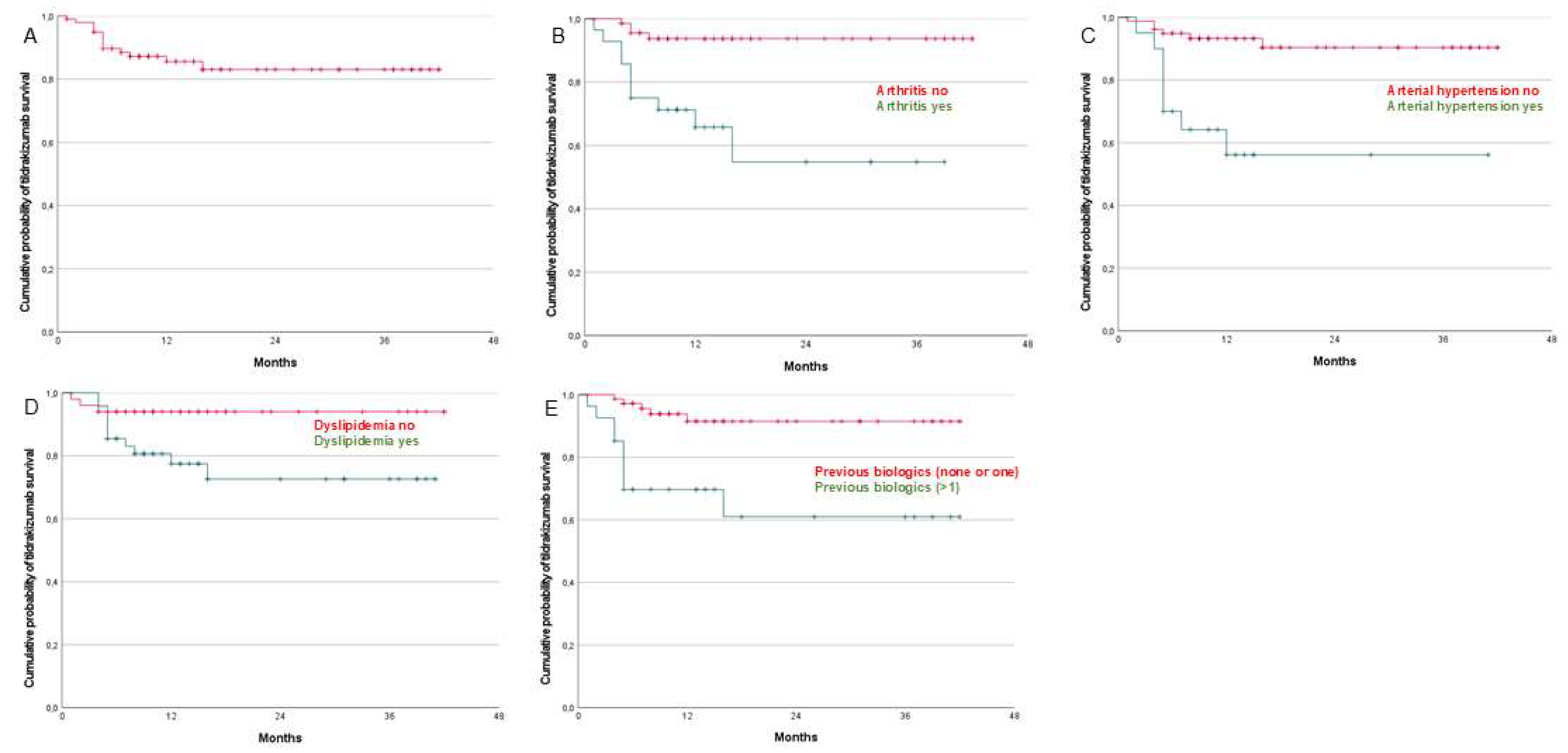
3.3. Univariate and Multivariate Analyses
3.4. Adverse Effects
3.5. Patients Discontinuing Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffiths, C.E.; Barker, J.N. Pathogenesis and Clinical Features of Psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Vičić, M.; Kaštelan, M.; Brajac, I.; Sotošek, V.; Massari, L.P. Current Concepts of Psoriasis Immunopathogenesis. Int. J. Mol. Sci. 2021, 22, 11574. [Google Scholar] [CrossRef] [PubMed]
- Ferrándiz, C.; Bordas, X.; García-Patos, V.; Puig, S.; Pujol, R.; Smandía, A. Prevalence of Psoriasis in Spain (Epiderma Project: Phase I). J. Eur. Acad. Dermatol. Venereol. 2001, 15, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, Y.; Cao, X. Global burden and future trends in psoriasis epidemiology: Insights from the global burden of disease study 2019 and predictions to 2030. Arch. Dermatol. Res. 2024, 316, 114. [Google Scholar] [CrossRef]
- Dogra, S.; Mahajan, R. Psoriasis: Epidemiology, Clinical Features, Co-Morbidities, and Clinical Scoring. Indian Dermatol. Online J. 2016, 7, 471–480. [Google Scholar] [CrossRef]
- Christophers, E. Psoriasis—Epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef]
- Sharma, A.; Upadhyay, D.K.; Gupta, G.D.; Narang, R.K.; Rai, V.K. IL-23/Th17 Axis: A Potential Therapeutic Target of Psoriasis. Curr. Drug Res. Rev. 2022, 14, 24–36. [Google Scholar] [CrossRef]
- Gladman, D.D. Psoriatic Arthritis. Dermatol. Ther. 2004, 17, 350–363. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761067Orig1s000MultdisciplineR.pdf (accessed on 12 May 2025).
- Dapavo, P.; Burlando, M.; Guarneri, C.; Megna, M.; Narcisi, A.; Talamonti, M.; Gisondi, P. Tildrakizumab: The Value of a Personalized and Flexible Approach for Treating Moderate-to-Severe Plaque Psoriasis in Patients with High Body Weight or High Disease Burden. Expert. Opin. Biol. Ther. 2024, 24, 133–138. [Google Scholar] [CrossRef]
- Banaszczyk, K. Tildrakizumab in the Treatment of Psoriasis—Literature Review. Reumatologia 2019, 57, 234–238. [Google Scholar] [CrossRef]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.H.; Prinz, J.C. The Role of IL-23 and the IL-23/TH 17 Immune Axis in the Pathogenesis and Treatment of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Seijo, P.; Dauden, E.; Carretero, G.; Ferrandiz, C.; Vanaclocha, F.; Gómez-García, F.-J.; Herrera-Ceballos, E.; De la Cueva-Dobao, P.; Belinchón, I.; Sánchez-Carazo, J.-L.; et al. Survival of Classic and Biological Systemic Drugs in Psoriasis: Results of the BIOBADADERM Registry and Critical Analysis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Van Den Reek, J.M.P.A.; Kievit, W.; Gniadecki, R.; Goeman, J.J.; Zweegers, J.; Van De Kerkhof, P.C.M.; Seyger, M.M.B.; De Jong, E.M.G.J. Drug Survival Studies in Dermatology:Principles, Purposes, and Pitfalls. J. Investig. Dermatol. 2015, 135, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Doval, I.; Dávila-Seijo, P. How Real Are “real-Life Studies” in Psoriasis, and the Uncertain Meaning of Drug Persistence. Br. J. Dermatol. 2019, 180, 15–16. [Google Scholar] [CrossRef]
- Carrascosa, J.M.; Notario, J. Supervivencia en terapia biológica. ¿Sabemos a qué nos referimos? ¿ Podemos usarla? Actas Dermo-Sifiliogr. 2014, 105, 729–733. [Google Scholar] [CrossRef]
- Becher, G.; Conner, S.; Ingram, J.A.; Stephen, K.E.; McInnes, A.C.; Heald, A.H.; Riley, P.A.; Davies, M.; Domenech, A.; Kasujee, I. A Retrospective Real-World Study of the Effectiveness and Tolerability of Tildrakizumab in UK Adults with Moderate-to-Severe Chronic Plaque Psoriasis. Dermatol. Ther. 2022, 12, 2343–2354. [Google Scholar] [CrossRef]
- Ruggiero, A.; Fabbrocini, G.; Cinelli, E.; Ocampo Garza, S.S.; Camela, E.; Megna, M. Anti-interleukin-23 for Psoriasis in Elderly Patients: Guselkumab, Risankizumab and Tildrakizumab in Real-world Practice. Clin. Experimental. Derm. 2022, 47, 561–567. [Google Scholar] [CrossRef]
- Ruggiero, A.; Picone, V.; Martora, F.; Fabbrocini, G.; Megna, M. Guselkumab, Risankizumab, And Tildrakizumab in the Management of Psoriasis: A Review of the Real-World Evidence. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1649–1658. [Google Scholar] [CrossRef]
- Burlando, M.; Maul, J.-T.; Salvi, I.; Simic, D.; Cozzani, E.; Ak, M.; Birkenmaier, I.; Parodi, A. Psoriasis Patients’ Characteristics Associated with High PASI Response to Tildrakizumab: An International Dual Center Study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6772–6776. [Google Scholar] [CrossRef]
- Burlando, M.; Castelli, R.; Cozzani, E.; Parodi, A. Treatment of Moderate-to-Severe Plaque Psoriasis with Tildrakizumab in the Real-Life Setting. Drugs Context 2021, 10, 2021-2-6. [Google Scholar] [CrossRef]
- Narcisi, A.; Valenti, M.; Gargiulo, L.; Ibba, L.; Amoruso, F.; Argenziano, G.; Bardazzi, F.; Burlando, M.; Carrera, C.G.; Damiani, G.; et al. Real-Life Effectiveness of Tildrakizumab in Chronic Plaque Psoriasis: A 52-Week Multicentre Retrospective Study-IL PSO (Italian Landscape Psoriasis). J. Eur. Acad. Dermatol. Venereol. 2023, 37, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Caldarola, G.; Galluzzo, M.; Bernardini, N.; Calabrese, L.; Grimaldi, M.; Moretta, G.; Pagnanelli, G.; Shumak, R.G.; Talamonti, M.; Tofani, L.; et al. Tildrakizumab in Moderate-to-Severe Plaque Psoriasis: A Multicenter, Retrospective, Real-Life Study. Dermatol. Ther. 2022, 35, e15488. [Google Scholar] [CrossRef] [PubMed]
- Heim, J.; Vasquez, J.G.; Bhutani, T.; Koo, J.; Mathew, J.; Gogineni, R.; Ferro, T.; Bhatia, N. Tildrakizumab Real-World Effectiveness and Safety Over 64 Weeks in Patients with Moderate-to-Severe Plaque Psoriasis. J. Drugs Dermatol. 2024, 23, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Heim, J.; Vasquez, J.G.; Bhutani, T.; Schenkel, B.; Gogineni, R.; Koo, J. Long-Term Quality of Life Outcomes from a Phase 4 Study of Tildrakizumab in Patients with Moderate-to-Severe Plaque Psoriasis in a Real-World Setting. J. Dermatolog. Treat 2024, 35, 2310631. [Google Scholar] [CrossRef]
- Cacciapuoti, S.; Potestio, L.; Gallo, L.; Musumeci, M.L.; Caldarola, G.; D’Amico, D.; Caudullo, F.; Papaianni, V.; De Simone, C.; Peris, K.; et al. Short-Term Efficacy of Tildrakizumab on Difficult-to-Treat Areas: A Real-World Experience. Int. J. Dermatol. 2024, 64, 319–324. [Google Scholar] [CrossRef]
- Bhutani, T.; Koo, J.; Heim, J.; Bhatia, N.; Mathew, J.; Ferro, T.; Vasquez, J.G. Improvements in Psoriasis-Related Work Productivity with Tildrakizumab: Results from a Phase 4 Real-World Study in Patients with Moderate-to-Severe Plaque Psoriasis. Dermatol. Ther. 2024, 14, 1019–1025. [Google Scholar] [CrossRef]
- Armstrong, A.; González-Cantero, A.; Khattri, S.; Muzy, G.; Malatestinic, W.N.; Lampropoulou, A.; Feely, M.; See, S.K.; Mert, C.; Blauvelt, A. Comparing Achievement of National Psoriasis Foundation Treatment Targets among Patients with Plaque Psoriasis Treated with Ixekizumab versus Other Biologics in Clinical and Real-World Studies. Dermatol. Ther. 2024, 14, 933–952. [Google Scholar] [CrossRef]
- Orsini, D.; Caldarola, G.; Dattola, A.; Campione, E.; Bernardini, N.; Frascione, P.; De Simone, C.; Richetta, A.G.; Galluzzo, M.; Skroza, N.; et al. Efficacy and Safety of Tildrakizumab in Elderly Patients: Real-World Multicenter Study (ESTER-Study). J. Dermatolog. Treat. 2024, 35, 2319304. [Google Scholar] [CrossRef]
- Campione, E.; Lambiase, S.; Gaeta Shumak, R.; Galluzzo, M.; Lanna, C.; Costanza, G.; Borselli, C.; Artosi, F.; Cosio, T.; Tofani, L.; et al. A Real-Life Study on the Use of Tildrakizumab in Psoriatic Patients. Pharmaceuticals 2023, 16, 526. [Google Scholar] [CrossRef]
- Burlando, M.; Salvi, I.; Parodi, A.; Cozzani, E. A 3-Year Experience with Tildrakizumab Treatment for Patients with Plaque Psoriasis in Clinical Practice. Dermatol. Ther. 2024, 14, 2645–2652. [Google Scholar] [CrossRef]
- Torres, T.; Varela, P.; Mendes Bastos, P.; Magina, S.; Henrique, M.; Ferreira, P. Tildrakizumab for the Treatment of Moderate-to-Severe Psoriasis: A 52-Week, Real-World Portuguese Multicentric Study. Drugs Context 2024, 13, 2023-12-15. [Google Scholar] [CrossRef] [PubMed]
- Heim, J.; Vasquez, J.G.; Schenkel, B.; Bhatia, N. Real-World Effectiveness and Safety of Tildrakizumab in Patients with Moderate-to-Severe Psoriasis: Week 28 Interim Analysis of a Phase 4 Study. J. Drugs Dermatol. 2023, 22, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Berenguer-Ruiz, S.; Aparicio-Domínguez, M.; Herranz-Pinto, P.; Ruíz-Villaverde, R.; López-Ferrer, A.; Santos-Juanes, J.; Rodríguez Fernández-Freire, L.; Hospital-Gil, M.; Arias-Santiago, S.; Carretero-Hernández, G.; et al. Effectiveness and Safety of Tildrakizumab for the Treatment of Psoriasis in Real-World Settings at 24 Weeks: A Retrospective, Observational, Multicentre Study by the Spanish Psoriasis Group. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Villaverde, R.; Rodriguez Fernandez-Freire, L.; Font-Ugalde, P.; Galan-Gutierrez, M. Tildrakizumab: Efficacy, Safety and Survival in Mid-Term (52 Weeks) in Three Tertiary Hospitals in Andalucia (Spain). J. Clin. Med. 2022, 11, 5098. [Google Scholar] [CrossRef]
- Elgaard, C.D.B.; Iversen, L.; Hjuler, K.F. Guselkumab, Tildrakizumab, and Risankizumab in a Real-World Setting: Drug Survival and Effectiveness in the Treatment of Psoriasis and Psoriatic Arthritis. J. Dermatolog. Treat 2023, 34, 2133531. [Google Scholar] [CrossRef]
- Melgosa Ramos, F.J.; Mateu Puchades, A.; Matáix-Díaz, J.; Schneller-Pavelescu, L.; Belinchón-Romero, I.; Santos Alarcón, S. 52-Week Mid-Term Efficacy of Tildrakizumab in Moderate-to-Severe Psoriasis: A Real-Life Multicenter Experience. Actas Dermosifiliogr. 2024, 115, T722–T726. [Google Scholar] [CrossRef]
- Gargiulo, L.; Ibba, L.; Malagoli, P.; Balato, A.; Bardazzi, F.; Burlando, M.; Carrera, C.G.; Damiani, G.; Dapavo, P.; Dini, V.; et al. Drug Survival of IL-12/23, IL-17 and IL-23 Inhibitors for Moderate-to-Severe Plaque Psoriasis: A Retrospective Multicenter Real-World Experience on 5932 Treatment Courses–IL PSO (Italian Landscape Psoriasis). Front. Immunol. 2024, 14, 1341708. [Google Scholar] [CrossRef]
- Tsianakas, A.; Schwichtenberg, U.; Pierchalla, P.; Hinz, T.; Diemert, S.; Korge, B. Real-World Effectiveness and Safety of Tildrakizumab in Long-Term Treatment of Plaque Psoriasis: Results from the Non-Interventional, Prospective, Multicentre Study TILOT. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 85–92. [Google Scholar] [CrossRef]
- Drerup, K.A.; Seemann, C.; Gerdes, S.; Mrowietz, U. Effective and Safe Treatment of Psoriatic Disease with the Anti-IL-23p19 Biologic Tildrakizumab: Results of a Real-World Prospective Cohort Study in Nonselected Patients. Dermatology 2022, 238, 615–619. [Google Scholar] [CrossRef]
- Galache Osuna, C.; Gómez-Vila, B.; Aubán Pariente, J.; Vázquez Losada, B.; Gómez de Castro, C.; Requena López, S.; de Dios Velázquez, Á.; Palacios García, L.; Ordoñez Fernández, L.; Gómez Diez, S.; et al. Ustekinumab Drug Survival in Patients with Psoriasis: A Retrospective Study of Real Clinical Practice. Medicina 2020, 56, 584. [Google Scholar] [CrossRef]
- Gómez-de Castro, C.; Mir-Bonafé, M.; Arias-Martínez, A.; Martínez-Camblor, P.; Díaz-Coto, S.; Santos-Juanes, J. Comment on ‘Baseline Patients’ Characteristics as Predictors for Therapeutic Survival and Response in Patients with Psoriasis on Biological Treatments’. Australas J. Dermatol. 2019, 60, 13016. [Google Scholar] [CrossRef] [PubMed]
- Palacios-García, L.; Gómez-de Castro, C.; Mir-Bonafé, M.; Calzón, C.; Galache, C.; Santos-Juanes, J. Comment on “Secukinumab Drug Survival in Patients with Psoriasis: A Multicenter, Real-World, Retrospective Study”. J. Am. Acad. Dermatol. 2019, 81, e81–e82. [Google Scholar] [CrossRef] [PubMed]
- Thaci, D.; Piaserico, S.; Warren, R.B.; Gupta, A.K.; Cantrell, W.; Draelos, Z.; Foley, P.; Igarashi, A.; Langley, R.G.; Asahina, A.; et al. Five-Year Efficacy and Safety of Tildrakizumab in Patients with Moderate-to-Severe Psoriasis Who Respond at Week 28: Pooled Analyses of Two Randomized Phase III Clinical Trials (reSURFACE 1 and reSURFACE 2). Br. J. Dermatol. 2021, 185, 323–334. [Google Scholar] [CrossRef]
- Ricceri, F.; Chiricozzi, A.; Peris, K.; Prignano, F. Successful Use of Anti-IL-23 Molecules in Overweight-to-Obese Psoriatic Patients: A Multicentric Retrospective Study. Dermatol. Ther. 2022, 35, e15793. [Google Scholar] [CrossRef]
- Griss, J.; Ratzinger, G.; Maul, J.-T.; Weger, W.; Thaçi, D.; Carrascosa, J.M.; Jonak, C. No Impact of Disease Duration on Response to Tildrakizumab Treatment among Patients with Moderate-to-Severe Plaque Psoriasis: Post Hoc Analyses from Two Phase 3 (reSURFACE 1 and reSURFACE 2) and One Phase 4 (TRIBUTE) Studies. Skin Health Dis. 2023, 3, e263. [Google Scholar] [CrossRef]
- Dattola, A.; Bernardini, N.; Svara, F.; Balato, A.; Caldarola, G.; D’Amico, D.; De Simone, C.; Di Brizzi, E.V.; Esposito, M.; Giofrè, C.; et al. Effectiveness of tildrakizumab 200 mg: An Italian multicenter study. J. Dermatolog. Treat. 2024, 35, 2420825. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.; Ibba, L.; Di Giulio, S.; Gargiulo, L.; Malagoli, P.; Balato, A.; Bardazzi, F.; Loconsole, F.; Burlando, M.; Cagni, A.E.; et al. Optimizing Tildrakizumab Dosing in Psoriasis: A 52-Week Multicenter Retrospective Study Comparing 100 mg and 200 mg-IL PSO (Italian Landscape Psoriasis). Dermatol. Ther. 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mastorino, L.; Dapavo, P.; Susca, S.; Cariti, C.; Siliquini, N.; Verrone, A.; Stroppiana, E.; Ortoncelli, M.; Quaglino, P.; Ribero, S. Drug Survival and Clinical Effectiveness of Secukinumab, Ixekizumab, Brodalumab, Guselkumab, Risankizumab, Tildrakizumab for Psoriasis Treatment. JDDG J. Dtsch. Dermatol. Ges. 2024, 22, 34–42. [Google Scholar] [CrossRef]
- Mastorino, L.; Cariti, C.; Susca, S.; Sciamarrelli, N.; Borriello, S.; Ortoncelli, M.; Stroppiana, E.; Verrone, A.; Dapavo, P.; Quaglino, P.; et al. Tildrakizumab in Real-Life Shows Good Efficacy in Moderate-to-Severe Psoriasis Regardless of Previous Use of Biologic Drugs and Joint Involvement. Dermatol. Ther. 2022, 35, e15818. [Google Scholar] [CrossRef]
- Galache-Osuna, C.; Reyes-García, S.; Salgueiro, E.; Bordallo-Landa, J.; Lozano, A.; Vázquez-López, F.; Santos-Juanes, J. Retrospective Study of Apremilast Drug Survival in Psoriasis Patients in a Daily Practice Setting: A Long-Term Experience. Dermatol. Ther. 2022, 35, e15583. [Google Scholar] [CrossRef]
- Di Brizzi, E.V.; Buononato, D.; Benvenuto, P.; Argenziano, G.; De Pasquale, R.; Fiorella, C.S.; Giofrè, C.; Musumeci, M.L.; Palazzo, G.; Zichichi, L.; et al. Effectiveness and Safety After a Switch to Tildrakizumab: A Real World Multicenter Italian Study in Psoriasis. Dermatol. Pract. Concept 2023, 13, e2023215. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.P.; Dauden, E.; Gerdes, S.; Lebwohl, M.G.; Menter, M.A.; Leonardi, C.L.; Gooderham, M.; Gebauer, K.; Tada, Y.; Lacour, J.P.; et al. Tildrakizumab Efficacy and Safety in Patients with Psoriasis and Concomitant Metabolic Syndrome: Post Hoc Analysis of 5-Year Data from reSURFACE 1 and reSURFACE 2. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Ibba, L.; Di Giulio, S.; Gargiulo, L.; Facheris, P.; Perugini, C.; Costanzo, A.; Narcisi, A.; Valenti, M. Long-term effectiveness and safety of risankizumab in patients with moderate-to-severe psoriasis with and without cardiometabolic comorbidities: A single-center retrospective study. J. Dermatolog. Treat. 2024, 35, 2425029. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, E.L.M.; Van den Reek, J.M.P.A.; Gaarn Du Jardin, K.; Barbero-Castillo, A.; De Jong, E.M.G.J.; Lubeek, S.F.K. Efficacy and Safety of Tildrakizumab in Older Patients: Pooled Analyses of Two Randomized Phase III Clinical Trials (reSURFACE 1 and reSURFACE 2) Through 244 Weeks. Acta. Derm. Venereol. 2023, 103, adv17752. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Coscarella, G.; Puig, L.; Vender, R.; Yeung, J.; Carrascosa, J.-M.; Piaserico, S.; Gisondi, P.; Lynde, C.; Ferreira, P.; et al. Age Affects Drug Survival Rates of Interleukin (IL)-17 and IL-23 Inhibitors in Patients with Plaque Psoriasis: Results from a Retrospective, Multicentric, Multi-Country, Cohort Study. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 2175–2185. [Google Scholar] [CrossRef]
- Galluzzo, M.; Talamonti, M.; Cioni, A.; Maffei, V.; Shumak, R.G.; Tofani, L.; Bianchi, L.; Campione, E. Efficacy of Tildrakizumab for the Treatment of Difficult-to-Treat Areas: Scalp, Nail, Palmoplantar and Genital Psoriasis. J. Clin. Med. 2022, 11, 2631. [Google Scholar] [CrossRef]
| Total Patients (n = 100) | |
|---|---|
| Sex (male), n (%) | 54 (54%) male |
| Age at start of biologic treatment (years), mean ± SD | 48.4 ± 15.6 |
| Positive family history of psoriasis (yes), n (%) | 63 (63%) |
| Onset before 40 years of age (%) | 74 (74%) |
| Duration of treatment (months); mean ± SD | 26.7 (IC95% = 23–29.72) |
| Initial PASI | 13.2 ± 6.1 |
| Tildrakizumab start age | 48.23 ± 15.26 |
| Age ≥ 65 | 16 (16%) |
| Comorbidities, n (%) | |
| Obesity (BMI ≥ 30) | 40 (30%) |
| Diabetes mellitus | 17% |
| Arterial hypertension | 21 (21%) |
| Dyslipidemia | 50 (50%) |
| Arthritis | 28 (28%) |
| Prior treatments with biologics (%) | 97 (97%) |
| One biologic | 69 (69%) |
| Two biologics | 18 (18%) |
| Three biologics | 4 (4%) |
| Four biologics | 3 (3%) |
| Five Biologics | 3 (3%) |
| Percentage (95% CI) | 1 Year | 2 Years | 3 Years | 4 Years |
|---|---|---|---|---|
| Global | 85 (78–93) | 81 (71–91) | 81 (71–91) | 81 (71–91) |
| Arthritis no | 93 (87–99) | 93 (87–99) | 93 (87–99) | 93 (87–99) |
| Arthritis yes | 68 (58–48) | 54 (42–66) | 54 (42–66) | 54 (42–66) |
| Univariate Analysis | HR (CI = 95%) | p-Value |
|---|---|---|
| Psoriasis onset ≥ 40 years 0.332 | 1.718 (0.575–5.135) | 0.332 |
| Sex (male) 0.810 | 1.137 (0.398–3.244) | 0.810 |
| Obesity: BMI ≥ 30 | 1.942 (0.674–5.598) | 0.219 |
| Arthritis: yes | 6.851 (2.141–21,920) | 0.001 |
| Hypertension arterial: yes | 6.275 (2.142–18.382) | <0.001 |
| Dyslipidemia: yes | 3.677 (1.026–13.185) | 0.030 |
| Family history: yes | 1.134 (0.380–3.384) | 0.822 |
| Diabetes: yes | 2.008 (0.628–6.413) | 0.240 |
| Previous biologics treatment > 1 | 5.581 (1.868–16.678) | <0.001 |
| Older than 65 | 1.436 (0.400–5.153) | 0.579 |
| Multivariate Analysis | HR (CI = 95%) | p-Value |
| Arthritis | 4.098 (1.206–13.925) | 0.024 |
| Hypertension arterial | 4.673 (1.410–15.488) | 0.012 |
| Previous biologic treatments > 1 | 3.704 (1.192–11.514) | 0.024 |
| Dyslipidemia | 1.245 (0.299–5.178) | 0.763 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Juanes Galache, R.; Reyes García, S.; Carrero Martín, J.; Nuñez Domínguez, Á.; López Pando, M.; Álvarez Losada, I.; de la Fuente Villaverde, I.; Lozano-Blazquez, A.; Salgueiro, E.; Bordallo, J.; et al. Real-World Study of Tildrakizumab Survival in Psoriasis: Impact of Arthritis, Hypertension, and Prior Biologic Use. Life 2025, 15, 789. https://doi.org/10.3390/life15050789
Santos-Juanes Galache R, Reyes García S, Carrero Martín J, Nuñez Domínguez Á, López Pando M, Álvarez Losada I, de la Fuente Villaverde I, Lozano-Blazquez A, Salgueiro E, Bordallo J, et al. Real-World Study of Tildrakizumab Survival in Psoriasis: Impact of Arthritis, Hypertension, and Prior Biologic Use. Life. 2025; 15(5):789. https://doi.org/10.3390/life15050789
Chicago/Turabian StyleSantos-Juanes Galache, Raquel, Sebastian Reyes García, Jimena Carrero Martín, Álvaro Nuñez Domínguez, Marta López Pando, Irene Álvarez Losada, Irene de la Fuente Villaverde, Ana Lozano-Blazquez, Esther Salgueiro, Javier Bordallo, and et al. 2025. "Real-World Study of Tildrakizumab Survival in Psoriasis: Impact of Arthritis, Hypertension, and Prior Biologic Use" Life 15, no. 5: 789. https://doi.org/10.3390/life15050789
APA StyleSantos-Juanes Galache, R., Reyes García, S., Carrero Martín, J., Nuñez Domínguez, Á., López Pando, M., Álvarez Losada, I., de la Fuente Villaverde, I., Lozano-Blazquez, A., Salgueiro, E., Bordallo, J., Santos-Juanes, J., & Galache Osuna, C. (2025). Real-World Study of Tildrakizumab Survival in Psoriasis: Impact of Arthritis, Hypertension, and Prior Biologic Use. Life, 15(5), 789. https://doi.org/10.3390/life15050789







